Abstract
Background:
Pheochromocytomas (PHEO) and paragangliomas (PGL) are derived from paraganglia of the sympathetic and parasympathetic nervous system. Most of the sympathetic PHEO/PGL secrete either catecholamine or their metabolites, metanephrines, whereas parasympathetic PHEO/PGL are nonsecretory. We assessed the utility of plasma free 3-methoxytyramine (3MT), normetanephrine (NM), and metanephrine (MN) for the diagnosis of PHEO/PGL.
Materials and Methods:
Sixty-five patients referred to endocrine/ENT clinics were enrolled. Twelve patients with von Hippel-Lindau (VHL), neurofibromatosis type 1 (NF1) and multiple endocrine neoplasia type 2 (MEN2) syndromes were excluded. Remaining 53 patients (39 patients with adrenal, abdominal, cervical and thoracic PHEO/PGL and 14 patients with head and neck PGL (HNPGL) were taken for this study. Sixty-five age- and sex-matched subjects were taken as controls. Plasma levels 3MT, NM, and MN were measured using high-performance liquid chromatography. Receivers operating characteristics was plotted and cut-off levels were established.
Results:
When compared with controls, there was a 36-, 8.7- and 9.5-fold increase in levels of NM, 3MT and MN in the patients with PHEO/PGL and 7.2- and 2.7-fold increase in 3MT and NM, in the patients with HNPGL, respectively. In malignant PHEO/PGL, there was a 99-, 16- and 20-fold increase and in benign PHEO/PGL, there was 19-, 6.8- and 6.4-fold increase in levels of NM, 3MT, and MN, respectively. NM in combination with MN was high in 97% of the patients with PHEO/PGL. All three metabolites in combination were high in 83% of patients with HNPGL. In malignant PHEO/PGL, 50% subjects had increased levels of both NM and 3MT.
Conclusions:
Measurement of plasma-free NM along with 3MT and MN provides a better tool for the diagnosis of PHEO/PGL as well as HNPGL. Further, NM in combination with 3MT can be used for the diagnosis of malignant PHEO/PGL.
Keywords: 3 methoxy tyramine, metanephrine, normetanephrine, paraganglioma, pheochromocytoma
INTRODUCTION
Paragangliomas (PGL) are rare neuroendocrine tumors that arise in the sympathetic and parasympathetic nervous system. Sympathetic PGLs are mainly present in adrenal medulla and termed as pheochromocytomas (PHEO).[1] Most of these tumors are secretary in nature and diagnosed by the secretion of catecholamine.[1] These catecholamine are metabolized by the tumor to form the ortho-methylated products, which are metanephrine (MN), normetanephrine (NM), and 3-methoxytyramine (3MT).[2,3,4,5] Some of these tumors may not secrete catecholamine as these are metabolized to metanephrines (MNs) within the tumor.[5] Therefore, the measurement of MTNs is the most sensitive and specific method to detect these tumors.[6] Parasympathetic PGLs are generally located in head and neck region.[1] Only 4% of these tumors are secretary, and others are diagnosed by their mass effect on neighboring organs.[7]
The biochemical diagnosis of PHEO/PGL is made by measuring the levels of catecholamine and their ortho-methylated metabolites (MTNs).[8] Measurement of plasma-free MTNs are the metabolites of choice over urinary MTNs, plasma and urinary catecholamine for the diagnosis of PHEO/PGL.
There are no data available regarding the succinate dehydrogenase mutations and their phenotype in the patients with PHEO/PGL from India. This study was carried out to study the biochemical phenotype in such patients.
MATERIALS AND METHODS
Recruitment of subjects
A total of 65 consecutive patients attending endocrine and ENT clinics, diagnosed with PHEO/PGL based on clinical symptoms, metaiodobenzylguanidine scan and magnetic resonance imaging reports were recruited in this study. The PHEO/PGL was confirmed in these patients by histopathological evaluation. A written informed consent was obtained from all the patients. The study was approved by the AIIMS Ethics Committee.
Exclusion criteria
The PHEO/PGL patients with a family history of MEN2, VHL and NF1 and all patients with the symptoms of MEN2, VHL, and NF1 were excluded from the study.
Study subjects
Of 65 patients, 12 patients with medullary thyroid cancer, multiple cyst, and renal cell carcinoma were excluded from the study. Fifty-three subjects with PHEO/PGL were taken for this study. Sixty-five age and sex matched, apparently healthy controls without the family history of PHEO/PGL were recruited for plasma free MTNs analysis.
Sample collection
Fasting blood samples from the subjects with PHEO/PGL and apparently healthy controls with no family history of PHEO/PGL, were collected after lying supine for 30 min. The diet which interferes with catecholamine synthesis (coffee, chocolate, banana, and nuts)[9] and their measurement like acetaminophen were avoided for 3 days prior to blood sampling. Six ml of venous blood samples were collected from these subjects in tubes containing ethylenediaminetetraacetic acid kept on the ice. The samples were centrifuged immediately at 3500 rpm at 4°C for 15 min and plasma was separated. The plasma samples were stored separately at −20°C until analysis.
Metanephrines measurements
Plasma free MTNs (MN, NM, and 3MT) were measured using high-performance liquid chromatography (HPLC) with electron-capture detection after solid phase extraction. The HPLC system (Waters India) and the column (X-Bridge C18 analytical reverse-phase column [P/N 186003044; Waters India]) were used to separate the metabolites.
Preparation of standards
D, L-MN hydrochloride, and DL-NM hydrochloride were purchased from SIGMA; St. Louis, MO, USA. 3MT hydrochloride and 3-hydroxy-4-methoxybenzylamine hydrochloride (HMBA) were purchased from Aldrich, St. Louis, MO, USA. Standards (NM, MN, 3MT and HMBA) were dissolved in 0.1 M perchloric acid at 1 mg/ml and stored at −20°C. A cocktail of NM, MN and 3MT was prepared by adding an equal concentration of these three standards at the following concentration and spiking with an internal standard (HMBA) at the final concentration of 20 ng/ml.
Preparation of mobile phase
Mobile phase was prepared as described by Guillemin et al.[10]
Extraction of plasma free metanephrines
The extraction of plasma free metanephrines was done using Lender's method[11] with some modifications.
Preparation of standard curve for metanephrines
The working standards for NM, MN and 3MT were prepared and 50 μl of the cocktail of these was injected at 4°C to the HPLC system using autosampler. The standard curve was plotted using the area under the curve for each metabolite, and the results of the unknown sample were calculated using Empower 2 software (Waters Corporation, Milford, MA, USA).
Determination of cut-off values for metanephrines in pheochromocytomas/paragangliomas
Receivers operating characteristics (ROC) curves were plotted to determine the cut-off values for MN, NM, and 3MT between the values in healthy controls and patients. The sensitivity and specificity to distinguish the patients with PHEO/PGL from control was calculated using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
Cut-off value for MN: At cut point ≥510 pg/ml, the sensitivity, and specificity were 35% and 90% respectively. At cut point ≥806 pg/ml, the sensitivity, and specificity were 33% and 96% respectively. Area under the curve: 0.654.
Cut-off value for NM: At cut point ≥668 pg/ml with a sensitivity and specificity 84% and 88%, respectively, in the patients with PHEO/PGL (including head and neck PGL [HNPGL]) and when the patients with HNPGL were excluded and only PHEO/PGL patient's data were used to determine the cut-off value of NM, at cut point ≥668 pg/ml, the sensitivity and specificity were 95% and 94%, respectively. Area under the curve for NM in PHEO/PGL and PHEO/PGL was 0.921 and 0.975, respectively.
Cut-off value for 3MT: At a cut-point ≥1850 pg/ml, the sensitivity and specificity of 3MT was 57% and 94%, respectively, in the patients with PHEO/PGL. Area under the curve for 3MT was 0.837.
Determination of cut-off values in malignant pheochromocytomas/paragangliomas
Cut-off value for NM: ROC was plotted for NM between the values from benign and malignant PHEO. At a cut-off point of 3471 pg/ml, the sensitivity and specificity were 75% and 79%, respectively, (3.5 times higher than 688 pg/ml) to distinguish malignant from the benign PHEO/PGL (area under the curve: 0.879).
Cut off value for 3MT: ROC was plotted for 3MT between the values from benign and malignant PHEO. The sensitivity and specificity of 3MT at a cut-off point of 3922 pg/ml was 63% and 76%, respectively, to distinguish malignant from benign PHEO/PGL.
Statistical analysis
All the statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. The descriptive statistics were used to determine the mean, SD and range of different parameters. The cut-off values of NM, MN and 3MT in PHEO/PGL, HNPGL, benign and malignant PGL were determined using the ROC. The levels of NM, MN and 3MT in controls and the patients were compared using Mann-Whitney test at 95% confidence intervals.
RESULTS
Plasma free metanephrines in pheochromocytomas/paragangliomas
The mean plasma levels of free MTNs in the patients with PHEO/PGL are given in Table 1 and Figures 1-3. The mean ± standard deviation (SD) levels of 3MT were 4836 ± 7039 and 555 ± 707 pg/ml in the patients with PHEO/PGL and controls, respectively. The mean ± SD levels of NM were 7561 ± 12575 and 210 ± 258 pg/ml in the patients with PHEO/PGL and controls, respectively. The mean + SD levels of MN in the patients with PHEO/PGL and controls were 1632 ± 3353 and 171 ± 584 pg/ml, respectively. The mean plasma concentration of NM were 36-fold higher in the patients with PHEO/PGL as compared to control [Table 1] and [Figures 1-3]. This increase was significantly (P < 0.01) larger than that of MN (9.5-fold) and 3MT (8.7-fold). The ROC analysis showed that NM levels were high in 95% patients with PHEO/PGL [Table 2], whereas MN and 3MT levels were high in 57% and 51% patients with PHEO/PGL, respectively. NM in combination with MN was high in 97% of the patients with PHEO/PGL [Table 2].
Table 1.
Comparison of NM, MN and 3MT values in different groups
Figure 1.
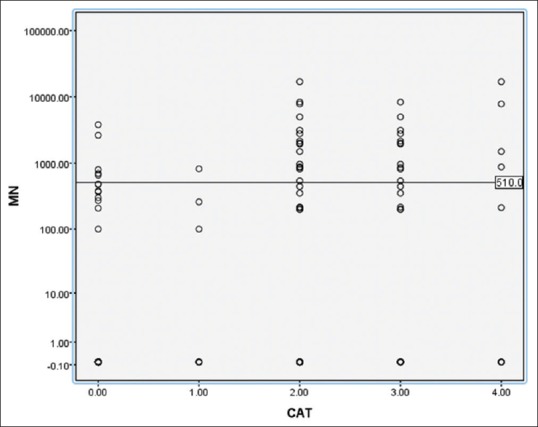
Comparison of metanephrine values in different groups using scatter plot Groups (0-control, 1-head and neck paragangliomas, 2-pheochromocytomas and paragangliomas [PHEO/PGL], 3-benign PHEO/PGL, 4-malignant pheochromocytomas/paragangliomas). In this scatter plot the metanephrine levels were compared between different groups
Figure 3.
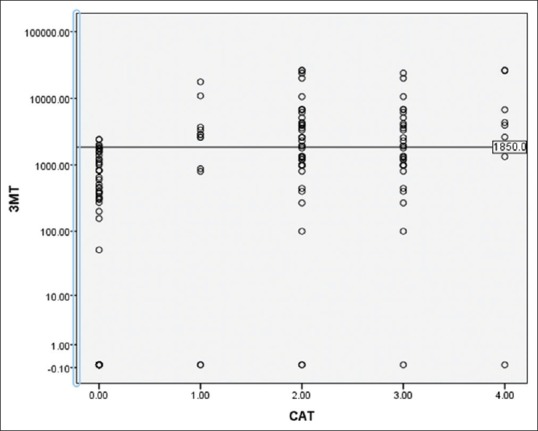
Comparison of 3-methoxytyramine values in different groups using scatter plot Groups (0-control, 1-head and neck paragangliomas, 2-pheochromocytomas and paragangliomas [PHEO/PGL], 3-benign PHEO/PGL, 4-malignant pheochromocytomas/paragangliomas). In this scatter plot the 3-methoxytyramine levels were compared between different groups
Table 2.
Diagnostic efficacy of metanephrines
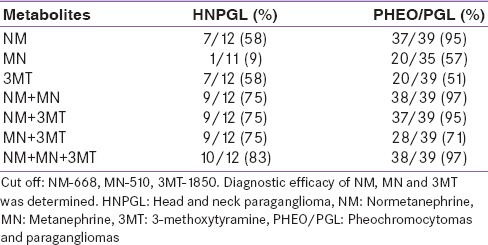
Figure 2.
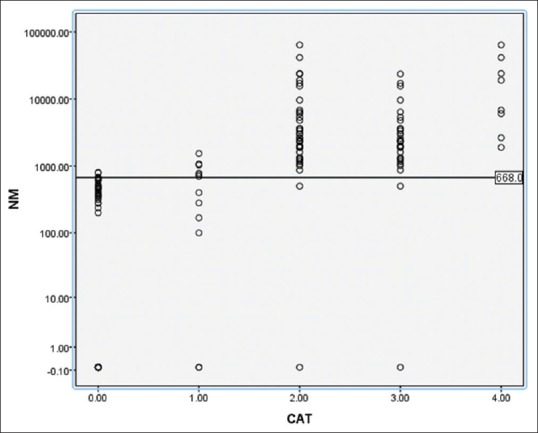
Comparison of normetanephrine values in different groups using scatter plot Groups (0-control, 1-head and neck paragangliomas, 2-pheochromocytomas and paragangliomas [PHEO/PGL], 3-benign PHEO/PGL, 4-malignant pheochromocytomas/paragangliomas). In this scatter plot the normetanephrine levels were compared between different groups
Plasma free metanephrines in head and neck paragangliomas
The mean plasma levels of free MTNs in the patients with HNPGL are given in Table 1 and Figures 1-3. The mean ± SD levels of 3MT in the patients with HNPGL and controls were 4014 ± 5160 and 555 ± 707 pg/ml, respectively. The mean + SD levels of NM in the patients with HNPGL and controls were 568 ± 490 and 210 ± 258 pg/ml, respectively. The mean + SD levels of MN in the patients with HNPGL and controls were 147 ± 286 and 171 ± 584 pg/ml, respectively. The mean plasma concentrations of 3MT increased 7.2-fold in the patients with HNPGL [Table 1]. This increase in 3MT levels was significantly larger than NM (2.7-fold) and MN (no increase) [Table 1] and [Figures 1-3]. Plasma 3MT and NM each were significantly high in 58% of the patients with HNPGL. Plasma 3MT in combination with NM was able to identify 75% of the patients with HNPGL, whereas, when all the three metabolites were used, 83% of the patients were identified with HNPGL [Table 2].
Plasma free metanephrines in malignant pheochromocytomas/paragangliomas
The mean plasma levels of free MTNs in the patients with benign and malignant PHEO/PGL are given in Table 1 and Figures 1-3. The mean ± SD levels of 3MT in the patients with benign and malignant PHEO/PGL were 3785 ± 5543 and 8920 ± 10854 pg/ml, respectively. The mean ± SD levels of NM were 4160 ± 5331 and 20740 ± 22068 pg/ml in the patients benign and malignant PHEO/PGL, respectively. The mean ± SD levels of MN in the patients with benign and malignant PHEO/PGL were 1105 ± 1899 and 3405 ± 6046 pg/ml respectively. The mean plasma concentrations of NM were increased to 4.9-fold in the patients with malignant as compared to benign PHEO/PGL [Table 1]. This increase in NM was significant as compared to 3MT (2.4-fold) and MN (3-fold) [Table 1].
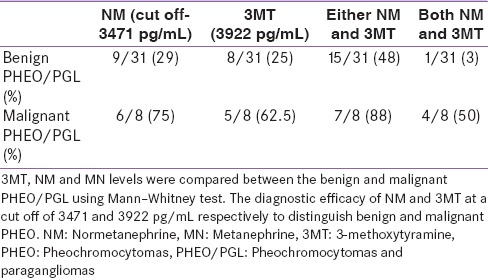
Plasma normetanephrine and 3-methoxytyramine to distinguish benign and malignant pheochromocytomas/paragangliomas
In combination, 3% patients showed an increase in both NM and 3MT in benign HNPGL, whereas 50% patients with malignant HNPGL showed increased levels of both NM and 3MT [Table 3].
Table 3.
Diagnostic efficacy of NM and 3MT to discriminate benign from malignant PHEO/PGL
DISCUSSION
The results of this study have showed that measurement of NM along with 3MT not only provide a useful biomarker for the diagnosis of PHEO/PGL, but is also useful in the diagnosis of HNPGL. We have further shown that measurement of NM in combination with 3MT improves the efficacy to distinguish benign from malignant PHEO/PGL.
Earlier studies have reported an increased levels of urinary and plasma catecholamine in the patients with PHEO/PGL. The secretion of catecholamine is associated with the symptoms like hypertension, headache, palpitations, abdominal pain and sweating, which are episodic. These catecholamine disappear from the blood very fast due to shorter half-life. Further, all PHEO/PGL don’t secrete catecholamine but all metabolize catecholamine to MTNs.[5] The MTNs have a longer half-life, which makes them better biomarkers for the diagnosis of PHEO/PGL.[12,13,14] Estimations of plasma MTNs is preferred over urinary MTNs because blood sample collection is easy as compared to the 24 h urine sample collection, and their sensitivity and specificity is greater than the catecholamine.[15,16,17] Eisenhofer et al., have reviewed the catecholamine metabolism in sympathetic, adrenal medulla and PHEO tissue and provided convincing evidence that plasma free MTNs are more specific for the diagnosis of PHEO/PGL. Further, urinary dopamine or its metabolite, 3MT has much lower signal strength as compared to plasma because more than 90% of the dopamine in urine is derived from renal excretion and decarboxylation of circulating 3,4-dihydroxyphenylalanine.[18] (Brown and Allison, 1981). Thus, compared to plasma measurements of dopamine and 3-MT, the measurement of urinary dopamine provide an insensitive biomarker of tumor dopamine (Eisenhofer et al., 2005).[19]
In the present study, plasma free NM levels were significantly higher in the patients with PHEO/PGL as compared to controls. Earlier studies have also reported a similar increase in the levels of NM the patients with PHEO/PGL.[11] Eisenhofer et al., has reported the high levels of 3MT in the patients with PHEO/PGL.[20,21]
In this study, the levels of plasma free NM were significantly higher in 37/39 patients with PHEO/PGL in contrast to MN being elevated in only 15/35 and 3MT in 20/39 patients indicating that plasma free NM is better biomarker for the diagnosis of PHEO/PGL than MN and 3MT. When these metabolites were analyzed in combination, we were able to diagnose PHEO/PGL in 38/39 patients. This is probably because the study was carried out in a tertiary care hospital, and all the subjects were prediagnosed with PHEO/PGL by imaging studies and confirmed by histopathology.
In the patients with HNPGL, 7/12 (58%) patients had a significant increase in plasma free 3MT and NM levels were elevated in 5/12 patients. HNPGL are rare tumors that are derived from the parasympathetic paraganglia and most of these tumors are nonsecretary and nonfunctional. Studies have reported that HNPGL are innervated with dopamine-secreting neurotransmitters[22] and also contain the enzyme catechol-O-methyltransferase, which metabolize dopamine to 3MT.[23] Thus, plasma 3MT can be a useful biochemical marker for the detection of HNPGL. Previous studies have also reported increased levels of 3MT in 23–28% of patients with HNPGL.[22,24]
The increased levels of NM in the patients with HNPGL suggest the presence of associated secretary PGL in these patients. An earlier study has reported 15% patients with HNPGL had elevated levels of catecholamine or their metabolite.[22] Several other reports observed the presence of associated tumors in these patients with HNPGL. Plasma free 3MT levels were high in around 67% of these subjects. Duinen et al.,[22] reported that HNPGL do not have the enzyme system to synthesize norepinephrine from dopamine. The dopamine synthesized by the HNPGL tissue is metabolized to 3MT, leading to increased levels of plasma 3MT.
In this study, plasma free NM and 3MT levels were significantly elevated in the subjects with malignant PHEO/PGL as compared to benign PHEO/PGL. In combination these two parameters were able to distinguish malignant PHEO/PGL in 50% of the subjects. The results of this study suggest that estimation of both NM and 3MT can be useful in the diagnosis of malignant PHEO/PGL. This observation is consistent with an earlier reports of 4.7-fold increase in 3MT levels in the patients with malignant PHEO/PGL.[21] In our study, all the patients with malignant PHEO/PGL were form PHEO/PGL group and no malignancy was present in the patients with HNPGL. The results of the present study suggest the role of catecholamine/MTNs in the transformation of benign tumors to malignant PHEO/PGL.
In conclusion, plasma free NM is the biomarker of choice for the diagnosis of PHEO/PGL and 3MT for the diagnosis of HNPGL. The efficacy of plasma NM and 3MT can be improved by analyzing these MTNs in combination. Plasma NM and 3MT in combination can also be used for the diagnosis of malignant PHEO/PGL [Table 3].
Footnotes
Source of Support: AIIMS, New Delhi
Conflict of Interest: None declared.
REFERENCES
- 1.DeLellis RA, Lloyd RV, Heitz PU, Eng C. Oxford, UK: Oxford University Press; 2004. Pathology and Genetics: WHO Classification of Tumors of Endocrine Organs. [Google Scholar]
- 2.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 3.Unger N, Pitt C, Schmidt IL, Walz MK, Schmid KW, Philipp T, et al. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409–17. doi: 10.1530/eje.1.02097. [DOI] [PubMed] [Google Scholar]
- 4.Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, et al. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med. 2000;160:2957–63. doi: 10.1001/archinte.160.19.2957. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, et al. Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998;83:2175–85. doi: 10.1210/jcem.83.6.4870. [DOI] [PubMed] [Google Scholar]
- 6.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: Which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 7.Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, et al. Benign paragangliomas: Clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001;86:5210–6. doi: 10.1210/jcem.86.11.8034. [DOI] [PubMed] [Google Scholar]
- 8.van Berkel A, Lenders JW, Timmers HJ. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur J Endocrinol. 2014;170:R109–19. doi: 10.1530/EJE-13-0882. [DOI] [PubMed] [Google Scholar]
- 9.de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: Implications for diagnosis of catecholamine-producing tumors. J Clin Endocrinol Metab. 2009;94:2841–9. doi: 10.1210/jc.2009-0303. [DOI] [PubMed] [Google Scholar]
- 10.Guillemin A, Troupel S, Galli A. Determination of catecholamines in plasma by high-performance liquid chromatography. Clin Chem. 1988;34:1913–4. [PubMed] [Google Scholar]
- 11.Lenders JW, Eisenhofer G, Armando I, Keiser HR, Goldstein DS, Kopin IJ. Determination of metanephrines in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1993;39:97–103. [PubMed] [Google Scholar]
- 12.Bühler HU, da Prada M, Haefely W, Picotti GB. Plasma adrenaline, noradrenaline and dopamine in man and different animal species. J Physiol. 1978;276:311–20. doi: 10.1113/jphysiol.1978.sp012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clutter WE, Bier DM, Shah SD, Cryer PE. Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest. 1980;66:94–101. doi: 10.1172/JCI109840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward MM, Mefford IN, Parker SD, Chesney MA, Taylor CB, Keegan DL, et al. Epinephrine and norepinephrine responses in continuously collected human plasma to a series of stressors. Psychosom Med. 1983;45:471–86. doi: 10.1097/00006842-198312000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hickman PE, Leong M, Chang J, Wilson SR, McWhinney B. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology. 2009;41:173–7. doi: 10.1080/00313020802579284. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan NM, Kramer NJ, Holland OB, Sheps SG, Gomez-Sanchez C. Single-voided urine metanephrine assays in screening for pheochromocytoma. Arch Intern Med. 1977;137:190–3. [PubMed] [Google Scholar]
- 17.Sawka AM, Jaeschke R, Singh RJ, Young WF., Jr A comparison of biochemical tests for pheochromocytoma: Measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553–8. doi: 10.1210/jc.2002-021251. [DOI] [PubMed] [Google Scholar]
- 18.Brown MJ, Allison DJ, Jenner DA, Lewis PJ, Dollery CT. Increased sensitivity and accuracy of phaeochromocytoma diagnosis achieved by use of plasma-adrenaline estimations and a pentolinium-suppression test. Lancet. 1981;1:174–7. doi: 10.1016/s0140-6736(81)90058-1. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, et al. Biochemical and clinical manifestations of dopamine--producing paragangliomas: utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90:2068–75. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, et al. Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57:411–20. doi: 10.1373/clinchem.2010.153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: A novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–49. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Duinen N, Steenvoorden D, Kema IP, Jansen JC, Vriends AH, Bayley JP, et al. Increased urinary excretion of 3-methoxytyramine in patients with head and neck paragangliomas. J Clin Endocrinol Metab. 2010;95:209–14. doi: 10.1210/jc.2009-1632. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhofer G, Goldstein DS, Sullivan P, Csako G, Brouwers FM, Lai EW, et al. Biochemical and clinical manifestations of dopamine-producing paragangliomas: Utility of plasma methoxytyramine. J Clin Endocrinol Metab. 2005;90:2068–75. doi: 10.1210/jc.2004-2025. [DOI] [PubMed] [Google Scholar]
- 24.van Duinen N, Corssmit EP, de Jong WH, Brookman D, Kema IP, Romijn JA. Plasma levels of free metanephrines and 3-methoxytyramine indicate a higher number of biochemically active HNPGL than 24-h urinary excretion rates of catecholamines and metabolites. Eur J Endocrinol. 2013;169:377–82. doi: 10.1530/EJE-13-0529. [DOI] [PubMed] [Google Scholar]


