Abstract
Homology-directed repair (HDR) of breaks induced by the RNA-programmed nuclease Cas9 has become a popular method for genome editing in several organisms. Most HDR protocols rely on plasmid-based expression of Cas9 and the gene-specific guide RNAs. Here we report that direct injection of in vitro–assembled Cas9-CRISPR RNA (crRNA) trans-activating crRNA (tracrRNA) ribonucleoprotein complexes into the gonad of Caenorhabditis elegans yields HDR edits at a high frequency. Building on our earlier finding that PCR fragments with 35-base homology are efficient repair templates, we developed an entirely cloning-free protocol for the generation of seamless HDR edits without selection. Combined with the co-CRISPR method, this protocol is sufficiently robust for use with low-efficiency guide RNAs and to generate complex edits, including ORF replacement and simultaneous tagging of two genes with fluorescent proteins.
Keywords: CRISPR-Cas9, genome editing, homology-directed repair, ribonucleoprotein complexes, C. elegans
THE CRISPR-Cas9 system is a bacterial adaptive immune system that has been harnessed as a powerful genome editing tool (Doudna and Charpentier 2014). Cas9 is a nuclease that functions with two small RNAs: CRISPR RNA (crRNA), which guides Cas9 to complementary target sequences, and trans-activating crRNA (tracrRNA), which binds to the crRNA and to Cas9 to form the ribonucleoprotein (RNP) complex (Deltcheva et al. 2011). For use in genome editing, the crRNA and tracrRNA are often combined into a single chimeric guide RNA (sgRNA) (Jinek et al. 2012). Expression of Cas9 and sgRNA in cells leads to cleavage of complementary genomic sequences. The double-strand breaks are repaired by endogenous cellular pathways, including end-joining mechanisms [e.g., nonhomologous end joining (NHEJ) and theta-mediated end joining (TMEJ)] and homology-dependent repair (HDR) mechanisms (van Schendel et al. 2015). End joining typically introduces random insertions/deletions at the DNA break site, which can disrupt gene activity. HDR, in contrast, is a more precise repair process that uses a repair template. If the repair template contains edits flanked by sequences that are homologous to the cleavage site (homology arms), the edits will be incorporated by gene conversion. The high efficiency of Cas9 and the simplicity of guide RNA design have made it possible to develop end-joining protocols to systematically knock out genes (Hsu et al. 2014; Shah et al. 2015). In principle, scalable HDR protocols also could be used for systematic knock-ins of custom edits [such as green fluorescent protein (GFP)]. Unfortunately, current HDR protocols are inefficient (Hsu et al. 2014). First, most HDR protocols require cloning to create a repair template and a guide RNA expression vector for each gene to be targeted. Second, the efficiency of HDR typically is low, requiring the screening of large number of animals or the use of selection markers that are integrated alongside the desired edits (Dickinson et al. 2013, 2015).
Recently, we found in Caenorhabditis elegans that linear DNAs with homology arms as short as 35 bases can support the efficient incorporation of HDR edits (Paix et al. 2014). This finding simplifies the construction of donor templates. For example, a donor template to introduce GFP can be synthesized using two 55-base oligos, each containing 35 bases that are homologous to the targeted locus and 20 bases that are homologous to GFP. The PCR amplicon is injected alongside plasmids coding for Cas9 and the sgRNA into the gonad of adult hermaphrodites, and their progeny are screened for GFP expression by visual inspection or by PCR screening. Using this method, we were able to recover GFP fusions at six of eight loci attempted. HDR frequencies were high enough to avoid the use of coselection makers but were still relatively low and variable (0.4–12%) (Paix et al. 2014). Other researchers also have reported difficulties in obtaining HDR edits, a problem that can be partially overcome by improvements in the design of guide RNAs and repair templates (Farboud and Meyer 2015; Katic et al. 2015).
Two additional factors are likely to also contribute to low edit frequency. First, the efficiency of expressing and assembling Cas9–sgRNA complexes from plasmids is not known and may be low given the tendency of C. elegans germ cells to silence foreign DNA (Kelly et al. 1997). In mouse embryos and mammalian cells, direct delivery of Cas9 complexes assembled in vitro has been reported to yield a higher frequency of edits (Lin et al. 2014; Aida et al. 2015; Liang et al. 2015). Injection of Cas9 complexes has been used in C. elegans to create edits by end-joining mechanisms (Cho et al. 2013) but has not yet been compared directly to plasmid delivery or used for HDR.
A second factor that contributes to the low edit frequency is that many injected hermaphrodites generate no edits. For reasons that remain unclear, we have found that edits tend to cluster among the progeny of a minority of injected mothers (jackpot broods) (Paix et al. 2014). Identification of jackpot broods before screening would eliminate the need to screen the broods of nonproductive hermaphrodites. Recently, three reports have described methods to enrich for desired edits by selecting or screening for editing at a second marker locus (Arribere et al. 2014; Kim et al. 2014; Ward 2015). In the method described by Arribere et al. (2014), a dominant mutation is introduced by HDR in the marker locus dpy-10, leading to a roller phenotype that is easily identified among the progeny of injected hermaphrodites.
Here we report the development of a new direct-delivery protocol that combines injection of in vitro–synthesized and –assembled Cas9-crRNA-tracrRNA complexes with the dpy-10 co-CRISPR approach of Arribere et al. (2014) to identify jackpot broods. The direct-delivery protocol is entirely cloning-free and generates edits at frequencies ranging from 2 to 70% of F1 progeny, a 10-fold improvement over our plasmid-based earlier method (Paix et al. 2014). This new protocol permits the use of inefficient sgRNAs that failed in our previous study and expands the repertoire of possible genome edits to include ORF swaps and fluorescent protein (FP) tagging of two genes at once.
Materials and Methods
Supplementary materials include protocols for direct-delivery editing (Supporting Information, File S1) and for Cas9 protein purification (File S2), as well as lists of plasmids (Table S2), crRNAs (Table S3), rescue templates (Table S4), and strains (Table S5).
Data availability
Strains and plasmids are available from the Caenorhabditis Genetics Center (CGC) and Addgene, or upon request.
Results and Discussion
Injection of Cas9 RNP complexes supports robust HDR
We developed a simple method to purify from Escherichia coli recombinant Cas9 [fused at its C-terminus with a nuclear localization sequence (Fu et al. 2014)] and assemble Cas9-crRNA-tracrRNA RNP complexes in the presence of repair templates and in a buffer suited for injection into C. elegans (File S1 and File S2). Cas9 also can be obtained from a commercial source, as in Cho et al. (2013). As in the co-CRISPR method of Arribere et al. (2014), we co-injected Cas9 complexes and repair templates targeting the marker gene dpy-10 and a second target locus. dpy-10 was repaired with a single-strand oligodeoxyribonucleotide (ssODN) that introduces a missense mutation in the dpy-10 ORF (Arribere et al. 2014). The missense mutation causes a dominant roller phenotype that is easily spotted under a dissecting microscope. For the second locus, we first used gtbp-1 (aka K08F4.2), a nonessential gene expressed in most tissues, which we targeted previously using plasmid delivery (Paix et al. 2014). We introduced a single cut near the C-terminus of gtbp-1 and repaired the lesion with an ∼700-base double-stranded PCR fragment containing the ORF for a fluorescent protein [red fluorescent protein (RFP) or GFP] flanked by 35-base arms that were homologous to sequences immediately surrounding the cut site (Figure 1).
Figure 1.
Co-CRISPR strategy. (A) We cotargeted (1) the dpy-10 locus with a ssODN repair template to introduce a missense mutation leading to the dominant roller phenotype (Arribere et al. 2014) and (2) a second locus (gene of interest) with a PCR repair template to insert a fluorescent protein (FP) near the C-terminus. (B) Experimental outline. The gonads of 10–20 hermaphrodites are injected, and their broods are examined for the presence of rollers (dpy-10 edits) and FP+ animals. In typical experiments, >50% of hermaphrodites segregate rollers. Jackpot broods are the broods with the highest numbers of rollers. Edits at the gene of interest (pink) are found in both roller and nonroller worms, but only among broods that contain rollers.
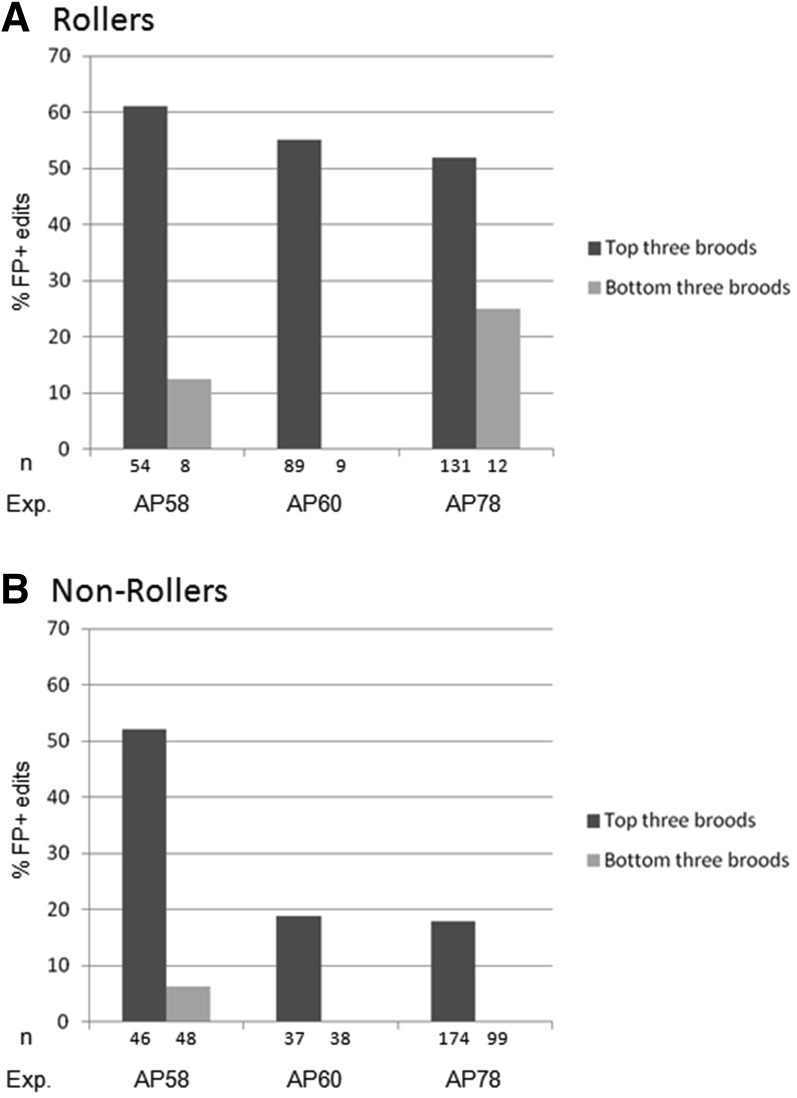
For three experiments (Table 1 and Table S1, experiments AP58, AP60, and AP78), we examined the broods of all injected hermaphrodites by visual inspection for the presence of roller and fluorescent (FP+) worms (Figure 2). We found that 50–61% of injected hermaphrodites segregated rollers (Table 1). Broods without rollers contained no FP+ edits (experiment AP58: 0 of 62 F1 progeny examined from five broods). Among broods with rollers, edits could be found in both rollers and their nonroller siblings (Table 1). For each experiment, we found that the frequency of FP+ edits among the three broods that segregated the highest number of rollers was higher than that observed among the three broods with the lowest numbers of rollers (Figure 2), as also reported by Arribere et al. (2014). For simplicity, in subsequent experiments, we analyzed only the three broods with the highest number of rollers (jackpot broods) and ignored all other broods. We conclude that direct delivery of Cas9 RNP complexes supports robust HDR and, like plasmid delivery, generates jackpot broods that are easily identified using the dpy-10 co-CRISPR marker (Arribere et al. 2014).
Table 1. Optimization of Cas9, crRNA, and rescue template concentrations.
| Experiment number | Experiment | Insertion in gtbp-1 (size) | Cas9 pmol/µl | Cas9 source | crRNA ratio (gtbp-1/dpy-10) | template ratio (gtbp-1/ dpy-10) | Percent P0 with rollers (n) | Percent edits rollers (n) | Percent edits nonrollers (n) |
|---|---|---|---|---|---|---|---|---|---|
| AP80 | Baseline | mCherry (700 bp) | 20.6 | Homemade | 1 (15.7/15.7) | 1.3 (0.56/0.43) | 80% (15) | 24.4% (131) | 2.9% (134) |
| AP78 | Increase crRNA ratio | mCherry (700 bp) | 20.6 | Homemade | 2.5 (39.4/15.7) | 1.3 (0.56/0.43) | 50% (20) | 51.9% (131) | 17.8% (174) |
| AP111 | Decrease Cas9 concentration | mCherry (700 bp) | 4.1 | Homemade | 2.5 (39.4/15.7) | 1.3 (0.57/0.43) | 33% (18) | 33.3% (12) | 12.7% (212) |
| AP96 | Increase template ratio | mCherry (700 bp) | 15.5 | Homemade | 2.5 (29.6/11.8) | 2.5 (1.13/0.44) | 45% (11) | 70.2% (84) | 22.1% (122) |
| AP108 | Lower crRNA concentrations | mCherry (700 bp) | 15.5 | Homemade | 2.5 (11.1/4.4) | 2.5 (1.13/0.44) | 10% (10) | 60% (10) | 10% (40) |
| AP112 | Plasmid delivery | mCherry (700 bp) | NA | Plasmid | 2.5 (0.025/0.01) | 1.3 (0.56/0.43) | 11% (17) | 83% (18) | 14% (120) |
| AP60 | Commercial Cas9 | eGFP (800 bp) | 4.1 | Commercial | 4.2 (39.4/9.4) | 0.9 (0.40/0.43) | 50% (30) | 55% (89) | 18.9% (37) |
| AP58 | Commercial Cas9 + Increase template ratio | eGFP (800 bp) | 4.1 | Commercial | 4.2 (39.4/9.4) | 2.1 (0.91/0.43) | 61.5% (25) | 61.1% (54) | 52.1% (46) |
All experiments were conducted as described in Figure 1 by cotargeting dpy-10 and gtbp-1. Concentrations are in picomoles per microliter. Percent P0 with rollers, percent of injected hermaphrodites that segregated rollers (n= total number of injected hermaphrodites); Percent edits rollers, percent FP+ edits at the gtbp-1 locus among rollers from the top three broods with the most rollers (n= number of rollers screened for FP expression); Percent edits nonrollers, percent FP+ edits at the gtbp-1 locus among nonrollers from the top three broods with the most rollers (n= number of nonrollers screened for FP expression).
Figure 2.
Jackpot broods. Broods with high numbers of dpy-10 edits contain higher percentages of gtbp-1 edits compared to broods with few dpy-10 edits. For three separate experiments, we compared the frequency of FP+ edits (insertion of GFP or RFP) at the gtbp-1 locus among rollers (A) and nonrollers (B) derived from the top three broods with the highest numbers of rollers (jackpot broods) compared to the bottom three broods with the lowest numbers of rollers (as depicted in Figure 1). The frequency of gtbp-1 edits is higher in the jackpot broods. For experiments AP58, AP60, and AP78, the top three jackpot broods contained 70.2 (33 of 47), 44.2 (49 of 111), and 71.6% (68 of 95) of all FP+ edits, respectively. Numbers refer to the number of F1 progeny screened.
Increasing the target/marker ratio of crRNAs and repair templates increases HDR frequency at the target locus
When using equimolar amounts of crRNA and templates for dpy-10 and gtbp-1 (Table 1, experiment AP80), we obtained FP+ edits at a higher frequency among rollers than among nonrollers (24 vs. 3%) (Table 1). For genes unlinked to dpy-10, edits of interest can be separated from the dominant dpy-10(Rol) edit by picking nonroller worms in the next generation. For genes linked to dpy-10, however, recovering the edit without the marker is more difficult, and it is therefore preferable to isolate the edit in nonroller F1 progeny. We reasoned that increasing the levels of gtbp-1 crRNA and template relative to that of dpy-10 should increase the frequency of gtbp-1 edits overall, including in the nonroller population. As expected, increasing the gtbp-1/dpy-10 ratio of crRNA (experiments AP80 and AP78) and template (experiments AP78 and AP96) increased the number of GFP+ edits (Table 1 and Table S1). We also found, however, that decreasing the concentration of dpy-10 crRNA below 5 pmol/µl made it difficult to obtain enough rollers for analysis (experiment AP108). Similarly, the need to maintain relatively high overall concentrations of Cas9 complexes limits the amount of excess target template that can be added to the injection mix (see protocol in File S1 and Table 1, experiments AP78 and AP111). Under the best conditions tested (experiment AP58, high gtbp-1/dpy-10 ratio for both the crRNAs and the repair templates), 61% of injected hermaphrodites segregated rollers, and the jackpot broods gave rise to 61% FP+ edits among rollers and 52% FP+ edits among nonrollers.
Direct injection of preassembled Cas9 RNP complexes generates more overall edits per injected hermaphrodite than plasmid delivery
The FP+ edit frequencies shown in Table 1 are four- to fivefold higher than those obtained using plasmid delivery at the same locus without co-CRISPR (12%) (Paix et al. 2014) or at a different locus using co-CRISPR and longer homology arms (14%) (Arribere et al. 2014). To directly compare the efficiency of direct delivery vs. plasmid delivery, we injected plasmids expressing Cas9 and sgRNAs targeting gtbp-1 and dpy-10 with the same high gtbp-1/dpy-10 ratio as in experiment AP78. Of 17 injected hermaphrodites, we obtained only two (11%) that segregated rollers, for a total of 18 rollers (Table 1, experiment AP112). This low roller frequency is consistent with that reported by Arribere et al. (2014). The frequency of FP+ worms across the two roller broods, however, was high (83% among rollers, 14% among nonrollers). These findings confirm that plasmid delivery can generate jackpot broods with high edit frequencies. Direct delivery of preassembled Cas9 RNP complexes, however, yields a higher number of productive injections, i.e., injected hermaphrodites that generate rollers (50 vs. 11%). In practice, this means that fewer hermaphrodites (∼10 instead of ∼50) need to be injected to obtain jackpot broods.
Application of the protocol to challenging edits
Low-efficiency sgRNAs:
We reasoned that the higher efficiency of our protocol might make it possible to use low-efficiency sgRNAs. Previously, using plasmid delivery and no co-CRISPR, we failed to recover GFP edits at two loci, glh-1 and htp-3, likely because the sgRNAs targeting those genes failed to support sufficient cutting to stimulate HDR (Paix et al. 2014). Both of these sgRNAs have a C at their 3′ ends, which is thought to be unfavorable (Doench et al. 2014). We retested the same sgRNAs and repair templates using complexes assembled in vitro at high target/dpy-10 crRNA and template ratios. We obtained 47% edits for glh-1 and 2% edits for htp-3 (Table 2 and Table S1, experiments AP106 and AP114). A recent report has shown that guide RNAs that end in GG consistently yield good editing efficiencies in C. elegans (Farboud and Meyer 2015). We tested such a sgRNA at the gtbp-1 locus and obtained 20% edits (Table 2, experiment AP92), a frequency comparable to that of the original sgRNA we used at that locus, which ends in a single G. We conclude that the higher efficiency of the new protocol expands the range of sgRNAs that can be used, although efficiencies still vary greatly (Table S3).
Table 2. Protocol performance with different types of edits.
| Experiment number | Edit | Target locus | Recipient strain | Size of insertion | Distance from cut | crRNA ratio (gof/dpy-10) | Template ratio (gof/dpy-10) | Percent edits rollers (n) | Percent edits nonrollers (n) |
|---|---|---|---|---|---|---|---|---|---|
| AP106 | RFP insertion with low-efficiency guide RNA | glh-1 | wt | 750 bp | 6 | 2.5 (29.6/11.8) | 1.8 (0.83/0.44) | 47.5% (101) | 16.6% (120) |
| AP114 | GFP insertion with low-efficiency guide RNA | htp-3 | wt | 900 bp | 8 | 2.5 (29.6/11.8) | 1.5 (0.70/0.44) | 2% (198) | 0.7% (282) |
| AP92 | RFP insertion with GG guide RNA | gtbp-1 | wt | 700 bp | 1 | 2.5 (39.4/15.7) | 1.3 (0.56/0.43) | 20.2% (69) | 15.8% (126) |
| AP83 | GFP insertion at distance from cut | gtbp-1 | wt | 800 bp | 28 | 2.5 (39.4/15.7) | 1.1 (0.48/0.43) | 6.0% (82) | 3.9% (76) |
| AP82 | Ollas insertion at distance from cut | gtbp-1 | wt | 27 nt | 28 | 2.5 (39.4/15.7) | 1.1 (0.51/0.43) | 2.1% (47) | 0% (47) |
| AP95 | Ollas insertion at distance from cut + increase ssODN template | gtbp-1 | wt | 27 nt | 28 | 2.5 (39.4/15.7) | 6 (2.58/0.43) | 8.8% (34) | 0% (34) |
| AP103 | Tagging two genes at once (GFP/RFP) | gtbp-1 and fbf-2 | wt | 750 and 750 bp | 1 and 1 | 1.8 (14.8/8.1) + 3.6 (29.6/8.1) | 0.4 (0.20/0.44) + 0.9 (0.41/0.44) | 28% (57) | 7.1% (223) |
| AP115 | Replacement of gtbp-1 ORF with GFP::H2B | gtbp-1 | wt | 1300 bp | 1 and 0 | 1.8 (22.2/11.8) + 1.8 (22.2/11.8) | 0.9 (0.41/0.44) | 58.1% (74) | 7.5% (120) |
| AP105 | Replacement of GFP with RFP | gtbp-1 | gtbp-1 | 750 bp | 0 and 1 | 1.8 (22.2/11.8) + 1.8 (22.2/11.8) | 1.3 (0.59/0.44) | 26.5% (79) | 2.7% (144) |
| ::eGFP | |||||||||
| ::3Xflag | |||||||||
| AP105D | Replacement of GFP with RFP | deps-1 | deps-1 | 750 bp | 0 and 1 | 1.8 (22.2/11.8) + 1.8 (22.2/11.8) | 1.3 (0.59/0.44) | 21.1% (52) | 5.9% (135) |
| ::GFP |
All experiments were conducted as described in Figure 1 by cotargeting dpy-10 and one (or two) target loci as indicated. Concentrations are in picomoles per microliter. gof, gene of interest; Percent edits rollers, % FP+ edits at the target locus among rollers in the top three broods with the most rollers (n= number of rollers screened); Percent edits nonrollers: % FP+ edits at the target locus among nonrollers in the top three broods with the most rollers (n= number of nonrollers screened).
Insertion at a distance from Cas9-induced cleavage site:
We previously noted that edits at a distance from the cleavage site (>10 bases) are incorporated less efficiently than edits closer to the cleavage site (Paix et al. 2014). We found that this observation still holds using the new protocol. We obtained 6% edits when inserting GFP in gtbp-1 28 bases away from the cleavage site (Table 2, experiment AP83) compared to 51% edits when inserted directly at the cleavage site under the same crRNA and template ratios (Table 1, experiment AP78). A low edit frequency also was observed when using a ssODN to insert a small tag at the same position away from the cleavage site (Table 2, experiment AP82). Increasing the ssODN concentration improved efficiency modestly (experiment AP95, 2 vs. 8.8%). We conclude that edits away from the cleavage site are incorporated less efficiently than edits close to the cleavage site, as reported previously (Arribere et al. 2014; Paix et al. 2014). This inefficiency can be compensated partially by increasing the template concentration in the injection mix.
Tagging two genes at once:
We tested whether the new protocol could be used to recover edits at two target loci simultaneously (in addition to the marker locus dpy-10). We targeted gtbp-1 and fbf-2 for GFP and RFP insertion, respectively. We obtained 28% GFP/RFP double edits among rollers and 7% GFP/RFP double edits among nonrollers (Table 2, experiment AP103). The frequency of GFP edits overall (63%) exceeded that of RFP edits (35%) almost by a factor of 2 (Table S1), possibly as a result of higher efficiency of the sgRNA targeting gtbp-1 compared to fbf-2, as also seen when these sgRNAs were used separately (Paix et al. 2014). We conclude that protein delivery can support robust HDR at three loci simultaneously (including dpy-10) even when using sgRNAs with different editing efficiencies.
ORF replacement:
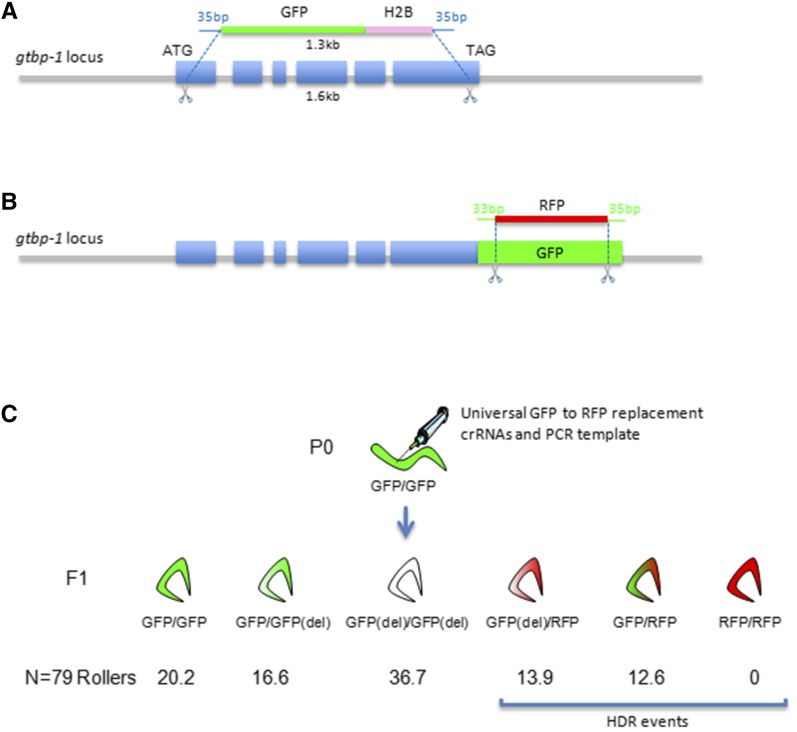
We showed previously that a repair ssODN could be used to insert a restriction site at the junction of a deletion generated by two cuts. To test whether a similar approach could be used to replace an entire ORF with another, we first attempted to replace the gtbp-1 ORF with a histone H2B::GFP fusion. We recovered 58% replacements among rollers and 7.5% replacements among nonrollers (Table 2, experiment AP115). Next, we attempted to replace GFP with RFP at two loci previously tagged with GFP. We used a pair of crRNAs that cut at the 5′ and 3′ ends of GFP and an RFP PCR repair template with 35-base homology arms directly flanking the cleavage sites. We obtained the desired GFP-to-RFP swap in 26% (gtbp-1 locus) and 21% (deps-1 locus) of rollers. Interestingly, we also recovered a significant number of rollers that were GFP− and RFP−, indicating a high degree of cutting in the GFP target without HDR (Figure 3 and Table S1). Because both maternal and paternal GFP copies were affected, these observations indicate that Cas9 complexes injected in the maternal germline persist into zygotes, where they can edit the paternal genome, as also reported by Cho et al. (2013). Consistent with these observations, in addition to rollers, in all our experiments we also obtained dumpy worms, which result from editing both copies of the dpy-10 locus (Arribere et al. 2014).
Figure 3.
Gene replacement strategy. (A) Replacement of the gtbp-1 ORF with GFP::H2B. Two cuts were made at either end of the gtbp-1 ORF and repaired using a PCR template containing GFP::H2B flanked by 35 bases that were homologous to the 5′ and 3′ ends of gtbp-1. (B) Replacement of GFP with RFP. Two cuts were made in GFP and repaired using a PCR template containing RFP flanked by 33 and 35 bases that were homologous to the 5′ and 3′ ends of GFP, respectively. (C) Experimental results for replacing GFP with RFP at the gtbp-1 locus. The percentages of each genotype among roller F1 progeny are indicated.
Conclusions
We report that injection in the C. elegans gonad of repair templates and preassembled Cas9-crRNA-tracrRNA complexes generates HDR edits with high efficiency (∼50% of injected hermaphrodites generate edits). Direct delivery allows us to take full advantage of the benefits of the co-CRISPR approach of Arribere et al. (2014), which provides a visual marker to identify broods with edits. By optimizing the ratio of Cas9 complexes (and repair templates) that target the gene of interest compared to the dpy-10 marker, we found that it is possible to obtain both a high yield of dpy-10-edited progeny and a high yield of dpy-10 edits that are also edited at the gene of interest (coedits, as high as 70%). Among broods that segregate the highest numbers of dpy-10 edits (jackpot broods), edits at the gene of interest also can be found in non-dpy-10-edited siblings (as high as 52%). The ability to recover edits in unmarked animals is particularly useful for linked loci that cannot be separated easily from the dominant dpy-10 marker in the next generation. Our findings also demonstrate that the co-CRISPR strategy works efficiently even when using different types of templates to repair the marker locus (ssODN to generate a point mutation) and the gene of interest (PCR amplicon to insert a fluorescent protein). The efficiency of editing, however, still remains variable from locus to locus, likely owing to differences in sgRNA efficiency and possibly differences in locus competency for HDR. We also continue to find that edits at a distance from the Cas9 cleavage site (>10 bases) are incorporated less efficiently than edits closer to the cleavage site, as reported previously (Arribere et al. 2014; Paix et al. 2014). Although protospacer adjacent motifs (PAMs) can be found within 30 bases of most edit sites, in cases where no PAMs are available, it may be preferable to use templates with longer homology arms and selection markers, as in Dickinson et al. (2015).
The efficiency of our direct-delivery protocol translates into several practical advantages: workload is reduced at both the injection (10–20 injected hermaphrodites vs. 50) and screening (<50 F1 progeny compared to hundreds) steps, low-efficiency sgRNAs can be used, and complex edits, including gene replacements and multigene edits, are possible. Replacement of an ORF with GFP, as we report here for the gtbp-1 locus, is an effective method to generate marked null alleles that can be maintained using the linked fluorescent marker. We have also developed universal crRNAs and rescue templates to convert GFP fusions to RFP fusions (File S1). Another advantage of direct delivery is that the entire protocol requires no cloning: tracrRNA, crRNAs, and ssODN repair templates for short edits (<50 bases) are all synthesized chemically, and longer repair templates are made by PCR using oligonucleotides to code for the short (35-base) homology arms. In principle, direct delivery of Cas9 RNP complexes could be combined with other CRISPR protocols (Dickinson et al. 2015; Ward 2015) and also should be advantageous for nematodes where promoters to drive expression of Cas9 and the sgRNAs are not readily available (Chiu et al. 2013; Witte et al. 2015).
Supplementary Material
Acknowledgments
We thank Mike Nonet and Andy Fire for the Cas9::NLS::His bacterial expression plasmid and members of the Seydoux Laboratory for comments on the manuscript. Some strains were provided by or deposited at the Caenorhabditis Genetics Center (CGC), which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). We also thank WormBase for gene annotations and sequences. This work was supported by National Institutes of Health grant R01HD37047. G.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Communicating editor: O. Hobert
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.179382/-/DC1
Literature Cited
- Aida T., Chiyo K., Usami T., Ishikubo H., Imahashi R., et al. , 2015. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol. 16: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere J. A., Bell R. T., Fu B. X., Artiles K. L., Hartman P. S., et al. , 2014. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics 198: 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H., Schwartz H. T., Antoshechkin I., Sternberg P. W., 2013. Transgene-free genome editing in Caenorhabditis elegans using CRISPR-Cas. Genetics 195: 1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. W., Lee J., Carroll D., Kim J. S., Lee J., 2013. Heritable gene knockout in Caenorhabditis elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics 195: 1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deltcheva E., Chylinski K., Sharma C. M., Gonzales K., Chao Y., et al. , 2011. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 471: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Ward J. D., Reiner D. J., Goldstein B., 2013. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., Goldstein B., 2015. Streamlined genome engineering with a self-excising drug selection cassette. Genetics 115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J. G., Hartenian E., Graham D. B., Tothova Z., Hegde M., et al. , 2014. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doudna J. A., Charpentier E., 2014. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346: 1258096. [DOI] [PubMed] [Google Scholar]
- Farboud B., Meyer B. J., 2015. Dramatic enhancement of genome editing by CRISPR/Cas9 through improved guide RNA design. Genetics 199: 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B. X., Hansen L. L., Artiles K. L., Nonet M. L., Fire A. Z., 2014. Landscape of target:guide homology effects on Cas9-mediated cleavage. Nucleic Acids Res. 42: 13778–13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S., Zhang F., 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic I., Xu L., Ciosk R., 2015. CRISPR/Cas9 genome editing in Caenorhabditis elegans: evaluation of templates for homology-mediated repair and knock-ins by homology-independent DNA repair. G3 115.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Ishidate T., Ghanta K. S., Seth M., Conte D., Jr, et al. , 2014. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics 197: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Potter J., Kumar S., Zou Y., Quintanilla R., et al. , 2015. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 208: 44–53. [DOI] [PubMed] [Google Scholar]
- Lin S., Staahl B. T., Alla R. K., Doudna J. A., 2014. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 3: e04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Wang Y., Smith H. E., Lee C. Y., Calidas D., et al. , 2014. Scalable and versatile genome editing using linear DNAs with microhomology to Cas9 Sites in Caenorhabditis elegans. Genetics 198: 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. N., Davey C. F., Whitebirch A. C., Miller A. C., Moens C. B., 2015. Rapid reverse genetic screening using CRISPR in zebrafish. Nat. Methods 12: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schendel R., Roerink S. F., Portegijs V., van den Heuvel S., Tijsterman M., 2015. Polymerase theta is a key driver of genome evolution and of CRISPR/Cas9-mediated mutagenesis. Nat. Commun. 6: 7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. D., 2015. Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair. Genetics 199: 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte H., Moreno E., Rodelsperger C., Kim J., Kim J. S., et al. , 2015. Gene inactivation using the CRISPR/Cas9 system in the nematode Pristionchus pacificus. Dev. Genes Evol. 225: 55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available from the Caenorhabditis Genetics Center (CGC) and Addgene, or upon request.