Abstract
Introduction:
Giant cell tumor (GCT) of bone is locally aggressive benign tumor involving the epiphysis of long bones in young adults. Various treatment options include intralesional curettage, extended curettage, wide resection, resection and reconstruction and amputation. The main variables to be considered for planning treatment include the site of involvement and Campanacci stage of the tumor. Functional and oncological outcomes of these treatment options vary widely, the predominant detrimental factor being tumor recurrence rate.
Aim:
A study was conducted to evaluate the long-term oncological and functional outcome of patients with GCT of the proximal femur that underwent tumor resection and endoprosthetic replacement.
Materials and Methods:
Eleven patients with Campanacci stage-III GCT of proximal femur who underwent wide excision of tumor and endoprosthesis replacement with a mean follow-up the duration of 10.6 years were assessed using standard proforma. The treatment outcome was evaluated using the Revised Musculoskeletal Tumor Society Rating Scale for the lower extremity.
Results:
At mean follow-up the duration of 10.6 years, none of the cases had tumor recurrence, infection, prosthesis loosening or dislocation. All the patients were community ambulators among whom eight patients were walking without support while three patients were using a cane for support. The mean total Musculoskeletal Tumor Society Score was 26.8 out of 30 indicating the good outcome.
Conclusions:
The authors recommend that wide resection and endoprosthetic replacement should be considered as a preferred treatment option for proximal femur GCT as the functional, and oncological outcome is satisfactory with this modality of treatment.
KEYWORDS: Endoprosthesis megaprosthesis, giant cell tumor of bone, proximal femur
INTRODUCTION
Giant cell tumor (GCT) represents 5% of neoplasms of bone. It is a benign locally aggressive tumor usually involving the distal end of the femur, proximal tibia and distal radius in young adults.[1,2,3] The main variables to be considered for planning treatment include the site of involvement and Campanacci stage of the tumor.[4] The proximal femur is a relatively rare site for primary GCT. Different treatment options are available which include intralesional curettage, extended curettage, wide resection and reconstruction.[5,6,7,8] Functional and oncological outcome of these treatment options varies widely, the predominant detrimental factor being tumor recurrence rate. The tumor recurrence is least with wide resection of the tumor with clear margins. Reconstruction of endoprosthesis after wide excision of the tumor offers good short-term and mid-term functional and oncological outcomes as established by previous studies.[2,5,9] This study was conducted to evaluate the long-term outcome of 11 patients with GCT who underwent wide excision and customized endoprosthetic replacement.
MATERIALS AND METHODS
This study included 11 patients (eight men and three women) aged 24–48 (mean 32) years with primary GCT of proximal femur Campanacci stage-III who were available for mean follow-up duration of 10.6 (range 10.2–14) years. All these patients were evaluated with standard preoperative work up. The definite diagnosis was established on histopathological confirmation with incisional biopsy. Computed tomography scan of chest and bone scan were done to rule out metastasis. None of the patients had pulmonary metastasis, and all the 11 patients had a solitary lesion in the proximal femur. These patients underwent wide resection of the tumor using postero-lateral approach to the proximal femur. Intraoperative frozen section and histopathological evaluation confirmed the clear tumor free margins. The proximal femur was reconstructed using a customized, titanium, cemented endoprosthesis [Figure 1]. Hip abductors, short external rotators and iliopsoas tendon were secured onto the prosthesis and hip capsule repair was performed. Postoperative rehabilitation protocol included nonweight bearing and abduction splinting of the limb for 6 weeks followed by nonweight bearing crutch walking for another 6 weeks. Once the hip abductors and quadriceps strength was regained weight bearing was allowed. Patients were serially followed up at 6 monthly intervals. Long-term functional outcome was evaluated at minimum 10 years duration using Revised Musculoskeletal Tumor Society Rating scale.[10]
Figure 1.
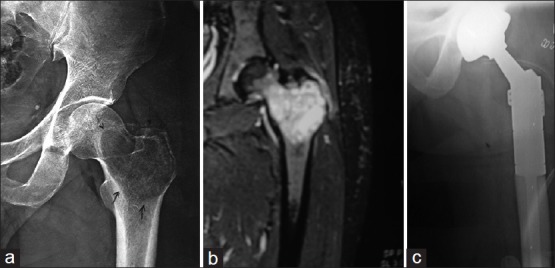
(a) Radiogragh showing giant cell tumor of proximal femur in a 34-year-old man. (b) Magnetic resonance imaging showing giant cell tumor of proximal femur with soft tissue extension. (c) Endoprosthetic replacement of proximal femur
RESULTS
None of the patients had tumor recurrence or deep infection. There were no instances of prosthesis related complications like aseptic loosening or dislocation. At the end of mean 10.6 years, eight patients had no pain whereas three had some occasional intermediate pain. Six patients had good hip function without any restrictions, four patients had intermediate functional restriction whereas one had the recreational restriction of function. Nine patients enthusiastically accepted the outcome of the procedure whereas two patients had satisfactory emotional acceptance. Eight patients were walking without any support with unlimited walking abilities whereas three patients were using a cane for support while walking. None of the patients had any major gait abnormalities. The mean is Revised Musculoskeletal Tumor Society Score was 26.8 out of 30 [Table 1].
Table 1.
Mean Revised Musculoskeletal Tumour Society Scores

DISCUSSION
Traditional treatment of GCT has been a difficult problem in orthopaedic oncology owing to high recurrence rates following conventional treatment with curettage or extended curettage.[4,6,7,8] Ideally treatment should ensure local control and maintain function. Currently with improvement in reconstructive surgical techniques and availability of high quality biomechanically designed megaprosthesis, wide resection of tumor with proximal femur endoprosthesis replacement is being considered as a treatment option for Campanacci stage-III lesions in proximal femur with extensive osteolysis and soft tissue extension.[2,5,9] It offers good local control of s with least recurrence rate and favorable functional outcome. However, the longevity of the prosthesis has been the main concern. The previous studies have shown satisfactory short- and mid-term functional and oncological outcomes.[2,9] This study shows good long-term functional, and oncological outcomes of the procedure and hence the authors recommended as an endoprosthetic replacement for advanced GCT of the proximal femur. Furthermore, randomized control trials are required to established this modality as a standard treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52:619–64. [PubMed] [Google Scholar]
- 2.Khan SA, Kumar A, Inna P, Bakhshi S, Rastogi S. Endoprosthetic replacement for giant cell tumour of the proximal femur. J Orthop Surg (Hong Kong) 2009;17:280–3. doi: 10.1177/230949900901700306. [DOI] [PubMed] [Google Scholar]
- 3.Kabukcuoglu Y, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement for primary malignant tumors of the proximal femur. Clin Orthop Relat Res. 1999;358:8–14. [PubMed] [Google Scholar]
- 4.Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: Oncological and functional results of long-term follow-up. Jpn J Clin Oncol. 1998;28:323–8. doi: 10.1093/jjco/28.5.323. [DOI] [PubMed] [Google Scholar]
- 5.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, et al. Giant cell tumor of bone:Treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–78. doi: 10.1007/s00432-008-0370-x. [DOI] [PubMed] [Google Scholar]
- 6.Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendrol M, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–7. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–33. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci M, Giunti A, Olmi R. Giant cell tumours of bone. A study of 209 cases with long term follow up in 130. Ital J Orthop Traumatol. 1975;1:153–80. [Google Scholar]
- 9.Natarajan M, Bose JC, Rajkumar G. Proximal femoral reconstruction with custom mega prosthesis. Int Orthop. 2003;27:175–9. doi: 10.1007/s00264-003-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–6. [PubMed] [Google Scholar]


