Abstract
Context:
The perception of non-alcoholic fatty liver disease (NAFLD) as an uncommon and benign condition is rapidly changing. Approximately, 70% type 2 diabetes mellitus (T2DM) patients have a fatty liver, which may follow an aggressive course with necroinflammation and fibrosis.
Aims:
To assess the profile of liver enzymes in subjects with impaired glucose tolerance (IGT), new onset treatment naive T2DM and normal glucose tolerance (NGT) with and without NAFLD.
Settings and Design:
Cross-sectional clinic-based study.
Subjects and Methods:
152 IGT and 158 recently detected T2DM subjects aged between 30 and 69 years, along with 160 age and gender matched controls with NGT. An ultrasonography scan of the upper abdomen was done in all patients in order to examine presence of fatty liver. Anthropometry, lipid profile, liver enzymes were also analyzed in all patients.
Statistical Analysis Used:
Unpaired t-test, Chi-square/Fisher Exact test (for categorical variables), Pearson/Spearmen correlation test to find significant difference, association and correlation between two or more groups respectively.
Results:
NAFLD was significantly associated with higher alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) but not ALP levels in IGT and T2DM patients. ALT, GGT significant correlated with waist circumference, body mass index, fasting insulin, homeostatic model assessment- insulin resistance, fasting blood glucose, high density lipoprotein cholesterol, triglyceride. 57% of NAFLD patients had normal ALT between 25 and 40 U/L, 53% of NAFLD subjects had normal GGT between 15 and 30 U/L. ALT <25 U/L and GGT <15 U/L had highest negative predictivity whereas ALT >40 U/L and GGT > 30 U/L had highest positive predictivity for presence of NAFLD in our study sample.
Conclusions:
Mild elevations of liver enzymes in the upper normal range are associated with features of metabolic syndrome and NAFLD even in IGT and recently detected T2DM patients. Novel cut-offs for liver enzymes are warranted in order to prevent unnecessary diagnostic work-ups and early detection of NAFLD to reduce the risk of cirrhosis, hepatocellular carcinoma and classical cardiovascular disease in T2DM and IGT patients.
Keywords: Impaired glucose tolerance, nonalcoholic fatty liver disease, novel cut-offs for liver enzymes, type 2 diabetes mellitus
INTRODUCTION
The prevalence of non-alcoholic fatty liver disease (NAFLD) has doubled during last 20 years and is the number one cause of liver disease in the western countries, but recent data confirm that NAFLD play an equally important role worldwide.[1] NAFLD is diagnosed in clinical settings using ultrasound imaging as a liver biopsy is not practically feasible. In developing countries like India, affordability of ultrasound imaging modalities may be a crucial factor. Thus World Gastroenterology Organisation guidelines prescribe a hierarchical resource-sensitive approach.[1] Aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT) and other markers of liver injury may be useful surrogate measures of NAFLD. NAFLD is closely related to insulin resistance, obesity, metabolic syndrome, and diabetes: Triggers for testing of liver enzymes and considering a diagnosis of NAFLD.[1] We need novel cut-offs for liver enzymes as a qualifying criteria for patients to undergo ultrasonography for detection of NAFLD, but there is a paucity of data from Indian literature in this regard. We studied the profile of liver enzymes in NAFLD in patients with impaired glucose tolerance (IGT) and newly detected untreated type 2 diabetes mellitus (T2DM).
SUBJECTS AND METHODS
This cross-sectional clinic-based study at a tertiary care teaching institute in eastern India, examined 152 IGT and 158 recently detected T2DM subjects aged between 30 and 69 years, never treated with any antihyperglycemic, antihypertensive, lipid lowering drugs. 160 age and gender matched controls (willing healthy spouse or unrelated attendants of patients) with normal glucose tolerance (NGT) were also selected.
Patients with osmotic symptoms, history of ketosis, weight loss of >3 kg in preceding 3 months, microvascular complications, recent (<1 year) MI, acute coronary syndrome, stroke, severe comorbid disease (cancer, renal failure), alcohol consumption, known liver disease, viral hepatitis, steatogenic medication, parenteral nutrition, ALT >3 times normal were excluded. We obtained detailed patient history of alcohol consumption, the critical threshold being <20 g/day in women, <30 g/day in men, as there are presently no diagnostic test can reliably distinguish between ASH and NASH.[1]
The criteria for definition of NAFLD requires that (a) there is evidence of hepatic steatosis, either by imaging or by histology and (b) there are no causes for secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders.[2]
Abdominal ultrasound scans are the most frequently used imaging tool for diagnosing NAFLD. Five point ultrasonographic criteria of diagnosis of fatty liver disease recommended by Dasharty's was used to diagnose NAFLD: (1) Increased hepatic brightness” or hyperechogenicity (He), (2) posterior attenuation of the right lobe (RLpA), (3) increased contrast between the right kidney and liver (HRC), (4) loss of visualization of right diaphragm and (5) diminished visibility of the intrahepatic vessels.[3] However, ultrasonography may fail to detect mild steatosis, cannot distinguish between steatosis and NASH is vulnerable to inter- and intra-observer variability.[4] In our study, conventional B-mode liver ultrasound was performed with a convex 3.5 MHz probe, by the same operator who was unaware of the aims of the study and blinded to laboratory values.
Anthropometric measurements included weight, height, waist circumferences (WC), body mass index (BMI). Fasting plasma glucose (FPG), insulin, lipid profile, liver function tests including liver enzymes, HBsAg, anti-HCV antibody, HEV Antibody, were analyzed.
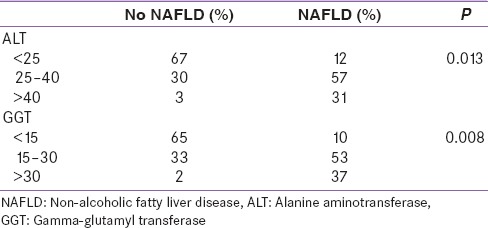
Serum liver enzymes ALT, AST, GGT, ALP were estimated by modified IFCC method, and normal kit ranges were: ALT: 10–40 international units per liter (IU/L), AST: 10–35 IU/L, GGT: 5–30 IU/L, ALP: 80–290 IU/L. The study subjects with or without NAFLD were divided into groups according to different ALT and GGT tertiles [Table 1].
Table 1.
Study subjects with or without NAFLD were divided into groups according to different ALT and GGT tertiles
RESULTS
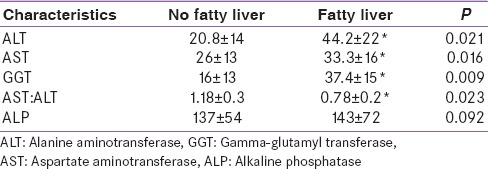
In our study, 48.3% of the total study cohort had fatty liver while 51.7% had no fatty liver. 64% (n = 101) of the type 2 diabetes mellitus [T2DM]) subjects, 52% (n = 79) of the IGT subjects had fatty liver, while only 20% (n = 32) of the NGT (control) subjects had fatty liver. Subjects with NAFLD had significantly higher ALT, AST, GGT and AST: ALT ratio than subjects without NAFLD as determined by unpaired t-test [Table 2].
Table 2.
Comparison of liver function test between fatty liver and no fatty liver cohort
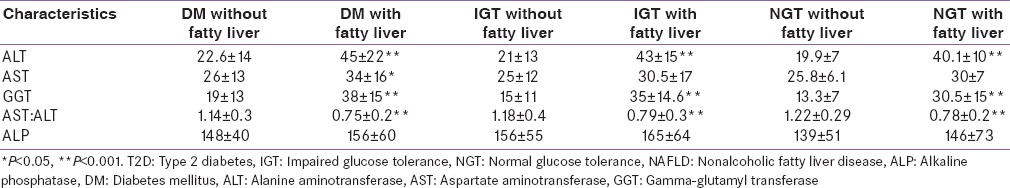
By Spearman correlation test, fatty liver was significantly (P < 0.023) correlated with higher ALT and GGT. ALT, GGT had statistically significant correlation with fasting insulin, WC, waist height ratio, BMI, waist hip ratio, high density lipoprotein cholesterol (HDL-C), triglyceride and fasting blood glucose. The association was strongest for homeostatic model assessment insulin resistance (HOMA-IR) and QUICKI. The profile of liver enzymes in T2D, IGT and NGT with or without NAFLD is described in Table 3.
Table 3.
Profile of liver enzymes in T2D, IGT and NGT with or without NAFLD
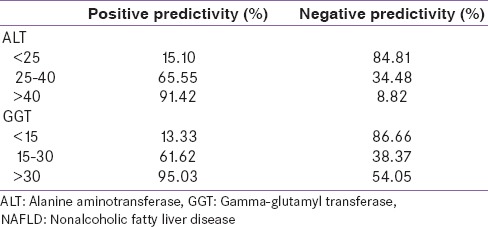
Significant association was found between NAFLD status with both ALT and GGT groups based on tertiles. 57% of NAFLD patients had normal ALT between 25 and 40 U/L, 53% of NAFLD subjects had normal GGT between 15 and 30 U/L. The positive and negative predictivity for different cut-offs of ALT and GGT are displayed in Table 4. ALT <25 U/L and GGT <15 U/L had highest negative predictivity whereas ALT >40 U/L and GGT >30 U/L had highest positive predictivity for presence of NAFLD in our study sample.
Table 4.
Positive and negative predictivity for different cut-offs of ALT and GGT for presence of NAFLD
DISCUSSION
Patients with NAFLD have increased liver-related and overall mortality commonly due to cardiovascular disease.[1] NAFLD is a major global public health problem, but there is insufficient evidence to justify screening for NAFLD in the general population.[1] Not all patients with risk factors will have NAFLD and not all patients with NAFLD will have standard risk factors. WGO global guidelines suggest that mortality from liver disease in general population is around 0.2%, with simple steatosis it is 0%, with NASH it is 1.6–6.8% and 10 year survival rate for simple steatosis are normal, for NASH 38% and for alcoholic steatohepatitis (ASH) 15%. While the simple steatosis seen in NAFLD does not correlate with increased short-term morbidity or mortality, progression to NASH dramatically increases the risks of cirrhosis, liver failure, and even hepatocellular carcinoma. While 10–20% of NAFLD can progress to NASH, only 0–4% progress to cirrhosis by 10–20 years. Although there is no definite way to know, which patient may progress to NASH from NAFLD, an abnormal ferritin level in the presence of normal transferrin saturation always hints to rule out NASH. Liver biopsy remains the gold standard for characterizing liver histology in NAFLD, but it is expensive and carries some morbidity and very rare mortality risk. Thus, it should be performed in those who would benefit the most from diagnostic, therapeutic guidance, and prognostic perspectives: Competing etiologies for hepatic steatosis; co-existing chronic liver diseases cannot be excluded; at risk for steatohepatitis and advanced fibrosis.[1]
An ultrasonographic study of patients with T2DM showed a 69% prevalence of NAFLD.[4] We have reported a high prevalence of NAFLD in T2DM (64%) and IGT (52%) similar to other studies.[5] ALT, GGT, AST are markers of liver injury and may be useful surrogate measures of NAFLD. ALT is located in the hepatocellular cytosol, whereas AST is mostly within the mitochondria. In fact, NAFLD and NASH have been reported to be most common causes of chronically elevated liver enzymes and is often the tipping point for further diagnostic evaluation.[6] A recent large UK study determined that NAFLD detected by USG, was the most common cause of abnormal liver biochemistry.[7] In our study NAFLD was significantly associated higher ALT and GGT. Diabetic subjects with NAFLD had significantly higher ALT, AST, GGT and significantly lower AST: ALT ratio in comparison with diabetic subjects without NAFLD, but there was no significant difference in ALP levels. IGT and NGT study group with or without NAFLD had similar features except that there was no difference in ALT.
Loannou et al. demonstrated classical cardiovascular disease (CVD) risk (by Framingham risk score) with elevated ALT.[8] The population-based Hoorn study showed that high normal ALT levels were associated with an increased 10-year risk of coronary heart disease independent of CVD risk factors indicating that ALT may be a useful marker in assessment of CVD risk in patients who may have NAFLD.[9] Asian-Indian men may be genetically predisposed to develop hepatic steatosis, hepatic insulin resistance and T2DM at a lower BMI with greater central obesity than other ethnic groups suggesting significant ethnic differences in the pathogenesis of insulin resistance.[10] A study showed that GGT and ALT even within the “normal” range, but not AST were significantly associated with BMI and WC, HOMA-IR, triglycerides, HDL-C, glucose even after further adjustment for BMI. In our study, ALT, GGT significant correlated with fasting insulin, WC, waist height ratio, BMI, waist-hip ratio, HDL-C, triglyceride, fasting blood glucose with the strongest association for HOMA-IR and QUICKI.
Previous studies based on liver enzymes screening consistently reported a lower prevalence of NAFLD (3–12%) than imaging or histology based studies.[11] Normal levels of liver enzymes have been demonstrated in subjects with the entire spectrum of NAFLD, and therefore ALT, ST, GGT have not been very useful in predicting NAFLD.[6] Current “normal” range of liver transaminases present in patients with NAFLD may, therefore, underestimate the presence of NAFLD. In our study, only 31% of our study subjects with NAFLD had ALT above laboratory normal limit of >40 U/L, 57% of our study subjects with NAFLD had ALT between 25 and 40 U/L, whereas 67% of the subjects without NAFLD had ALT <25 U/L, but 30% of non-NAFLD subjects had ALT between 25 and 40 U/L. Only 37% of our study subjects with NAFLD had GGT above laboratory normal limit of >30 U/L, 53% of study cohorts with NAFLD had GGT between 15 and 30 U/L, while 65% of the subjects without NAFLD had GGT <15 U/L, but 33% had GGT between 15 and 30 U/L.
WGO global guidelines suggest that in morbid obesity, diabetes and ALT/AST more than 27 IU/L are associated with high risk of progression to NASH. Prati et al. have proposed a new ALT upper limits of normal for healthy males and females to be 30 U/L and 19 U/L, respectively, to be diagnostically useful in NAFLD.[12] Kunde et al. found a significant increase in biopsy-proven NAFLD with the application of the new standard.[13] Mofrad et al. found that the entire histologic spectrum of NAFLD can be seen in individuals with normal ALT and diabetes was the only factor independently associated with increased risk of advanced fibrosis and normal ALT.[14] The diagnostic value of a test is reflected by its predictivities. A screening test should have high negative predictivity whereas a confirmatory test should have high positive predictivity. In our study [Table 4], ALT <25 U/L and GGT <15 U/L was found to have a high negative predictivity of 84.81% and 86.66% respectively for NAFLD detection which supports the contention that ALT <25 U/L and GGT <15 U/L cut-offs can be accurately used to exclude NAFLD in this subset of patients. Routine ultrasound examination of the abdomen for the screening of hepatic steatosis in T2DM patients may not be feasible in common practice in most countries, so new hepatic enzymes standards may detect early stages of NAFLD and thus may be useful for early treatment and reduction of CVD and cirrhosis risk. WGO global guidelines suggest that metabolic syndrome, diabetes, elevated ALT and AST are independent predictors for progression and mortality in NAFLD[1] In our study, ALT >40 U/L and GGT >30 U/L have high positive predictivity of 91.42% and 95.03% respectively suggesting patients having liver enzymes higher than these cut-offs could qualify for ultrasonography for detection of NAFLD in order to avoid unnecessary expenditure, especially in a developing country like India.
Limitations of the study
This study is a cross-sectional study with a limited number of subjects, and we did not perform a liver biopsy. We need large-scale prospective studies to validate our observations.
Ultrasound is the usual screening test for fatty liver although it is insensitive, operator dependent and reliably diagnose NAFLD only if steatosis is >33%. Even with MRI, results are variable and not well verified. Even MRI which is costly and less available, cannot distinguish steatosis and fibrosis or NASH/ASH or can stage the disease. MRI is also insensitive if there is <33% steatosis. In fact without a liver biopsy, no imaging study can identify liver fat accurately if it is <33% or distinguish NASH from ASH. Ultimately, NAFLD/NASH is a diagnosis of exclusion, and liver biopsy is required to confirm the diagnosis, stage the disease, rule out other liver diseases, and determine the need for and urgency of aggressive therapy. Although liver biopsy may indicate the severity of the disease, but only fibrosis, and not inflammation or necrosis, has been confirmed to predict the disease prognosis. Moreover, there are no adequate prospective, double-blind, controlled trials to provide the data necessary to create an evidence-based guidelines, and all NAFLD patients should first be treated with diet and exercise.[1]
CONCLUSIONS
Mild elevations of liver enzymes in the upper normal range are associated with features of metabolic syndrome and NAFLD even in IGT and recently detected T2DM patients, which should prompt clinicians to initiate further diagnostic work-up. Novel cut-offs for liver enzymes are warranted in order to prevent unnecessary diagnostic work-ups as well as early detection of NAFLD to reduce the risk of cirrhosis, hepatocellular carcinoma and CVD in T2DM and IGT patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Review Team, LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–73. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: A prospective study. J Hepatol. 2009;51:1061–7. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology. 2011;54:1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–8. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 6.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56:234–40. doi: 10.1016/j.jhep.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–51. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–7. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, et al. Alanine aminotransferase predicts coronary heart disease events: A 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191:391–6. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 12.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–6. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 14.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–92. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]


