Abstract
Aim:
To determine whether pioglitazone is associated with an increased risk of bladder cancer among Indian type 2 diabetic patients.
Methods:
A retrospective data analysis of 2222 type 2 diabetic patients was conducted. The study subjects were divided into two equal groups: 1111 pioglitazone users and 1111 pioglitazone non-users. The safety of pioglitazone therapy was analyzed in terms of occurrence of bladder and other types of cancers along with its efficacy in terms of glycemic control. Parameters for assessing safety were duration of disease, duration of usage and total dose of pioglitazone consumed across age groups, glycemic control, obesity and family history of any cancer. Bladder cancer prevalence was analyzed on the basis of urinary cytology, urine routine and microscopy, hematuria, urinary nuclear matrix protein 22 analysis and ultrasonography.
Results:
Of the 2222 cases analysed, there was no evidence of bladder cancer in any of the studied groups, (p=not significant) which was also evident among 1111 patients on Pioglitazone therapy with a cumulative dose consumption of 2737 mg to 1,31,400 mg. On subgroup analysis, there was no evidence of bladder cancer amongst patients with age >60 years, duration of diabetes > 10 years and uncontrolled diabetics (HbA1c >8%) with cumulative pioglitazone consumption of >28,000 mg. A significant number of patients achieved good glycemic control (HbA1c <7.5%) with pioglitazone therapy.
Conclusion:
Pioglitazone therapy was not associated with occurrence of bladder cancer among Indian type 2 diabetic patients and demonstrated good glycemic control.
Keywords: Pioglitazone, bladder cancer, Type 2 diabetes mellitus, India, cumulative dose
INTRODUCTION
In India, type 2 diabetes mellitus (T2DM) is growing in an epidemic proportions, affecting younger people and is associated with a spectrum of complications.[1,2,3] Oral anti-diabetic drugs play a key role in diabetes management. Thiazolidinediones, the insulin sensitizers, are synthetic ligands for peroxisome proliferator-activated receptors that alter the transcription of genes influencing carbohydrate and lipid metabolism.[4] Pioglitazone demonstrated a beneficial effect on cardiovascular risk profile.[5] However, due to the publication of a longitudinal cohort study reporting an increased risk of bladder cancer with pioglitazone, there has been a lot of controversy generated over the positioning and use of pioglitazone.[6,7] Sudden banning of the molecule and subsequent revocation of the ban in India has left many prescribers and patients uncertain about its usage. Few Indian studies have already re-affirmed the safety of the drug in Indian patients.[8,9] However, many physicians are still apprehensive about the drug.
MATERIALS AND METHODS
Present study was conducted at a Tertiary Care Centre in Central India. The study was approved by the Ethics committee and data were collected after taking the informed consent from all the subjects. Data of 2222 type 2 diabetics were retrospectively analyzed. Studied subjects were divided into two equal groups; 1111 pioglitazone users and 1111 pioglitazone nonusers (who have never used pioglitazone), and was analyzed for demographical characteristics, duration of T2DM, patients on pioglitazone or other antidiabetic therapy, total dose and duration of pioglitazone consumed, glycemic control achieved, and prevalence of bladder cancer among pioglitazone users across all age groups. Prevalence of bladder cancer was assessed by taking history and detailed urinary analysis including urine routine, microscopy, hematuria, and cytology examination. Urinary nuclear matrix protein 22 (NMP-22) assay is a specific and important, cost-efficient point-of-care proteomic assay (biomarker test) for detecting bladder cancer. It has been observed that the concentration of NMP-22 is increased in patients with bladder cancer.[10,11] Urinary measurement of NMP-22 in urine samples was conducted in randomly selected 120 subjects whose cumulative dose consumption was >28000 mg, while ultrasonography abdomen was done in all pioglitazone users with cumulative dose consumption of >28000 mg. Among pioglitazone nonusers, the prevalence of bladder cancer was detected by conducting urine routine, microscopy, and detection of hematuria. History of occurrence of other malignancies apart from bladder cancer was also analyzed in all patients irrespective of pioglitazone usage. It has been suggested that before starting pioglitazone therapy, several risk factors for the development of bladder cancer like elderly patients, people with long duration of diabetes, past or family history of malignancy, etc., should be assessed to minimize the chances of its occurrence.[12] Thus, all subjects were also evaluated for association of bladder cancer with cumulative risk of bladder cancer in terms of age, duration of diabetes, hemoglobin A1c (HbA1c), family history of malignancy and body mass index (BMI). Statistical analysis was performed using Chi-square tests and ANOVA model.
RESULTS
The age of patients analyzed ranged from 28 to 83 years with a mean age being 53.89 years for pioglitazone users and 50.18 years for pioglitazone nonusers which was comparable, P = NS. Among total pioglitazone users, 62.6% cases were male and 37.4% were female, which was comparable to pioglitazone nonuser group having 64.4% male cases and 35.6% female cases (P = NS). Weight, height, and BMI of patients were comparable between both the groups and difference was not statistically significant [Table 1].
Table 1.
Demographical data
Dose of pioglitazone consumed
The mean dose of pioglitazone consumed in this study population was 22,322.8 mg (Standard deviation = 18,181). The minimum and maximum dose consumption was 2737 mg and 131,400 mg, respectively.
Analysis of safety parameters
Prevalence of bladder cancer
There was no evidence of bladder cancer among both pioglitazone users and nonusers [Table 2].
Table 2.
Prevalence of bladder cancer between two groups

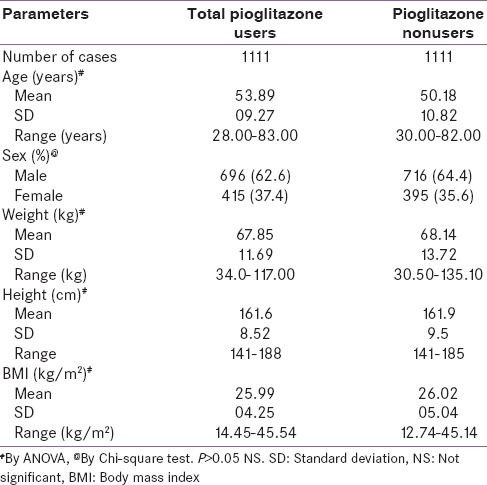
Association between duration of therapy and patients with bladder cancer
Of the total number of patients taking pioglitazone, 77% of patients were on therapy for >2 years and 33% were on therapy for <2-year duration. There was no evidence of bladder cancer in either of patient population [Table 3].
Table 3.
Duration of pioglitazone use and prevalence of bladder cancer
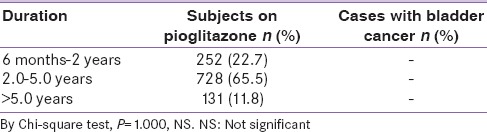
Association between cumulative dose consumption and patients with bladder cancer
Of the total pioglitazone users, 18.8% consumed <10000 mg, around 56.6% of patients consumed a dose of 10,000–28,000 mg, and 24.6% consumed >28,000 mg of pioglitazone. No evidence of bladder cancer was reported in this patient population (P = NS). Furthermore, there was no evidence of bladder cancer at a high cumulative dose of more than 50,000 mg, which was 7% of total pioglitazone users [Table 4].
Table 4.
Association between pioglitazone dose consumption and patients with bladder cancer
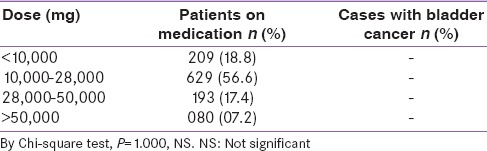
Association between age, cumulative pioglitazone dose and bladder cancer
Pioglitazone usage was assessed as per different age groups and dose consumed. The mean pioglitazone dose consumption by subjects >60 years of age was 24961.03 mg and in subjects with >70 years of age it was 25501.97 mg. However, this analysis did not reveal any evidence of bladder cancer in these elderly subjects [Table 5].
Table 5.
Association between age group, mean pioglitazone dose consumption, duration of diabetes and prevalence of bladder cancer
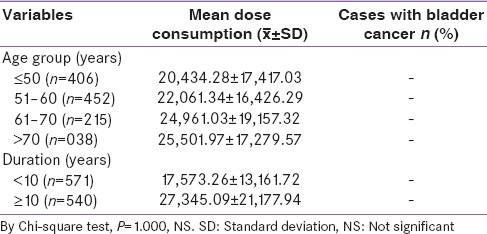
Association between duration of type 2 diabetes mellitus, cumulative pioglitazone dose, and bladder cancer
As per the study the mean dose of consumption of pioglitazone by patients with more than 10 years of T2DM was 27345.09 mg, whereas patients having diabetes for <10 years consumed a mean dose of 17573.26 mg. Neither of the doses was associated with any occurrence of bladder cancer (P = NS) [Table 5].
Association between Hemoglobin A1c and patients with bladder cancer
Totally 785 patients (70.7%) had an HbA1c of ≥7.5%. There was no occurrence of bladder cancer in this group as well (Chi-square test; P = 1.000, NS).
Association between age, duration of diabetes, mean dose of pioglitazone consumed, and level of glycemic control with bladder cancer
In a separate analysis consisting of patients with uncontrolled diabetes (HbA1c >8%), elderly patients (>60 years) and patients with long-standing diabetes (>10 years) with a mean cumulative pioglitazone dose consumption of 28250.99 mg, there was no occurrence of bladder cancer.
Association between body mass index and family history of cancer with bladder cancer
The association of bladder cancer was also evaluated among pioglitazone users who were obese (BMI ≥23 kg/m2) or who had a family history of any cancer. Pioglitazone was not associated with bladder cancer in these two groups as well.
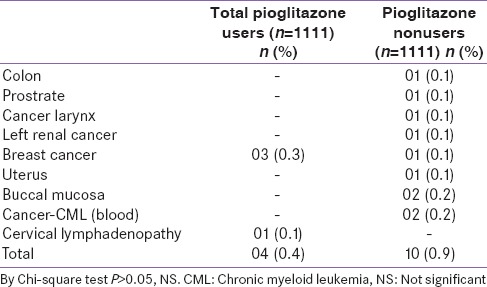
Prevalence of other types of cancer between the two groups
Apart from analyzing the occurrence of bladder cancer, an independent analysis was conducted to determine the association of pioglitazone use with other types of cancers. The analysis revealed that 0.4% of total pioglitazone users developed other types of cancer, which was comparable with 0.9% occurrence in nonusers (P: NS). Among pioglitazone users, 0.3% of patients developed breast cancer which was higher as compared to nonusers (0.1% cases), but was found to be statistically nonsignificant on further analysis [Table 6].
Table 6.
Profile of types of cancer between the 2 groups
Analysis of efficacy parameters changes in proportion of patients with high hemoglobin A1c after the treatment
Among pioglitazone users, at baseline 14.7% of subjects demonstrated HbA1c value of <7.5%. After pioglitazone therapy significantly higher number of subjects (33.3%) had HbA1c <7.5%. Furthermore, prior to pioglitazone therapy, 31.7% of subjects had a fasting blood glucose (FBG) level of ≤120 mg/dL. Post therapy, significant higher number of subjects (41.4%) achieved FBG of ≤120 mg/dL. Similar findings were noted for postprandial blood glucose (PPBG) levels. Before treatment, 3.1% of patients had PPBG <140 mg/dL. Postpioglitazone treatment 92.4% achieved PPBG level of <140 mg/dL.
Urinary nuclear matrix protein 22 analysis
As stated earlier, there is an increase in the level of urinary NMP 22 in patients with bladder cancer. In this study, NMP 22 analysis was carried out on 120 randomly selected subjects with >28,000 mg consumption of pioglitazone. NMP-22 was tested negative in all 120 patients, which in turn meant that bladder cancer was not evident in these patients.
DISCUSSION
With the publication of a longitudinal cohort study reporting an increased risk of bladder cancer with pioglitazone, there has been a lot of controversy generated over the positioning and use of pioglitazone in India.[7] This retrospective study aimed to analyze the probable link between pioglitazone and incidence of bladder cancer in a T2DM patient cohort. As per the data analyzed, pioglitazone usage was not associated with the occurrence of bladder cancer. These results are consistent with other recently published studies in which an association between pioglitazone and bladder cancer has not been established.
As per a study conducted by Song et al., relationship between pioglitazone use and incidence of bladder cancer was not observed in Korean diabetic patients.[13] Another study analyzed reimbursement databases of all Taiwanese patients with T2DM, who received oral anti-diabetic agents or insulin from 1996 to 2009 retrieved from the National Health Insurance. This study stated that pioglitazone and kidney cancer were not significantly associated in unadjusted, age-sex-adjusted, and fully adjusted models. None of the dose-response parameters showed a significant trend of risk association, with all P-trends >0.10.[14] A 10-year observational cohort study as well as a nested case–control study were conducted in patients with diabetes who are members of Kaiser Permanente Northern California (KPNC) health plan. Patients selected in this study had diabetes mellitus and were >40 years of age at study entry. Patients with bladder cancer prior to study entry or within 6 months of joining KPNC were excluded. The cohort included 193,099 patients with diabetes. A planned 5-year interim analysis was performed with data collected from January 1, 1997 to April 30, 2008. The median duration of therapy among pioglitazone-treated patients was 2 years (range 0.2–8.5 years). The study investigators did not observe a statistically significant association between pioglitazone usage and increased risk of bladder cancer (hazard ratio = 1.2, 95% confidence interval: 0.9–1.5).[7] In a recently published multipopulation pooled, cumulative exposure analysis, the cumulative use of pioglitazone was not associated with the incidence of bladder cancer.[15]
Nuclear matrix protein 22 test is a double monoclonal antibody test designed to measure quantitatively the nuclear mitotic apparatus (NuMA) protein which is over expressed by bladder cancer and is released into the urine in increased quantity.[16] NMP 22 test is superior to cytology for detection of Grades 1 and 2 bladder cancer but with lower specificity.[11] The specificity of this test can be enhanced by excluding the following 6 criteria namely benign inflammatory or infectious conditions, renal or bladder calculi, foreign body (stent or nephrostomy tube), bowel interposition, other genitourinary cancer, and instrumentation.[11] In the present study, NMP 22 test was conducted among 120 randomly selected patients taking pioglitazone. This particular analysis also showed results in favor of pioglitazone as no patients reported the presence of NMP 22.
These recent trends in favor of pioglitazone recommend an appropriate reassessment of the so-called bladder cancer association with pioglitazone. Not a single trial based on this issue is a prospective well-balanced one where pioglitazone was started after screening for cancer. Diabetes itself is a precancerous condition; carcinogenesis may be due to other drugs used concomitantly with pioglitazone.[17] The major correlation between pioglitazone and bladder cancer is the duration of therapy of >24 months and cumulative dose of >28000 mg. Average daily dose of pioglitazone used in India is <30 mg/day. To achieve a cumulative dose of 28,000 mg it would take more than 5 years, if 15 mg/day were used and if 7.5 mg/day is used it would take more than 10 years. Low dose pioglitazone has of late been very popular in India due to its efficacy and lesser side effects like weight gain and fluid retention.[6] However, in the present study, even this dose was not associated with the occurrence of bladder cancer. Increasing age, longer duration of diabetes, obesity, and uncontrolled hyperglycemia are the common risk factors for any malignancy in people with type 2 diabetes.[12] In the present study, among pioglitazone users with all these risk factors there was no increased risk of bladder cancer.
The European Medicines Agency (EMA) concludes that the “benefit/risk balance remains positive in a limited population of type 2 diabetics” and that “the small increased risk could be reduced by appropriate patient selection and exclusion, including a requirement for periodic review of the efficacy and safety of the individual patient's treatment.” Risk factors for bladder cancer should be “investigated,” particularly in elderly patients, before physicians move forward with the choice of pioglitazone, the EMA alert states.[18]
USFDA recommends not to use pioglitazone in patients with active bladder cancer and to use it with caution in patients with prior history of bladder cancer. Benefits of glycemic control versus unknown risks for cancer recurrence with pioglitazone should be considered in patients with a prior history of bladder cancer.[19] The Indian authorities also have revoked the ban on pioglitazone and has allowed the manufacture and sale but with a box warning in bold red letters.[20]
Pioglitazone remains a useful blood glucose-lowering therapy and offers good glycemic control without any incidences of bladder cancer in Indian subjects. This retrospective data concludes that pioglitazone was not associated with bladder cancer across age groups, dose and duration of therapy. Though very few cases of other cancers have been reported in this study, but such cases have additional risk factors accompanying them. Hence, the direct association is further minimized. Unlike, many other countries, we do not have any Indian diabetes central registry for such assessment and thus, a retrospective clinical data, like ours may help us to consolidate our knowledge and confidence on the molecule. To have better understanding, we will continue to capture more data in future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kaveeshwar SA, Cornwall J. The current state of diabetes mellitus in India. Australas Med J. 2014;7:45–8. doi: 10.4066/AMJ.2013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IDF Diabetes Atlas Sixth Edition. [Last cited on 2014 Dec 30]. Available from: http://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf .
- 3.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 4.Smith U. Pioglitazone: Mechanism of action. Int J Clin Pract Suppl. 2001 Sep;121:13–8. [PubMed] [Google Scholar]
- 5.Vallarino C, Perez A, Fusco G, Liang H, Bron M, Manne S, et al. Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clin Drug Investig. 2013;33:621–31. doi: 10.1007/s40261-013-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panikar V. Pioglitazone and bladder cancer: The pros and cons. J Assoc Physicians India. 2012;60:72–3. [PubMed] [Google Scholar]
- 7.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–22. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mithal A, Kaur P, Bansal B, Mishra SK, Wasir JS, Jevalikar G, et al. Usage of pioglitazone at Medanta, the Medicity. Indian J Endocrinol Metab. 2014;18:111–2. doi: 10.4103/2230-8210.126588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balaji V, Seshiah V, Ashtalakshmi G, Ramanan SG, Janarthinakani M. A retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–7. doi: 10.4103/2230-8210.131223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau P, Chin JL, Pautler S, Razvi H, Izawa JI. NMP22 is predictive of recurrence in high-risk superficial bladder cancer patients. Can Urol Assoc J. 2009;3:454–8. doi: 10.5489/cuaj.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shariat SF, Karam JA, Lotan Y, Karakiewizc PI. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev Urol. 2008;10:120–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S. Diabetes and cancer. In: Chandalia HB, editor. RSSDI Textbook of Diabetes Mellitus. 3rd ed. New Delhi: Jaypee Brothers Med Publisher (P) Ltd; 2014. pp. 1127–42. [Google Scholar]
- 13.Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. The risk of bladder cancer in Korean diabetic subjects treated with pioglitazone. Diabetes Metab J. 2012;36:371–8. doi: 10.4093/dmj.2012.36.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng CH. Pioglitazone does not affect the risk of kidney cancer in patients with type 2 diabetes. Metabolism. 2014;63:1049–55. doi: 10.1016/j.metabol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Levin D, Bell S, Sund R, Hartikainen SA, Tuomilehto J, Pukkala E, et al. Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Diabetologia. 2015;58:493–504. doi: 10.1007/s00125-014-3456-9. [DOI] [PubMed] [Google Scholar]
- 16.Sturgeon CM, Duffy MJ, Hofmann BR, Lamerz R, Fritsche HA, Gaarenstroom K, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin Chem. 2010;56:e1–48. doi: 10.1373/clinchem.2009.133124. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee S. Pioglitazone safe, so save. J Assoc Physicians India. 2012;60:62–6. [PubMed] [Google Scholar]
- 18.European Medicines Agency Recommends New Contraindications and Warnings for Pioglitazone to Reduce Small Increased Risk of Bladder Cancer. [Last cited on 2014 Dec 30]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/07/WC500109176.pdf .
- 19.Actos (Pioglitazone): Ongoing Safety Review-Potential Increased Risk of Bladder Cancer. [Last cited on 2014 Dec 30]. Available from: http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm226257.htm .
- 20.Minutes of the 64th Meeting of Drugs Technical Advisory Board (DTAB) [Last cited on 2014 Dec 30]. Available from: http://www.cdsco.nic.in/writereaddata/Minutes%20of%2064th%20DTAB%20 meeting.pdf .


