Abstract
AIM: To develop a novel 3-dimensional (3D) virtual hepatectomy simulation software, Liversim, to visualize the real-time deformation of the liver.
METHODS: We developed a novel real-time virtual hepatectomy simulation software program called Liversim. The software provides 4 basic functions: viewing 3D models from arbitrary directions, changing the colors and opacities of the models, deforming the models based on user interaction, and incising the liver parenchyma and intrahepatic vessels based on user operations. From April 2010 through 2013, 99 patients underwent virtual hepatectomies that used the conventional software program SYNAPSE VINCENT preoperatively. Between April 2012 and October 2013, 11 patients received virtual hepatectomies using the novel software program Liversim; these hepatectomies were performed both preoperatively and at the same that the actual hepatectomy was performed in an operating room. The perioperative outcomes were analyzed between the patients for whom SYNAPSE VINCENT was used and those for whom Liversim was used. Furthermore, medical students and surgical residents were asked to complete questionnaires regarding the new software.
RESULTS: There were no obvious discrepancies (i.e., the emergence of branches in the portal vein or hepatic vein or the depth and direction of the resection line) between our simulation and the actual surgery during the resection process. The median operating time was 304 min (range, 110 to 846) in the VINCENT group and 397 min (range, 232 to 497) in the Liversim group (P = 0.30). The median amount of intraoperative bleeding was 510 mL (range, 18 to 5120) in the VINCENT group and 470 mL (range, 130 to 1600) in the Liversim group (P = 0.44). The median postoperative stay was 12 d (range, 6 to 100) in the VINCENT group and 13 d (range, 9 to 21) in the Liversim group (P = 0.36). There were no significant differences in the preoperative outcomes between the two groups. Liversim was not found to be clinically inferior to SYNAPSE VINCENT. Both students and surgical residents reported that the Liversim image was almost the same as the actual hepatectomy.
CONCLUSION: Virtual hepatectomy with real-time deformation of the liver using Liversim is useful for the safe performance of hepatectomies and for surgical education.
Keywords: Liver, Surgery, Simulation, Virtual hepatectomy, Real-time deformation, Surgical education
Core tip: We aimed to develop a novel 3D virtual hepatectomy simulation software, Liversim, to visualize the real-time deformation of the liver. Eleven patients received virtual hepatectomies using Liversim; these virtual hepatectomies were performed both preoperatively and during actual surgery. The perioperative outcomes of the hepatectomies using Liversim were analyzed. There were no obvious discrepancies during the resection process between our simulation and the actual surgery. There were no significant differences in perioperative outcomes between the conventional 3D simulation software and the Liversim. Virtual hepatectomy with real-time liver deformation using Liversim is useful for the safe performance of hepatectomies.
INTRODUCTION
Recently, many institutes in Japan have constructed 3-dimensional (3D) images from the multidetector computed tomography (MDCT) datasets of patients who have undergone hepatectomies to share the visual information with the surgical staff and predict the liver resection volume for the hepatectomy[1-10]. The normal liver is non-rigid and is usually deformed during liver resection as a result of posture changes and the surgical procedure. We have used the SYNAPSE VINCENT medical imaging system (Fujifilm Medical, Tokyo) for the 3D visualization and virtual resection of the liver. However, in this system, the reconstructed 3D liver model is fixed and rigid. In addition, although we were able to observe the simulated cut surface of the liver, we were not able to observe every moment of cutting or the intrahepatic vessels. Therefore, we aimed to develop a novel surgical simulation software program to represent the real-time motion and deformation of the liver. We also investigated whether this software was useful for educating surgical residents and medical students.
MATERIALS AND METHODS
Development of liversim
We developed a novel real-time virtual hepatectomy simulation software program, Liversim, to present the real-time motion and deformation of the liver, the inferior vena cava (IVC), the intrahepatic vessels, tumors and the gallbladder. The main purpose of the software is to observe the location and orientation of the intrahepatic vessels moment by moment during surgery. To achieve this goal, the software provides 4 basic functions: viewing 3D models from arbitrary directions and distances, changing the colors and opacities of the models, deforming the models based on user interaction, and incising the liver parenchyma and intrahepatic vessels based on user operations. In addition to these basic functions, the Liversim software has the capacity to estimate and display the vascular territory of a selected hepatic vessel branch.
The Liversim software accepts a set of polygonal meshed surface models consisting of a liver, IVC, hepatic vein, portal vein, hepatic artery, tumor and gallbladder. The liver, IVC, tumor and gallbladder models are converted into tetrahedral meshed models and are considered in the calculation of physical simulations via the finite element method (FEM; Figure 1A). The FEM calculation is performed by the Sofa framework, which is the foundation of the Liversim software[11]. The liver model can be transformed by pulling up a specific point on the liver. The vessel surface models are placed inside the liver model, and their positions are altered according to the deformation of the liver model (Figure 1B). The reconstructed 3D liver models on Liversim were evaluated by two skilled surgeons in our group to confirm that the physical characteristics were similar to those of an actual liver.
Figure 1.
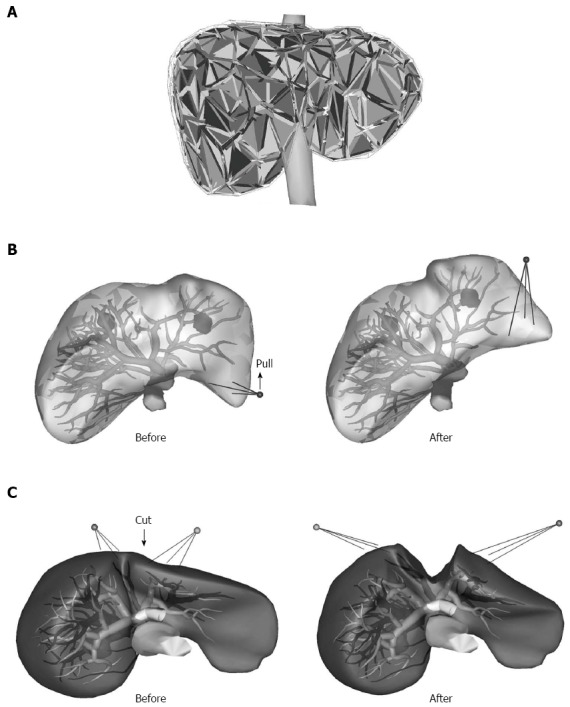
Three-dimensional tetrahedral meshed model of a liver. A: The liver, inferior vena cava, tumor and gallbladder models were converted into tetrahedral meshed models and considered in the calculation of the physical simulation via the finite element method; B, C: The vessel surface models were placed inside the liver model, and their positions changed according to the deformation of the liver model.
Two types of incision functions are implemented at the top of the Sofa framework. One is used for cutting the tetrahedral meshes used for the incisions of the liver parenchyma model[12], and the other, for cutting the polygonal meshes of the hepatic and portal veins. Each incision made during surgery virtually creates a rectangular cutting plane at a user-specified position and orientation within the 3D space of the liver model. The plane subdivides each crossing tetrahedral and triangular element in the liver and vein models into 4 to 9 tetrahedral and 5 triangular subelements according to the predefined subdivision patterns shown in Figure 2. When we place a line on a simulated resection line in the liver model, we can open up the incised part of the model by pulling the surrounding liver parenchyma outward and removing the exposed portions of the vessels (Figure 1C). This function enables us to represent the real-time motion and deformity of the 3D liver model. In this case, “real-time” means that we are able to observe this process 1-2 s after the incision is made. The capability to display an estimated vascular territory of specified segments of vessels is implemented to present the affected region in the liver after the vessels are cut. The vascular territory is represented by a set of cells adjacent to the selected vessel segments, as constructed from a Voronoi tessellation applied to the paths of the intrahepatic vessels (Figure 3).
Figure 2.
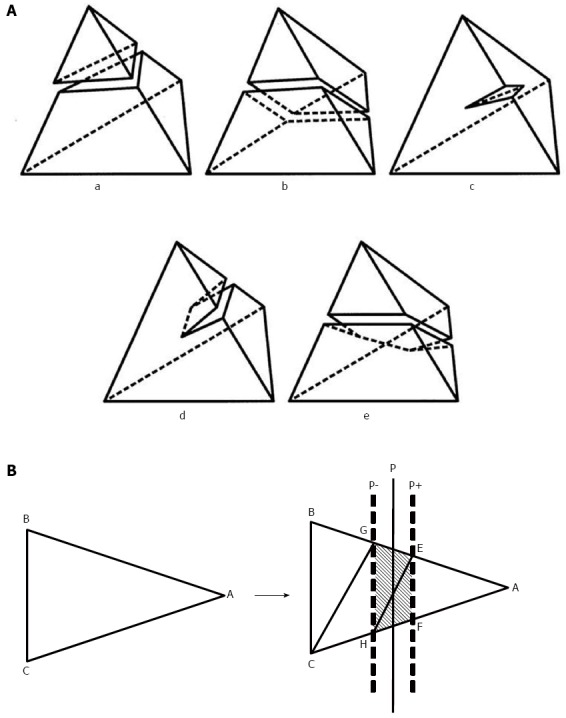
Incision function. A: The incision function of the liver parenchyma. Incision functions are implemented at the top of the Sofa framework. This function is used for cutting the tetrahedral meshes used in the incisions of the liver parenchyma. The patterns used for cutting the tetrahedral meshes were divided into five types; B: A typical example of triangle subdivision. The triangle ABC above is subdivided by two sheets of cut planes, P+ and P-, into five triangles: AEF, EFH, EHG, GHC, and GCB, as below. Planes P+ and P- are defined by the slightly translating plane P, which cuts the tetrahedrons. Each of the triangles plays a different role. The three triangles between P+ and P- are exposed on the outside of the liver during simulation, whereas the other two are placed inside. Liversim allows the user to delete the exposed triangles to simulate the process of removing the exposed vessels in the cut surface of the liver. Using these functions, a user can cut open the liver parenchyma and remove the exposed segments of the hepatic vessels.
Figure 3.
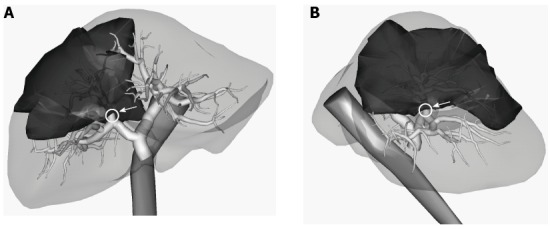
Territory of the anterior portal vein branch is described as deep color territory. The vascular territory is constructed from a Voronoi tessellation applied to the paths of the intrahepatic vessels. A: Three-dimensional image view from the front of the patient; B: Three-dimensional image view from the patient's right side.
Preoperative virtual hepatectomy (rehearsal)
The patients’ 2D CT images were transferred to the workstation (SYNAPSE VINCENT, Fujifilm Medical, Tokyo, Japan), and one of the authors (Y. O.) generated the 3D images. After the OBJ data of the 3D liver model were input into the Liversim software, we were able to perform a virtual hepatectomy with real-time liver deformity. The choice of resection was made on the basis of the tumor size, tumor location, liver function (ICGR15), and surgical margins based on the virtual hepatectomy.
Virtual hepatectomy simultaneously with an actual hepatectomy (navigation)
We also simultaneously performed virtual hepatectomies on a laptop computer using the Liversim software. The surgical team was able to observe the virtual hepatectomy on a large-screen display during the actual hepatectomy. During the actual hepatectomy, the operator using the Liversim software and the surgical team were able to communicate and to discuss the surgical process and the surgical liver anatomy, such as the appearance of the next vessel branches on the cut line of the liver. The Liversim software operator served as the navigator for the actual hepatectomy.
Virtual hepatectomy using liversim
Between April 2012 and November 2013, 11 patients received virtual hepatectomies preoperatively or during surgery using Liversim at the University of Tsukuba Hospital. The characteristics of the 11 patients who underwent virtual hepatectomies are detailed in Table 1. The 11 patients consisted of 9 men and 2 women with a median age of 60 years (range, 50 to 77 years). Of these patients, 5 had a preoperative diagnosis of hepatocellular carcinoma (HCC), 2 had intrahepatic cholangiocarcinoma (ICC), and 4 had metastatic liver tumor. The surgical procedure performed depended on the location of the primary tumor. Three patients underwent a right hepatectomy, two underwent extended left hepatectomies, and two underwent posterior sectionectomies. One patient underwent a left hepatectomy, one underwent an anterior sectionectomy, one underwent a lateral sectionectomy, and one underwent a S6 segmentectomy. The median operation time was 397 min (range, 232 to 497 min). The median intraoperative bleeding volume was 470 mL (range, 130 to 1600 mL), and the median postoperative stay was 13 d (range, 9 to 21 d).
Table 1.
Cases of the virtual hepatectomy using Liversim
| Cases | Age (yr) | Gender | Diagnosis | Surgery | Operation time (min) | Bleeding (mL) | Postoperative stay (d) |
| 1 | 68 | M | HCC | Lateral sectionectomy | 339 | 320 | 13 |
| 2 | 56 | M | Metastatic tumor(rectal cancer) | Extended left hepatectomy | 406 | 507 | 9 |
| 3 | 62 | M | Metastatic tumor(colon cancer) | Right hepatectomy | 374 | 1200 | 15 |
| 4 | 60 | M | Metastatic tumor(gastric cancer) | Posterior sectionectomy | 397 | 780 | 21 |
| 5 | 77 | M | HCC | Left hepatectomy | 290 | 370 | 13 |
| 6 | 60 | M | HCC | Anterior sectionectomy | 497 | 1600 | 15 |
| 7 | 69 | M | ICC | Segmentectomy (S6) | 232 | 235 | 9 |
| 8 | 50 | M | Metastatic tumor(colon cancer) | Right hepatectomy | 454 | 470 | 10 |
| 9 | 71 | F | ICC | Extended left hepatectomy | 436 | 1570 | 9 |
| 10 | 55 | M | HCC | Posterior sectionectomy | 342 | 130 | 19 |
| 11 | 56 | F | HCC | Right hepatectomy | 481 | 460 | 12 |
| Median | 60 | 397 | 470 | 13 |
HCC: Hepatocellular carcinoma; ICC: Intrahepatic cholangiocarcinoma; M: Male; F: Female.
Virtual hepatectomy using SYNAPSE VINCENT
From April 2010 through 2013, 99 patients underwent preoperative virtual hepatectomies using SYNAPSE VINCENT. The patients included 78 men and 21 women with a median age of 65 years (range, 21-84 years). Among these patients, 61 had a preoperative diagnosis of HCC, 14 had ICC, and 24 had metastatic liver tumors. Twenty-four patients underwent hemihepatectomy or other advanced procedures; 27 underwent a sectionectomy, and 48 patients underwent a segmentectomy or a more minor procedure (Table 2).
Table 2.
Characteristics of the patients n (%)
| Characteristics | VINCENT (n = 99) | Liversim (n = 11) |
| Age (yr) | ||
| Median (range) | 65 (21-84) | 60 (50-77) |
| Gender | ||
| Male | 78 (79) | 9 (82) |
| Female | 21 (21) | 2 (18) |
| Diagnosis | ||
| HCC | 61 (62) | 5 (45) |
| ICC | 14 (14) | 2 (18) |
| Metastasis | 24 (24) | 4 (36) |
| Procedure | ||
| Hemihepatectomy or larger | 24 (24) | 6 (55) |
| Sectionectomy | 27 (27) | 4 (36) |
| Segmentectomy or smaller | 48 (48) | 1 (9) |
HCC: Hepatocellular carcinoma; ICC: Intrahepatic cholangiocarcinoma.
Questionnaire-based survey: We conducted a simple survey using a self-administered questionnaire to determine the utility of the Liversim software. The questionnaire consisted of three questions: (1) Is presurgery rehearsal with Liversim before each hepatectomy useful for you? (response items: 1 to 10, with 10 indicating the greatest agreement); (2) Is the Liversim image seen during the rehearsal similar to the image of the actual hepatectomy? (response items: 1 to 10, with 10 indicating the greatest similarity, considering the following: the cut line of the liver; the portal vein; and the hepatic vein); and (3) Describe your opinion of, impression of, or suggestions for Liversim.
Participants and data collection: We identified attending medical students at the University of Tsukuba who were learning at bedside and gastroenterological surgical residents (3-7 years postgraduate) who worked at the Department of Surgery, Division of Gastroenterological and Hepatobiliary Surgery and Organ Transplantation at University of Tsukuba Hospital. The medical students and surgeons who met the above criteria (medical students: n = 24, surgical residents: n = 18) participated in the survey. All of the data were confidentially and anonymously stored. This study was approved by the ethics committee of the University of Tsukuba.
Data analysis
The data were analyzed in a descriptive format for both the quantitative and qualitative items. The mean responses and confidence intervals for each question item was assessed using 10-point Likert scales and calculated using statistical analysis software (SPSSII 11.0, SPSS, Inc., Chicago, IL, United States). We used the Mann-Whitney U test to compare scores and perioperative outcomes. P values less than 0.05 were considered statistically significant. Additional qualitative data provided via free-text responses were recorded after each question and analyzed using qualitative content analysis techniques to generate a descriptive summary of the participants’ responses.
RESULTS
Virtual hepatectomy used preoperatively and during surgery (navigation)
The Liversim software enabled us to demonstrate the real-time motions of the drawing, deformation, and resection of the liver preoperatively and during surgery. There was no obvious discrepancy during the resection process (e.g., in the emergence of branches of the portal vein or hepatic vein or in the depth and direction of the resection line) between our simulation and the actual surgery. Preoperative virtual hepatectomy using Liversim was able to predict the appearance of the vessels on the cut surface of the liver during the resection process. Virtual hepatectomies conducted simultaneously with surgery in the operation room were able to visualize the appearance of the vessels just before they were approached during the actual surgical procedure. A Liversim software operator was able to serve as a surgical navigator by reporting the appearance of the upcoming vessels on the resection line to the surgical staff. We were able to display the Liversim images on a large display in the operating room to allow surgical navigation. Accordingly, it was possible to share the anatomical images with the surgical staff during the actual hepatectomy.
Case 1
Case 1 (Table 1) shows a lateral sectionectomy (resection of segments 2 and 3) (Figure 4A). At first, we based our choice of resection on clinical information, such as the tumor size, tumor location, liver function, and surgical margin. After determining which surgical procedure to use, we set and pulled certain points from both sides of the established incision line and then cut onto the liver parenchyma. Then, the segment 3 portal vein (P3) appeared, and we cut the branch. Because we were able to change the transparency of the liver model, we could confirm the characteristics of the exposed branch by observing it from various points of view. With the next cut, the segment 2 portal vein (P2) appeared. We were able to identify these entities in the live patient’s actual liver in the operation room because they were similar to the Liversim images. Figure 4A shows the actual liver surface and the virtual liver surface. We were able to identify the segment 3 glissonean pedicle (G3) and the segment 2 glissonean pedicle (G2)
Figure 4.
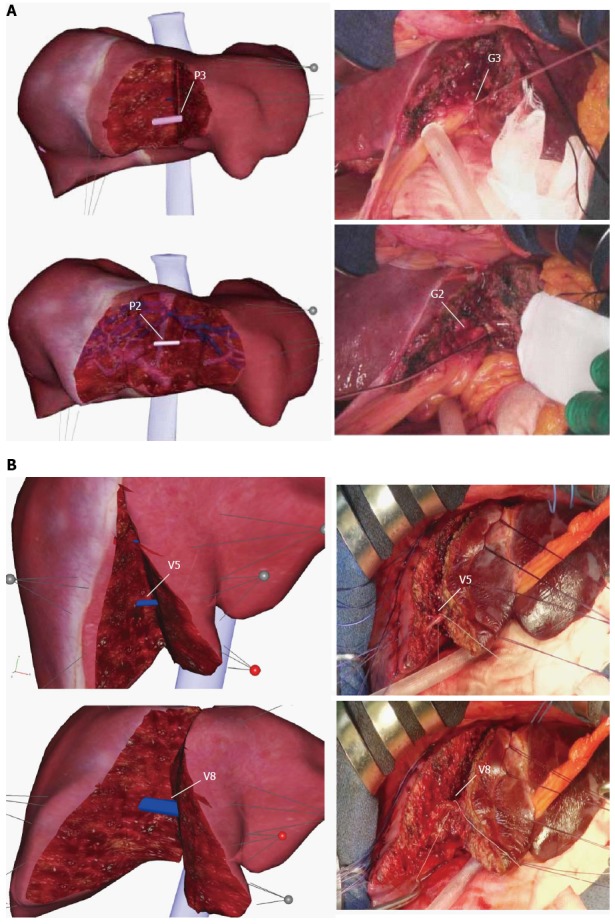
Pictures for comparisons between a virtual hepatectomy and an actual hepatectomy. The positional relationship of the exposed portal veins and the hepatic veins on the raw surface of the liver is consistent with the virtual hepatectomy using Liversim. A: Case 1, a lateral sectionectomy, is displayed. There was no obvious discrepancy (e.g., in the emergence of P3 (G3) and P2 (G2) or the depth and direction of the resection line) during the resection process between our simulation and the actual surgery; B: Case 2 underwent an extended left hepatectomy, as shown. A good resemblance can be observed between the positional relationship of the exposed V5 and V8 shown in the 3D simulation and observed during the actual hepatectomy. P3: The segment 3 portal branch; P2: The segment 2 portal branch; G3: The segment 3 glissonean pedicle; G2: The segment 2 glissonean pedicle; V5: The hepatic venous branches that drain segment 5; V8: The hepatic venous branches that drain segment 8.
Case 2
Case 2 (Table 1) shows an extended left hepatectomy. On the Liversim software, we first cut the established incision line and then were able to recognize the small hepatic venous branches that drain segment 5 (V5). As we proceeded with the cut, the large hepatic venous branches that drain segment 8 (V8) appeared. Figure 4B shows the actual cut liver surface and the virtual cut liver surface on Liversim. A good resemblance can be observed between the virtual liver surface on Liversim and the actual liver surface. We were able to predict the location of the V5, V8 and other hepatic venous branches inside the liver parenchyma.
Perioperative outcomes
The perioperative outcomes are summarized in Table 3. The median operating time was 304 min (range, 110 min to 846 min) in the VINCENT group and 397 min (range, 232 min to 497 min) in the Liversim group. The median intraoperative bleeding volume was 510 mL (range, 18 mL to 5120 mL) in the VINCENT group and 470 mL (range, 130 mL to 1600 mL) in the Liversim group. The median postoperative stay was 12 d (range, 6 d to 100 d) in the VINCENT group and 13 d (range, 9 d to 21 d) in the Liversim group. There were no significant differences in the operative time, intraoperative bleeding volume, or postoperative stay between the two groups.
Table 3.
Perioperative outcomes
| Perioperative outcomes (median) | VINCENT (n = 99) | Liversim (n = 11) | P value |
| Operation time (min) | 304 (110-846) | 397 (232-497) | 0.30 |
| Blood loss (mL) | 510 (18-5120) | 470 (130-1600) | 0.44 |
| Postoperative stay (d) | 12 (6-100) | 13 (9-21) | 0.36 |
Questionnaire
Usefulness of Liversim before surgery: The scores regarding the usefulness of Liversim were 9.33 ± 0.17 for the medical student group (n = 24) and 9.39 ± 0.26 for the surgical resident group (n = 18; Figure 5). All of the respondents thought that this software was valuable, and there were no significant differences between the two groups.
Figure 5.
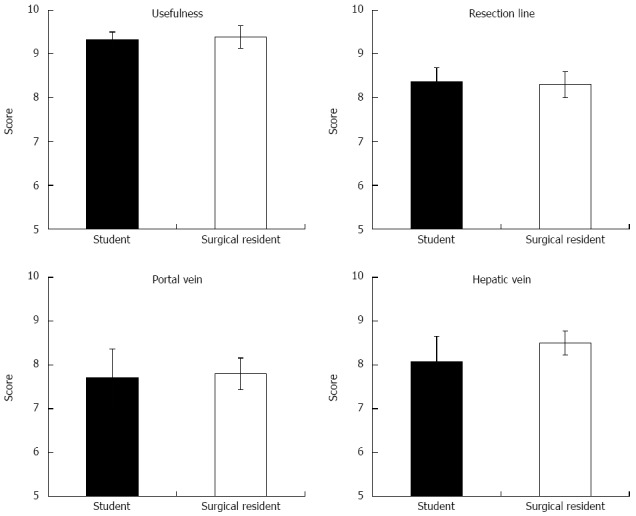
Utility of Liversim, a novel preoperative 3-dimensional liver surgery simulation software program, was assessed using a questionnaire. In particular, the conformity between the Liversim images and actual surgical observations was evaluated.
Assessment of the conformity between Liversim rehearsal images and actual surgical images: The scores for the conformity of the hepatic resection line were 8.36 ± 0.32 among the medical students (n = 14) and 8.3 ± 0.3 among the surgeons (n = 10). The scores for the conformity of the portal vein were 7.71 ± 0.65 among the students (n = 14) and 7.8 ± 0.36 among the surgical residents (n = 10). The scores for the conformity of the hepatic vein were 8.07 ± 0.58 among the students (n = 14) and 8.5 ± 0.27 among the surgeons (n = 10) (Figure 5). There were no significant differences between the two groups. Although the respondents perceived a discrepancy of approximately 20% between the Liversim image and the observations during the actual surgical procedure, they reported that the Liversim image was almost the same as an actual hepatectomy.
Free response questionnaire answers
Surprisingly, the questionnaire respondents stated that “the Liversim visual simulation image is more similar to an actual surgery than I could have imagined” in the open-ended free-text responses. They also reported having a greater interest in liver surgery than they previously had because the blood vessels were visualized at all times during the actual hepatectomy the way they were during the preoperative simulations using Liversim. In addition, the surgical residents and students said that Liversim made it easier to understand hepatectomies than previous surgical manuals or 3D simulation software did.
DISCUSSION
To perform safe and reliable liver surgery, it is important to grasp the positional relationship between the tumorous lesion and the blood vessels. Sharing the visual information and images of the liver anatomy among the entire surgical team is difficult because of the need to reconstruct a 3D CT image in each physician’s mind. Recently, several 3D simulation software programs have been developed to address the enormous quantity of imaging data acquired with advanced imaging modalities, such as MDCT[7,13-19]. At present, several software packages, such as OVA (Hitachi Medical Corporation, Japan), SYNAPSE VINCENT (FUJIFILM, Japan), Hepa Vision (Mevis, Germany), Ziostation (Ziosoft, Japan), VirtualPlace (AZE, Japan), and VR-Render (IRCAD, France), are available for computer-assisted liver surgery. In Japan, operative evaluations using image-processing software for liver surgery have been covered by universal healthcare insurance since 2012. Each patient is charged approximately $200 for this volumetric evaluation[20]. Until now, we have used the SYNAPSE VINCENT medical imaging system for the 3D visualization and virtual resection of the liver. We had considered that 3D reconstructed liver model clear, easily visible, and easy to understand.
Previously, several reports have indicated that newly developed 3D software could provide visual information, including information about the hepatic anatomy, and that furthermore, it could improve predictions of the resected volume for hepatectomy and liver transplantation[6,21-31]. However, several problems have emerged. In the previous 3D simulation software, the 3D reconstructed liver model was fixed and rigid, whereas an actual normal liver is non-rigid and can be deformed during liver resection. Moreover, we were unable to visualize the blood vessels inside the liver using these programs. Consequently, these 3D reconstructed liver models deviated far from the actual liver. To address these shortcomings, we developed a novel 3D virtual hepatectomy simulation software program that is able to visualize the real-time deformation of the liver.
As the result, there were no obvious discrepancies during the ongoing resection process, such as the emergence of portal vein branches covered in Glisson’s sheath or by the hepatic vein or in the depth and direction of the resection line, between our simulation and the actual surgery. Therefore, we were able to locate the intrahepatic vessels preoperatively. We hoped to prevent accidental injury and bleeding by visualizing the exposed blood vessels, including vascular anomalies, on the cutting surface of the liver preoperatively. The results of this analysis of the perioperative outcomes showed that virtual hepatectomy using Liversim had no significant benefit compared with virtual hepatectomy using the previous software, SYNAPSE VINCENT. In other words, Liversim was not found to be clinically inferior to SYNAPSE VINCENT. Still, Liversim is thought to be helpful for performing a safe hepatectomy and for educating surgical residents and medical students. Additionally, the primary advantage of Liversim was that the surgical team was able to use visual information about a patient’s liver anatomy to determine the appropriate surgical procedure. The surgical team was also able to discuss several critical points (i.e., the surgical margin and the relationship between the tumor and the hepatic vein and Glisson’s sheath) by observing the Liversim images during the actual hepatectomy. Needless to say, further retrospective and prospective studies should be conducted to confirm this utility. In the future, we plan to use Liversim on patients after obtaining their informed consent.
Liversim is useful as an educational tool and for performing virtual hepatectomies preoperatively and during surgery. Liversim was very popular with the surgical residents and medical students who used it, particularly because their young ages increased their familiarity with computers. There was a tendency towards higher scoring of the portal vein in Glisson’s sheath and of the hepatic vein among surgical residents than among students. The main reason for this issue was associated with the fact that the medical students had greater difficulty recognizing the anatomy of the liver because for many of them, it was the first time they had observed the actual liver of a patient undergoing surgery.
Several limitations of this novel modality should be addressed. First, at present, this approach requires a somewhat long time (approximately 1-2 h) to create detailed images. Second, certain small vessel branches may be missed by Liversim because not all of the vessels can be visualized with CT for each patient. Third, this study involved only 11 patients; therefore, our results will need to be confirmed with larger populations.
In conclusion, virtual hepatectomy with real-time deformation of the liver using Liversim preoperatively and during surgery is useful for performing a safe hepatectomy and for educating surgical residents and medical students.
ACKNOWLEDGMENTS
The authors thank Mr. Itoh M and Karasawa T of LEXI Co., Ltd., for their kind support.
COMMENTS
Background
The shape of the liver changes during liver resection due to the posture change and the surgical procedure. However, in conventional software, 3-dimensional (3D) liver models are fixed and rigid. In addition, the authors were not able to observe every moment of the cutting of the liver and intrahepatic vessels.
Research frontiers
New innovative softwares in virtual system will be created step by step in the future. No surgical simulation software to represent the real-time motion and deformation of the liver.
Innovations and breakthroughs
The main purpose of the novel software is to observe the location and orientation of the intrahepatic vessels moment by moment during surgery. To achieve this goal, the software provides 4 basic functions: viewing 3D models from arbitrary directions and distances, changing the colors and opacities of the models, deforming the models based on user interaction, and incising the liver parenchyma and intrahepatic vessels based on user operations.
Applications
Liversim is useful for educating surgical residents and medical students about performing a safe hepatectomy. Additionally, the primary advantage of Liversim is that the surgical team is able to use visual information that is identical to the patient’s liver anatomy to determine the appropriate surgical procedure.
Terminology
The finite element method (FEM) calculation is performed by the Sofa framework, which is the foundation of the Liversim software. The liver, inferior vena cava, tumor and gallbladder models are converted into tetrahedral meshed models and are considered in the calculation of physical simulations via the FEM.
Peer-review
Liversim system provides specialized functions for liver surgery planning. This function enables us to represent the real time motion and display an estimated vascular territory of segments.
Footnotes
Institutional review board statement: This study was approved by a research ethics committee at University of Tsukuba Hospital.
Informed consent statement: This study was conducted in accordance with the patients’ informed consent.
Conflict-of-interest statement: All authors indicated no potential conflicts of interest.
Data sharing statement: The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 13, 2015
First decision: April 13, 2015
Article in press: July 3, 2015
P- Reviewer: Boscá L, Hibi T, Wang CC, Wang DS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Sasaki R, Kondo T, Oda T, Murata S, Wakabayashi G, Ohkohchi N. Impact of three-dimensional analysis of multidetector row computed tomography cholangioportography in operative planning for hilar cholangiocarcinoma. Am J Surg. 2011;202:441–448. doi: 10.1016/j.amjsurg.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 2.Oshiro Y, Sasaki R, Nasu K, Ohkohchi N. A novel preoperative fusion analysis using three-dimensional MDCT combined with three-dimensional MRI for patients with hilar cholangiocarcinoma. Clin Imaging. 2013;37:772–774. doi: 10.1016/j.clinimag.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Sasaki R, Kondo T, Oda T, Murata S, Ohkohchi N. Preoperative 3D volumetric analysis for liver congestion applied in a patient with hilar cholangiocarcinoma. Langenbecks Arch Surg. 2010;395:761–765. doi: 10.1007/s00423-009-0572-y. [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297–1304. doi: 10.1002/hep.20684. [DOI] [PubMed] [Google Scholar]
- 5.Endo I, Shimada H, Sugita M, Fujii Y, Morioka D, Takeda K, Sugae S, Tanaka K, Togo S, Bourquain H, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007;142:666–675. doi: 10.1016/j.surg.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Ariizumi S, Takahashi Y, Kotera Y, Omori A, Yoneda G, Mu H, Katagiri S, Egawa H, Yamamoto M. Novel virtual hepatectomy is useful for evaluation of the portal territory for anatomical sectionectomy, segmentectomy, and hemihepatectomy. J Hepatobiliary Pancreat Sci. 2013;20:396–402. doi: 10.1007/s00534-012-0573-z. [DOI] [PubMed] [Google Scholar]
- 7.Takamoto T, Hashimoto T, Ogata S, Inoue K, Maruyama Y, Miyazaki A, Makuuchi M. Planning of anatomical liver segmentectomy and subsegmentectomy with 3-dimensional simulation software. Am J Surg. 2013;206:530–538. doi: 10.1016/j.amjsurg.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto J, Yamanaka J. Liver resection and transplantation using a novel 3D hepatectomy simulation system. Adv Med Sci. 2006;51:7–14. [PubMed] [Google Scholar]
- 9.Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249–1255. doi: 10.1007/s00268-007-9020-8. [DOI] [PubMed] [Google Scholar]
- 10.Endo K, Sata N, Ishiguro Y, Miki A, Sasanuma H, Sakuma Y, Shimizu A, Hyodo M, Alan L, Yasuda Y. A patient-specific surgical simulator using preoperative imaging data: an interactive simulator using a three-dimensional tactile mouse. J Comput Surg. 2014;3:10. [Google Scholar]
- 11.Allard J, Cotin S, Faure F, Bensoussan PJ, Poyer F, Duriez C, Delingette H, Grisoni L. SOFA--an open source framework for medical simulation. Stud Health Technol Inform. 2007;125:13–18. [PubMed] [Google Scholar]
- 12.Mor A. Progressive Cutting with Minimal New Element Creation of Soft Tissue Models for Interactive Surgical Simulation. Doctoral dissertation, Tech. Report CMU-RI-TR-01-29. Pittsburgh: Robotics Institute, Carnegie Mellon University; 2001. [Google Scholar]
- 13.Lamadé W, Glombitza G, Fischer L, Chiu P, Cárdenas CE, Thorn M, Meinzer HP, Grenacher L, Bauer H, Lehnert T, et al. The impact of 3-dimensional reconstructions on operation planning in liver surgery. Arch Surg. 2000;135:1256–1261. doi: 10.1001/archsurg.135.11.1256. [DOI] [PubMed] [Google Scholar]
- 14.Selle D, Preim B, Schenk A, Peitgen HO. Analysis of vasculature for liver surgical planning. IEEE Trans Med Imaging. 2002;21:1344–1357. doi: 10.1109/TMI.2002.801166. [DOI] [PubMed] [Google Scholar]
- 15.Hiroshige S, Shimada M, Harada N, Shiotani S, Ninomiya M, Minagawa R, Soejima Y, Suehiro T, Honda H, Hashizume M, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation. 2003;75:1561–1564. doi: 10.1097/01.TP.0000053755.08825.12. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto R, Oshiro Y, Hashimoto S, Kohno K, Fukunaga K, Oda T, Ohkohchi N. Three-dimensional imaging identified the accessory bile duct in a patient with cholangiocarcinoma. World J Gastroenterol. 2014;20:11451–11455. doi: 10.3748/wjg.v20.i32.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enkhbold Ch, Shimada M, Utsunomiya T, Ishibashi H, Yamada S, Kanamoto M, Arakawa Y, Ikemoto Z, Morine E, Imura S. One-stop shop for 3-dimensional anatomy of hepatic vasculature and bile duct with special reference to biliary image reconstruction. Hepatogastroenterology. 2013;60:1861–1864. doi: 10.5754/hge13536. [DOI] [PubMed] [Google Scholar]
- 18.Xiang C, Liu Z, Dong J, Sano K, Makuuchi M. Precise anatomical resection of the ventral part of Segment VIII. Int J Surg Case Rep. 2014;5:924–926. doi: 10.1016/j.ijscr.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shindoh J, Mise Y, Satou S, Sugawara Y, Kokudo N. The intersegmental plane of the liver is not always flat--tricks for anatomical liver resection. Ann Surg. 2010;251:917–922. doi: 10.1097/SLA.0b013e3181d773ae. [DOI] [PubMed] [Google Scholar]
- 20.Mise Y, Tani K, Aoki T, Sakamoto Y, Hasegawa K, Sugawara Y, Kokudo N. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci. 2013;20:157–164. doi: 10.1007/s00534-012-0574-y. [DOI] [PubMed] [Google Scholar]
- 21.Rau HG, Schauer R, Helmberger T, Holzknecht N, von Rückmann B, Meyer L, Buttler E, Kessler M, Zahlmann G, Schuhmann D, et al. Impact of virtual reality imaging on hepatic liver tumor resection: calculation of risk. Langenbecks Arch Surg. 2000;385:162–170. doi: 10.1007/s004230050260. [DOI] [PubMed] [Google Scholar]
- 22.Wigmore SJ, Redhead DN, Yan XJ, Casey J, Madhavan K, Dejong CH, Currie EJ, Garden OJ. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221–226. doi: 10.1097/00000658-200102000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang H, Radtke A, Hindennach M, Schroeder T, Frühauf NR, Malagó M, Bourquain H, Peitgen HO, Oldhafer KJ, Broelsch CE. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005;140:629–638; discussion 638. doi: 10.1001/archsurg.140.7.629. [DOI] [PubMed] [Google Scholar]
- 24.Yamanaka J, Saito S, Iimuro Y, Hirano T, Okada T, Kuroda N, Sugimoto T, Fujimoto J. The impact of 3-D virtual hepatectomy simulation in living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:363–369. doi: 10.1007/s00534-005-1075-z. [DOI] [PubMed] [Google Scholar]
- 25.Radtke A, Sotiropoulos GC, Molmenti EP, Schroeder T, Peitgen HO, Frilling A, Broering DC, Broelsch CE, Malago’ M. Computer-assisted surgery planning for complex liver resections: when is it helpful? A single-center experience over an 8-year period. Ann Surg. 2010;252:876–883. doi: 10.1097/SLA.0b013e3181fdd012. [DOI] [PubMed] [Google Scholar]
- 26.Karlo C, Reiner CS, Stolzmann P, Breitenstein S, Marincek B, Weishaupt D, Frauenfelder T. CT- and MRI-based volumetry of resected liver specimen: comparison to intraoperative volume and weight measurements and calculation of conversion factors. Eur J Radiol. 2010;75:e107–e111. doi: 10.1016/j.ejrad.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 27.van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG, Dejong CH. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010;34:2426–2433. doi: 10.1007/s00268-010-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dello SA, Stoot JH, van Stiphout RS, Bloemen JG, Wigmore SJ, Dejong CH, van Dam RM. Prospective volumetric assessment of the liver on a personal computer by nonradiologists prior to partial hepatectomy. World J Surg. 2011;35:386–392. doi: 10.1007/s00268-010-0877-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto D, Dohi T, Tsuzuki M, Horiuchi T, Ohta Y, Chinzei K, Suzuki M, Idezuki Y. Development of a computer-aided surgery system: three-dimensional graphic reconstruction for treatment of liver cancer. Surgery. 1991;109:589–596. [PubMed] [Google Scholar]
- 30.van Leeuwen MS, Noordzij J, Fernandez MA, Hennipman A, Feldberg MA, Dillon EH. Portal venous and segmental anatomy of the right hemiliver: observations based on three-dimensional spiral CT renderings. AJR Am J Roentgenol. 1994;163:1395–1404. doi: 10.2214/ajr.163.6.7992736. [DOI] [PubMed] [Google Scholar]
- 31.Marescaux J, Clément JM, Tassetti V, Koehl C, Cotin S, Russier Y, Mutter D, Delingette H, Ayache N. Virtual reality applied to hepatic surgery simulation: the next revolution. Ann Surg. 1998;228:627–634. doi: 10.1097/00000658-199811000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]


