Abstract
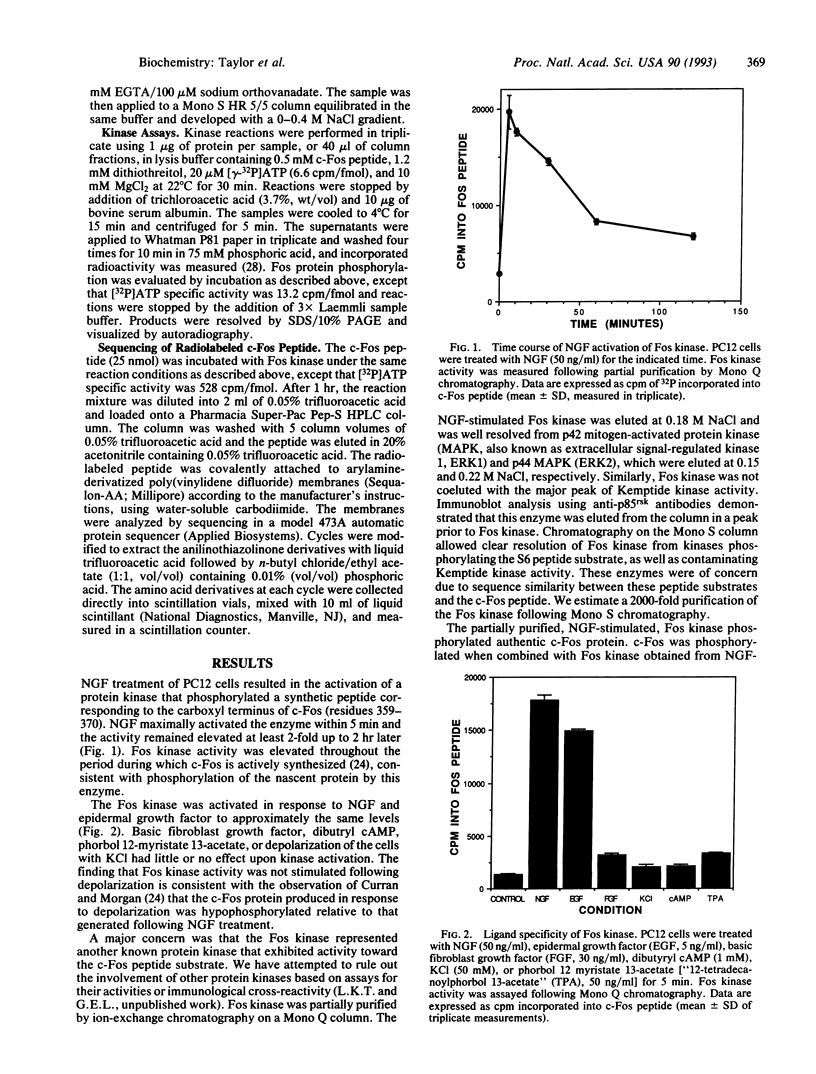
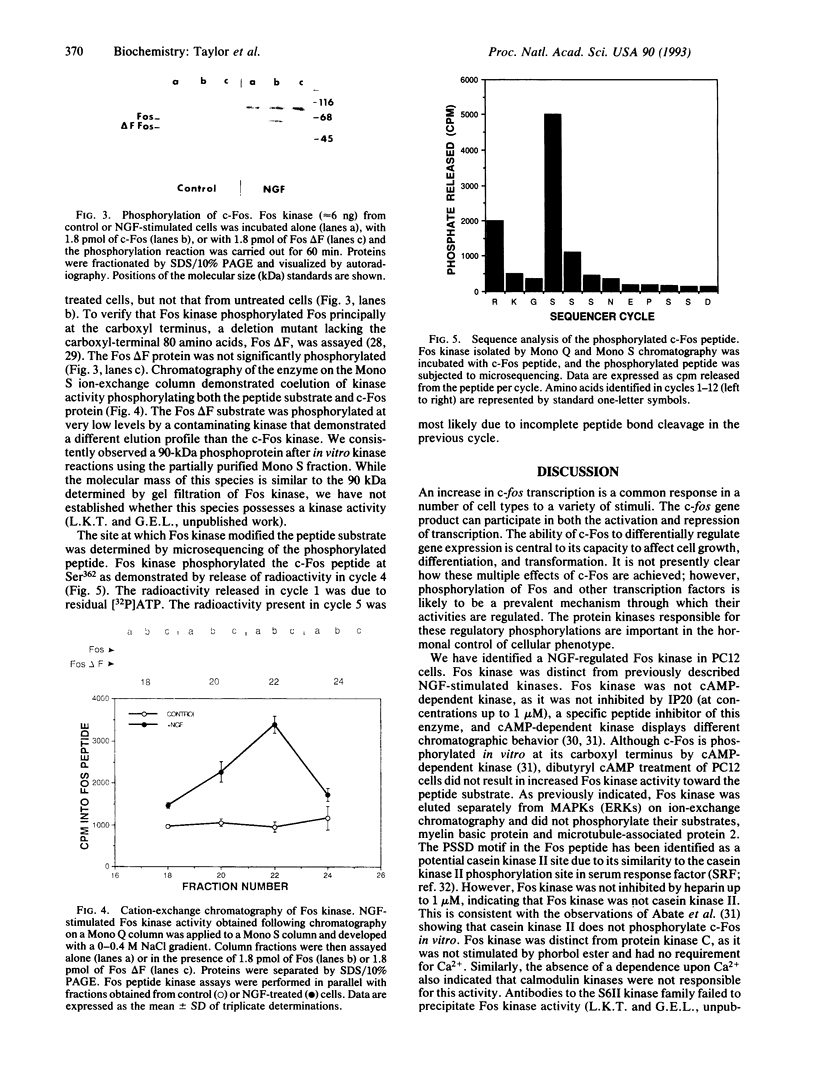
Nerve growth factor (NGF) treatment of rat pheochromocytoma (PC12) cells induces the synthesis of the transcription factor c-Fos, which becomes highly phosphorylated relative to that produced as a result of depolarization of the cell. A peptide derived from the carboxyl terminus of c-Fos (residues 359-370, RKGSSSNEPSSD) containing putative phosphorylation sites was used to detect a NGF-stimulated Fos kinase. NGF treatment of PC12 cells resulted in a rapid activation of a protein kinase which phosphorylated both the c-Fos peptide and authentic c-Fos at its carboxyl terminus. The kinase was selectively activated by NGF and epidermal growth factor but was not induced by depolarization or other agents. The c-Fos peptide was phosphorylated at a serine corresponding to Ser362, a site critically implicated in the capacity of c-Fos to exhibit transrepressive activity [Ofir, R., Dwarki, V. J., Rashid, D. & Verma, I. M. (1990) Nature (London) 348, 80-82)]. The NGF-stimulated Fos kinase may play an important role in regulating the expression and transforming potential of c-Fos.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. A ubiquitous nuclear protein stimulates the DNA-binding activity of fos and jun indirectly. Cell Growth Differ. 1990 Oct;1(10):455–462. [PubMed] [Google Scholar]
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate C., Marshak D. R., Curran T. Fos is phosphorylated by p34cdc2, cAMP-dependent protein kinase and protein kinase C at multiple sites clustered within regulatory regions. Oncogene. 1991 Dec;6(12):2179–2185. [PubMed] [Google Scholar]
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Barium modulates c-fos expression and post-translational modification. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8521–8524. doi: 10.1073/pnas.83.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Detection of nerve growth factor and epidermal growth factor-regulated protein kinases in PC12 cells with synthetic peptide substrates. Mol Pharmacol. 1989 Mar;35(3):331–338. [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Schubert D., Verma I. M. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth G. E., Smith D. S., McCabe C., Gittinger C. Characterization of a nerve growth factor-stimulated protein kinase in PC12 cells which phosphorylates microtubule-associated protein 2 and pp250. J Neurochem. 1990 Aug;55(2):514–523. doi: 10.1111/j.1471-4159.1990.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Manak J. R., Prywes R. Mutation of serum response factor phosphorylation sites and the mechanism by which its DNA-binding activity is increased by casein kinase II. Mol Cell Biol. 1991 Jul;11(7):3652–3659. doi: 10.1128/mcb.11.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T., Rudkin B. B., Koizumi S., Guroff G. Nerve growth factor, a differentiating agent, and epidermal growth factor, a mitogen, increase the activities of different S6 kinases in PC12 cells. J Biol Chem. 1988 Nov 5;263(31):15853–15856. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Ofir R., Dwarki V. J., Rashid D., Verma I. M. Phosphorylation of the C terminus of Fos protein is required for transcriptional transrepression of the c-fos promoter. Nature. 1990 Nov 1;348(6296):80–82. doi: 10.1038/348080a0. [DOI] [PubMed] [Google Scholar]
- Race H. M., Wagner J. A. Nerve growth factor affects cyclic AMP metabolism, but not by directly stimulating adenylate cyclase activity. J Neurochem. 1985 May;44(5):1588–1592. doi: 10.1111/j.1471-4159.1985.tb08799.x. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. P., Varon S., Shooter E. M. Multiple forms of the nerve growth factor protein and its subunits. Biochemistry. 1968 Sep;7(9):3259–3268. doi: 10.1021/bi00849a032. [DOI] [PubMed] [Google Scholar]
- Smith D. S., King C. S., Pearson E., Gittinger C. K., Landreth G. E. Selective inhibition of nerve growth factor-stimulated protein kinases by K-252a and 5'-S-methyladenosine in PC12 cells. J Neurochem. 1989 Sep;53(3):800–806. doi: 10.1111/j.1471-4159.1989.tb11776.x. [DOI] [PubMed] [Google Scholar]
- Tratner I., Ofir R., Verma I. M. Alteration of a cyclic AMP-dependent protein kinase phosphorylation site in the c-Fos protein augments its transforming potential. Mol Cell Biol. 1992 Mar;12(3):998–1006. doi: 10.1128/mcb.12.3.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Fos C-terminal mutations block down-regulation of c-fos transcription following serum stimulation. EMBO J. 1988 Dec 20;7(13):4193–4202. doi: 10.1002/j.1460-2075.1988.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]