Abstract
Purpose
We investigated the effects of GW559090, a novel, competitive, and high-affinity α4 integrin antagonist, in a murine model of dry eye. Through interaction with vascular cell adhesion molecule 1 (VCAM-1) and fibronectin α4β1 integrin is involved in leukocyte trafficking and activation.
Methods
Female C57BL/6 mice, aged 6 to 8 weeks, were subjected to desiccating stress (DS). Bilateral topical twice daily treatment with GW559090 was compared to vehicle-treated controls. Treatment was initiated at the time of DS induction. Treatment effects were assessed on corneal staining with Oregon Green Dextran (OGD) and expression of inflammatory markers in ocular surface tissues by real time PCR. Dendritic cell activation was measured in draining cervical lymph nodes (CLN) by flow cytometry. Separate groups of mice received GW559090 subcutaneously to evaluate the effects of systemic administration on corneal staining and cells in CLN.
Results
Topical GW559090 significantly reduced corneal uptake of OGD compared to vehicle-treated disease controls in a dose-dependent manner (1, 3, 10, and 30 mg/mL) with 30 mg/mL showing the greatest reduction in OGD staining. When administered topically, corneal expression of IL-1α, matrix metalloproteinase (MMP)–9, chemokine ligand 9 (CXCL9), and TGF-β1 was reduced in GW559090-treated eyes. Topical treatment with GW559090 decreased dendritic cell activation in lymph nodes. The effects on corneal staining and cellular composition in CLN were not reproduced by systemic administration of GW559090, suggestive of a local role for integrin antagonism in the treatment of dry eye.
Conclusion.
The novel α4 integrin antagonist, GW559090, improved outcome measures of corneal staining and ocular surface inflammation in this murine model of dry eye. These results indicate the potential of this novel agent for the treatment of dry eye disease.
Keywords: experimental dry eye; α4β1 integrin antagonist, inflammation
Dry eye disease (DED) is one of the most common and discomforting eye disorders. It has been defined as a multifactorial ocular surface disease more prevalent in women and the elderly. Dry eye disease is associated with symptoms of discomfort, visual disturbance, tear film instability, and inflammation of the ocular surface leading to potential damage to the ocular surface tissues.1 The proinflammatory milieu is characterized by increased levels of cytokines and chemokines in the tear film, cornea, and conjunctiva, and increased autoreactive T-cell infiltration of the conjunctival epithelium and sometimes lacrimal gland2–4; reviewed by Stern et al.5,6 Alteration of the tear film composition (mucins, lipids, proteins) and decreased volume lead to tear film abnormalities that contribute to the disease cycle.
Subjecting mice to a controlled environment of desiccating stress results in ocular surface pathology reminiscent of human DED in patients in many respects.3,7–9 As of today, this model represents the best characterized animal model to study DED.
Integrins are heterodimeric glycoproteins consisting of one α- and one β-subunit. Expressed on the cell surface of leukocytes, integrins have a role in their recruitment to sites of inflammation. The association of a specific α- and β-subunit determines the ligand specificity of the integrin. The α4 integrin subunit (CD49d) is a constituent of Very Late Antigen-4 (VLA-4, integrin a4b1, CD49d/CD29), and α4β7 (CD49d/CD103). In the case of integrin α4β1 the corresponding ligands are the immunoglobulin superfamily adhesion molecule vascular cell adhesion molecule 1 (VCAM-1) on vascular endothelial cells and the extracellular matrix glycoprotein fibronectin, which are responsible for the homing, trafficking, differentiation, priming, activation, and survival of integrin α4β1 expressing cells. Integrin α4β1 is expressed on lymphocytes, monocytes, macrophages, NK cells, and eosinophils. Integrin α4β7 and its corresponding ligand Mucosal Addressin Cell Adhesion Molecule-1 (MAdCAM) selectively regulate leukocyte trafficking to the gut and consequently are unlikely to be involved in the effects described herein.
Natalizumab, an antibody directed against the α4 integrin subunit, has been shown to profoundly inhibit inflammation and improve clinical outcomes in multiple sclerosis10 and Crohn's disease,11 which also are T-cell–mediated diseases. Lifitegrast, a small molecule antagonist, directed against a different adhesion molecule (LFA-1, integrin αLβ2), has been shown to reduce corneal staining and improve symptoms when delivered topically to dry eye patients.12 Furthermore, a specific antagonist to integrin α4β1, BIO-8809, had been shown to decrease corneal fluorescein staining, conjunctival T-cell infiltrates, and TNFα expression in cornea and conjunctiva in a murine dry eye model.13 Taken together these considerations provided a rationale for further exploring the blockade of integrin α4 in an animal model of DED. In the current study, we tested the hypothesis using GW559090, a potent integrin α4 antagonist that previously had been clinically investigated in asthma patients by the oral inhalation route.14
Materials and Methods
Compounds
Compound GW559090 ([S])-3-[4-([4-carbamoylpiperidine-1-carbonyl]oxy)phenyl]-2-[(S)-4-methyl-2-(2-[otolyloxy]acetamido)pentanamido]propanoic acid) was synthesized by GSK. Compound [3H]-GW559090 was custom synthesized at Amersham Pharmacia Biotech, Inc. (Piscataway, NJ, USA) to contain five 3H atoms per molecule giving a specific activity of 76 Ci/mmol.
Animals
All animal studies were conducted according to the GSK Policy on the Care, Welfare, and Treatment of Laboratory Animals after review by the GSK and BCM Institutional Animal Care and Use Committees, and conformed to the standards in the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. We purchased C57BL/6 mice, 6 to 8 weeks old, from Jackson Labs (Bar Harbor, ME, USA).
Binding and Cell Adhesion
Jurkat J6 cells (human lymphoblast cell line) were grown as a suspension culture in RPMI 1640 supplemented with fetal calf serum (FCS, 10%) and glutamine (2 mM). RPMI 8866 cells (human B lymphoid cell line) were grown as a suspension culture in RPMI 1640 supplemented with FCS (10%) and glutamine (2 mM). Rat basophilic cell line RBL-2H3 cells were cultured in Eagle's minimal essential medium (MEM) plus Earles salts supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 1 × nonessential amino acids, 1 mM sodium pyruvate 10% heat-inactivated fetal calf serum.
J6 Saturation Assay (Filtration Assay)
Binding of GW559090 was characterized in human J6 cells, which express VLA-4. J6 cells were harvested by centrifugation for 5 minutes at 500g and resuspended in assay buffer (50 mM HEPES, pH 7.5, 100 mM NaCl, 2 mM glucose, 1 mM MnCl2). Each well contained 1 × 106 cells and either 10 μM nontritiated GW559090, to define the NSB (nonspecific binding), or buffer. Then, [3H]-GW559090 (0.02–50 nM) was added in a final volume of 500 μL and incubated for 2 hours at 37°C. Bound [3H]-GW559090 was separated from free by rapid vacuum filtration through presoaked Whatman GF/B filters, followed by three washes in ice-cold buffer, scintillant then was added to filter discs, and disintegrations per minute measured on a Beckman scintillation counter. The actual amount of [3H]-GW559090 added for each concentration of the saturation curve was measured by counting disintegrations from a 50 μL aliquot of the label dilution range.
Saturation Binding Assay in RBL-2H3 Cells (SPA Assay)
The assay buffer contained 50 mM HEPES, 100 mM NaCl, 1 mM MnCl2, pH 7.5 (with NaOH). We used WGA SPA beads at 1 mg/well. Cells were harvested and resuspended in assay buffer, and 1 million cells were added per well in a white bottomed plate. Nontritiated GW559090 to give a final assay concentration of 20 μM (to define nonspecific binding) or buffer alone was added, and then [3H]-GW559090 across a concentration range across the plate was added (nominal [3H]-GW559090 concentration range was 0.01 to 200 nM). The final assay volume was 250 μL. The plate then was incubated at 37°C for 2 hours. Disintegrations were counted by scintillation (from the WGA SPA beads) in a Wallac Microbeta plate reader.
The actual amount of [3H]-GW559090 added for each concentration of the saturation curve was measured by counting disintegrations per minute from a 50-μL aliquot of the label dilution range.
VCAM Cell Adhesion Assay
Polystyrene 96-well microtiter plates were coated with IgG at a concentration of 0.05 mg/mL in bicarbonate buffer for 2 hours at 37°C. The solution was aspirated and the plates washed twice with PBS. The plates then were incubated overnight at 4°C with a 1:4000 dilution of zzVCAM-1 in 3% BSA in PBS; “zz” refers to a protein tag (Protein A) that aids in binding of the tagged adhesion molecule to IgG on the culture plate. Before use the zzVCAM-1 was aspirated and the plates washed twice with PBS.
The J6 or RPMI cells (as required) were labeled with the fluorescent dye BCECF-AM (10 μM and 6 × 106 cells/mL) for 10 minutes at 37°C before the excess was removed by centrifugation at 500g for 5 minutes and the cells resuspended at a cell concentration of 1.2 × 107 cells/mL in Hank's balanced salt solution (HBSS). Equal volumes HBSS containing GW559090 (over concentration range 38.1 pM to 10 μM) and cells were added to the VCAM-1 coated plates. After a 30-minute incubation at 37°C, non- or loosely adhering cells were removed by inverting the plate and blotting on tissue paper. Two washes with PBS and blotting were followed by addition of Triton X-100 (2% vol/vol). The plates were counted in a Wallac Viktor. Compounds that inhibited adhesion resulted in a lower fluorescence reading.
MAdCAM Cell Adhesion Assay
Polystyrene 96-well microtiter plates were coated with IgG at a concentration of 0.05 mg/mL in bicarbonate buffer for 2.5 hours at 37°C. The solution was aspirated and the plates washed twice with PBS. The plates then were incubated overnight at 4°C with zzMAdCAM at a concentration of 204.0 ng/mL in 3% BSA in PBS. Before use the zzMAdCAM was aspirated and the plates washed twice with PBS. Then, J6 or RPMI cells (as required) were labeled with the fluorescent dye BCECF-AM (10 μM and 6 × 106 cells/mL) for 10 minutes at 37°C before the excess was removed by centrifugation at 500g for 5 minutes and the cells resuspended at a cell concentration of 1.2 × 107 cells/mL in HBSS. Equal volumes of HBSS containing GW559090 (over concentration range 38.1 pM to 10 μM) and cells were added to the MAdCAM-coated plates. Adhesion took place over a 30-minute incubation at 37°C. Non- or loosely adhering cells were removed by inverting the plate and blotting on tissue paper. Two washes with PBS and blotting were followed by addition of Triton X-100 (2% vol/vol). The plates were counted in a Wallac Viktor.
CS-1 Cell Adhesion Assay
Polystyrene 96-well microtiter plates were coated with Connecting-Segment 1 (CS-1), binding domain on fibronectin for integrin α4β1,15 at a concentration of 0.01 mg/mL in bicarbonate buffer overnight at 4°C. The solution was aspirated and the plates washed twice with PBS. The plates then were incubated at room temperature in the presence of 3% BSA for 60 minutes, inverted, and tapped to expel the BSA and washed twice in bicarbonate buffer. We labeled J6 or RPMI cells (as required) with the fluorescent dye BCECF-AM (10 μM and 6 × 106 cells/mL) for 10 minutes at 37°C before the excess was removed by centrifugation at 500g for 5 minutes and the cells were resuspended at a cell concentration of 1.2 × 107 cells/mL in HBSS. Equal volumes of HBSS containing GW559090 (over concentration range 19.0 pM to 5 μM) and cells were added to the CS-1 coated plates. Adhesion took place over a 30-minute incubation at 37°C. Non or loosely adhering cells were removed by inverting the plate and blotting on tissue paper. Two washes with PBS and blotting were followed by addition of Triton X-100 (2% vol/vol). The plates were counted in a Wallac Viktor.
Induction of Desiccating Stress, Treatment Regimen
Female C57BL/6 mice, aged 6 to 8 weeks, were subjected to desiccating stress (DS) for 5 days (DS5) as described previously.8,9 Topical bilateral treatment with 1 eye drop per eye (2 μL volume) or subcutaneous (SC) injection (4 μL volume), 2 times per day, was initiated on day 1 concurrently with DS and continued through day 4. Treatment with GW559090 or dexamethasone phosphate 0.1% was compared to correspondingly treated vehicle controls. Unless otherwise noted, vehicle formulations were PBS (pH 7.0; osmolality approximately 300 mMol/kg) for GW559090 and Alcon BSS Sterile Irrigating Solution (BSS) for dexamethasone. Mice were randomized to receive one of the test articles. Control mice were kept in a nonstressed (NS) environment maintained at 50% to 75% relative humidity without exposure to airflow or scopolamine, and were not treated with test or control article. Treatment effects were assessed on corneal staining with Oregon Green Dextran (OGD); expression of inflammatory markers in ocular surface tissues by real time PCR; cell population analysis in draining cervical lymph nodes by fluorescence-activated cell sorting (FACS) analysis.
Corneal OGD Staining
On the morning of the fifth day, mice received one SC dose of scopolamine. Two hours later corneal staining was assessed using Oregon Green Dextran (OGD-488), which is a conjugated fluorescent dye of a 70 kDa molecular size (Invitrogen-Molecular Probes, Eugene, OR, USA) as described previously.8 The procedure consisted of instillation of 0.5 μL of OGD on the cornea using a glass capillary pipette, 1 minute before euthanasia. Mice were euthanized by inhalation of isoflurane gas followed by cervical dislocation. Eyes then were rinsed with 2 mL of BSS. Excess liquid was blotted carefully from the ocular surface with filter papers without touching the cornea. Digital pictures of both eyes were taken under 470 nm excitation and 488 nm emission wave lengths using a Nikon (Tokyo, Japan) SMZ-1500 stereo microscope, with an exposure time of 2 seconds. Both eyes from each animal were evaluated; the right eye always first followed by the left eye. The mean intensity in the central cornea was evaluated from digital images using NIS Elements (version 3.0) by placing a fixed region of interest (a 2-mm diameter circle) on the central cornea. The mean intensity of the fluorescence was read by the software and stored in a database (Excel; Microsoft, Redmond, WA, USA). This fluorescence measurement in the central ring was done independently by 2 masked observers, for each mouse eye. By the conclusion of the experiment, results were averaged from both observers using all data collected during all study weeks (Excel; Microsoft). Results are presented as geometric mean and upper and lower 95% confidence limit (CL) of gray levels.
RNA Isolation and Reverse Transcription
Total RNA was isolated from corneal and conjunctival epithelia that was collected and pooled from 2 eyes (right and left) at each time point from untreated control mice, DS5 mice topically treated with GW559090 (30 mg/mL), and vehicle treated animals (n = 7/group) using a PicoPure RNA Isolation Kit (Acturus Bioscience, Inc., Mountain View, CA, USA) following the manufacturer's protocol. Briefly, corneal epithelial cells were scraped, and whole conjunctiva was cut and placed in 100 μL of extraction buffer and incubated at 42°C for 30 minutes. The cell extract was loaded onto a preconditioned purified column, which was centrifuged, treated with DNase (Qiagen, Valencia, CA, USA), and washed twice with two different wash buffers. The RNA was eluted in 12 μL of low ionic strength buffer. The RNA concentration was measured by absorption at 260 nm using a spectrophotometer (NanoDrop 2000; Thermo Scientific, Wilmington, DE, USA) and samples were stored at −80°C until use. First-strand cDNA was synthesized from 1 μg of total RNA with random hexamers using M-MuLV reverse transcriptase (Ready-To-Go You-Prime First-Strand Beads; Amersham Pharmacia Biotech, Inc.) as described previously.16
Absolute Real Time PCR
A cDNA aliquot (1–4 μL) from samples was used for real time PCR in a total volume of 10 μL containing the following per reaction: 0.3 μL of gene specific Taqman probes used and 5 μL of 2X Taqman Fast PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). Real time PCR was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems) and the parameters consisted of predenaturation at 95°C for 22 seconds, followed by 40 cycles of denaturation at 95°C for 1 second, annealing, and extension at 60°C for 20 seconds. Samples and standards were assayed in duplicate. A nontemplate control and total RNA without retrotranscription were included in all the experiments to evaluate PCR and DNA contamination of the reagents used. The following mouse Taqman probes were used: IL-1α (Mm00439620); matrix metalloprotein–9 (MMP-9) (Mm00442991); chemokine ligand 9 (CXCL9; Mm004434946); TGF-β1 (Mm00441724), and hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT-1; Mm00446968). The HPRT-1 gene was used as an endogenous reference for each reaction. The results of quantitative PCR were analyzed by the comparative CT method where target change = 2–ΔΔ CT. The results were normalized by the CT value of HPRT-1 of the untreated control group.8
Dissection of Draining Lymph Nodes
Cervical draining lymph nodes (CLN) were surgically excised, placed in a round culture plate with approximately 8 mL of complete RPMI media, and crushed between two frosted slides.
Flow Cytometry
Single-cell suspensions of CLN from C57BL/6 mice, treated under DS conditions for 1 day, were prepared, centrifuged, and sequentially filtered, as described previously.3 Subsequently, 1.0 mL of ACT solution was added for 30 seconds followed by 2.0 mL of complete RPMI. The cells were centrifuged once again and the supernatant aspirated. Cells were then resuspended in 2.0 mL of complete RPMI and prepared for counting. Ten μL of Trypan Blue were used for counting and the cells were divided in tubes at 1 × 106 cells/mL. Subsequently, the cells were incubated on ice in 20 μL unconjugated anti-mouse CD16/CD32 (BD Pharmingen, San Diego, CA, USA) followed by 80 μL of directly conjugated primary antibody or isotype. Finally the samples were washed with 1 mL PBS/1% FBS, centrifuged, and resuspended in 0.3 mL PBS/1% FBS containing 1:1000 of propidium iodide (PI). The samples were stored at 4°C until the analysis was performed with an A BD LSRII Benchtop cytometer. The data was analyzed using BD Diva Software (BD Pharmingen) and FlowJo (TreeStar, Inc., Ashland, OR, USA).
Statistical Analysis
Due to skewed distribution of OGD data, the analysis was done on the log10 scale to make the data more normally distributed. Log10 OGD data were averaged across two observers and the left and right eyes. The mixed effects ANOVA model included treatment group as a fixed effect and week as a random effect. The 95% and 99% CLs were constructed for the disease (5-day desiccating stress versus untreated group kept in separate vivarium) and compound treatment effects (treatment versus vehicle). As these comparisons were all preplanned (comparing each treatment to its vehicle group), no adjustment was made for multiplicity. Log10 scale treatment effect estimates and CLs were converted back to the original OGD scale; thus, representing the estimate for the ratio of group geometric means and their CLs. For gene expression analysis, an unpaired t-test was performed to compare drug and vehicle treatment. For flow cytometry analysis, a 2-way ANOVA with fixed treatment and random experiment effects was used to test the treatment differences. The analysis was followed by Dunnett's multiple comparison procedure to compare each of the treatment groups with the vehicle group.
Results
Pharmacology of GW559090
Binding of [3H]-GW559090 to human J6 cells was saturable and was described in these experiments by a single binding site with a mean Kd of 0.19 nM (0.08–0.43; geometric mean and 95% CL) derived from 4 separate experiments. A single high affinity binding site for [3H]-GW559090 also was shown in rat RBL-2H3 cells, which express rat α4β1, mean Kd 1.04 nM (0.58–1.89, Table 1).
Table 1.
Binding of [3H]-GW559090 to α4 Integrins and Inhibition of Cell Adhesion
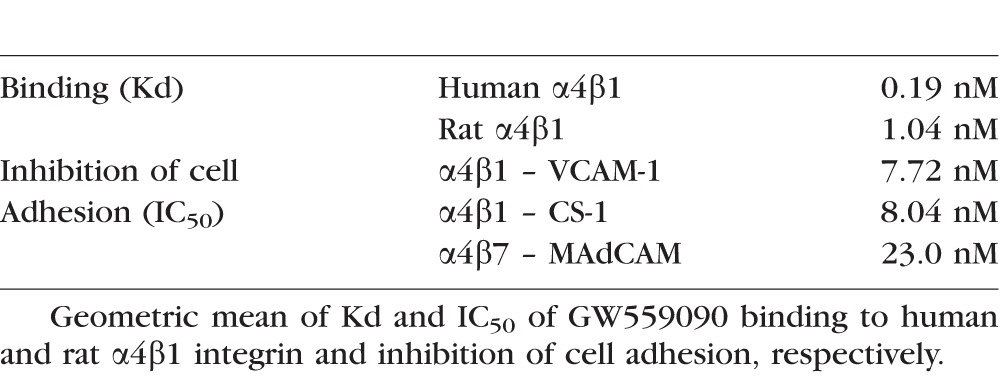
Inhibition of cell adhesion was determined for α4β1 (Jurkat J6 cells) to VCAM-1 and CS-1 (fibronectin domain); for α4β7 (RPMI 8866 cells) to MAdCAM in coated microtiter plates. GW559090 inhibited J6 cell adhesion to VCAM-1 in a monophasic fashion with a mean IC50 of 7.72 nM (2.39 −24.9). Also, GW559090 inhibited J6 cell adhesion to CS-1 with a mean IC50 of 8.04 nM (3.05-21.2) and to MAdCAM in a biphasic manner, supporting the presence of a high and low affinity site for MAdCAM – GW559090 binding in J6 cells (Table 1). The RPMI 8866 MAdCAM binding predominantly measures α4β7-mediated cell adhesion. Also, GW559090 inhibited RPMI 8866 cell adhesion to MAdCAM with an IC50 of 23.0 nM (20.0–26.4, Table 1), and GW559090 inhibited RPMI 8866 binding to VCAM-1, and CS-1 in a simple monophasic manner with respective IC50s of 4.81 nM (2.82–8.20) and 24.5 nM (identical duplicate values).
Additionally, GW559090 had been reported as highly selective versus non-α4 integrins, including LFA-1.14 No significant inhibition was observed by GW559090 (at 10 μM) in radioligand binding assays on 53 receptors and 4 transporters in an MDS Pharma screen (report on file at GSK).
Topical Treatment With GW559090 Prevents Desiccation-Induced Corneal Barrier Disruption
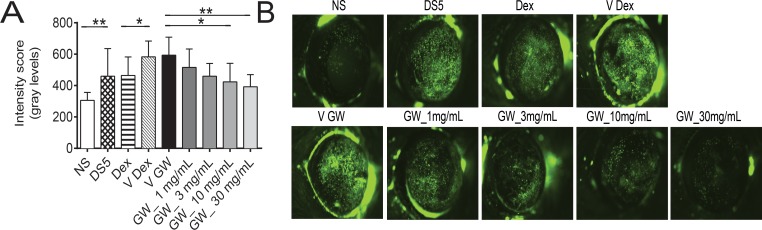
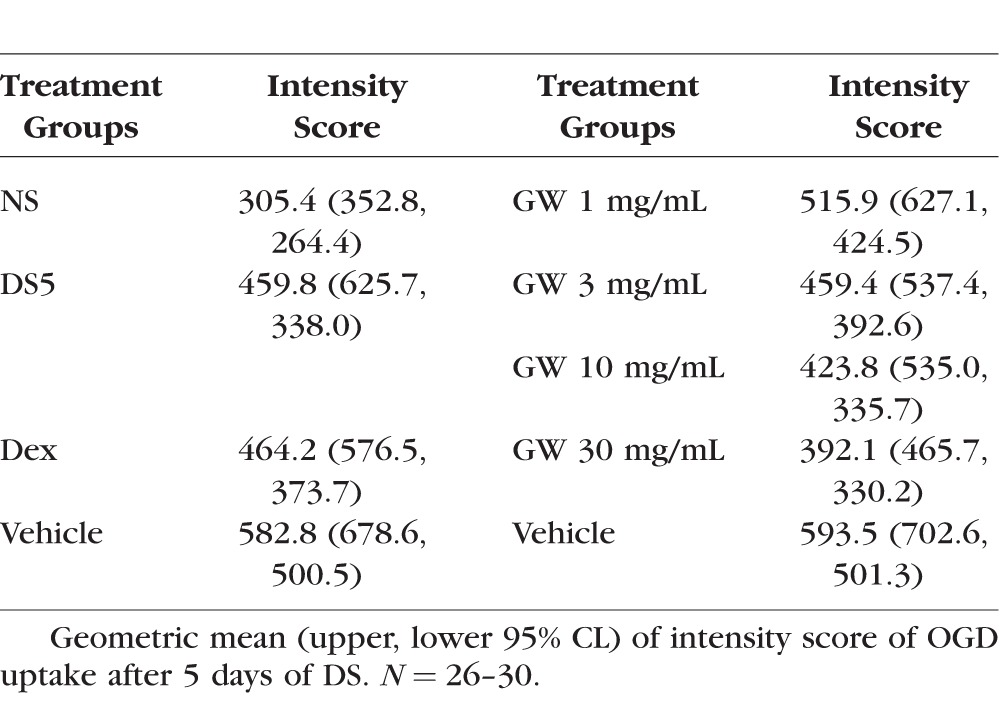
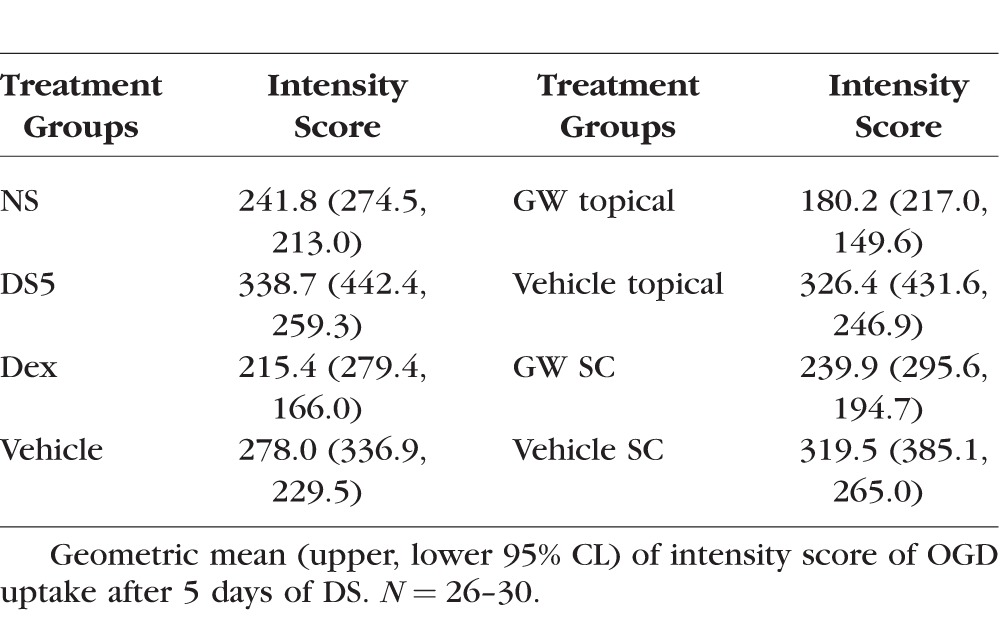
We found a significant increase in corneal permeability measured by OGD staining between the untreated NS and dry eye control groups (DS5, Fig. 1 and Table 2). Because corticosteroid therapy has been reported to improve corneal epithelial disease of dry eye in humans and mice,8,16,17 we used topical treatment with dexamethasone (Dex) as positive control. Treatment with 0.1% Dex significantly improved OGD intensity scores compared to vehicle. A range of doses of GW559090, as low as 1 mg/mL and as high as 30 mg/mL, was investigated. A significant decrease in OGD intensity scores was noted with increasing concentrations of GW559090 versus its vehicle. The most efficacious concentration was 30 mg/mL (Fig. 1, Table 2) which, therefore, was used in subsequent studies. Figure 1B shows representative examples of OGD-stained mouse corneas at the conclusion of the study period.
Figure 1.
Topical treatment with GW559090 dose-dependently prevents DS-induced corneal barrier disruption. (A) Geometric mean and upper 95% CL of intensity score of OGD uptake after 5 days of DS. (B) Representative images of OGD stained mouse corneas. NS indicates nonstressed, untreated control. DS5 indicates DS for 5 days without topical treatment; all other groups are DS5 with topical treatment. N = 26–30. *P < 0.05; **P < 0.01 compared to control (DS5 versus NS; Dex versus Dex vehicle; GW versus GW vehicle; mixed effects ANOVA of Log10 OGD data). V Dex, vehicle for dexamethasone (BSS); V GW, vehicle for GW559090; GW_1 mg/mL, GW559090 (1 mg/mL); GW_3 mg/mL, GW559090 (3 mg/mL); GW_10 mg/mL, GW559090 (10 mg/mL); GW_30 mg/mL, GW559090 (30 mg/mL).
Table 2.
Corneal OGD Staining, Dose-Response Study
GW559090 Acts Topically on the Ocular Surface
To explore the effect of systemic GW559090 on corneal staining, two routes of administration, topical and SC, were compared. An identical dose was given SC (120 μg as one 4 μL bolus) and topically (60 μg as a 2-μL drop to each eye). Mice receiving GW559090 systemically also were given vehicle eye drops as SHAM topical treatment. As shown in Figure 2 and Table 3, and similar to Figure 1, Dex decreased OGD uptake. Topical treatment with GW559090 again significantly decreased DS-induced corneal barrier disruption compared to its vehicle. This effect was neither as pronounced nor significant when GW559090 was administered systemically.
Figure 2.
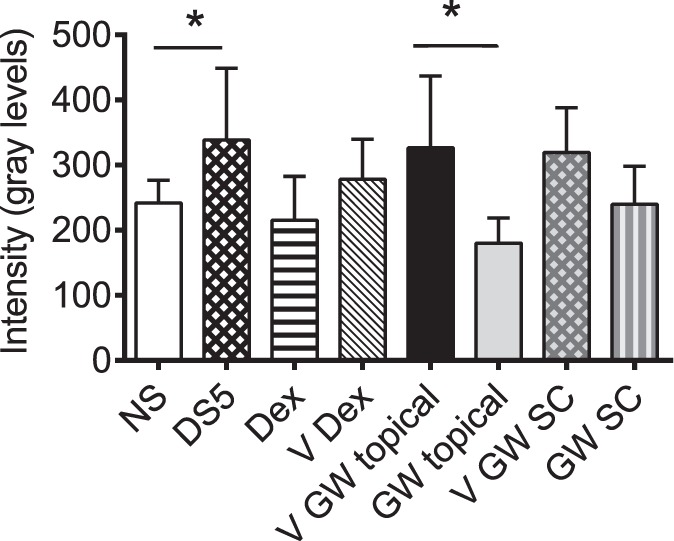
GW559090 acts primarily locally, not systemically, to improve DS-induced corneal barrier disruption. Geometric mean and upper 95% CL of intensity score of OGD uptake after 5 days of DS. N = 26–30. *P < 0.05; **P < 0.01 compared to control (DS5 versus NS; GW versus GW vehicle; mixed effects ANOVA of Log10 OGD data).
Table 3.
Corneal OGD Staining, Comparison of Topical and Systemic Treatment
These results indicated that the local therapeutic effect of GW559090 is achieved by topical administration as traditional eye drops.
Topical Treatment With GW559090 Decreases Inflammatory Markers on the Ocular Surface
Dry eye disease often is accompanied by increased T-cell related cytokines, MMPs, and inflammatory cytokines in cornea and conjunctiva.3,18,19 We investigated the expression of IL-1α, TGF-β1, MMP-9, and CXCL9 in cornea and conjunctiva using mice that were treated topically with GW559090 at 30 mg/mL during 5 days of DS and compared to vehicle-dosed mice. These genes were chosen since they are highly inducible by DS.3,8,16,18,20 Both experimental groups and the untreated, nonstressed control group had similar expression levels of the housekeeping gene, HPRT-1. Experimental groups were calibrated to nonstressed control. The results are presented in Figure 3.
Figure 3.
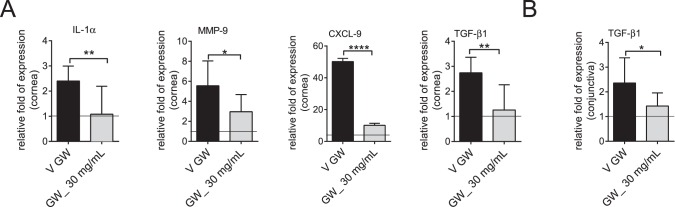
Topical treatment with GW559090 decreases inflammatory markers. (A) Relative fold of expression ± SD of IL-1α, MMP-9, CXCL9, TGF-β1 genes in cornea. (B) Relative fold of expression ± SD of TGF-β1 gene in conjunctiva. Line indicates nonstressed, untreated control. N = 7–8. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 (Unpaired t-test). V, vehicle.
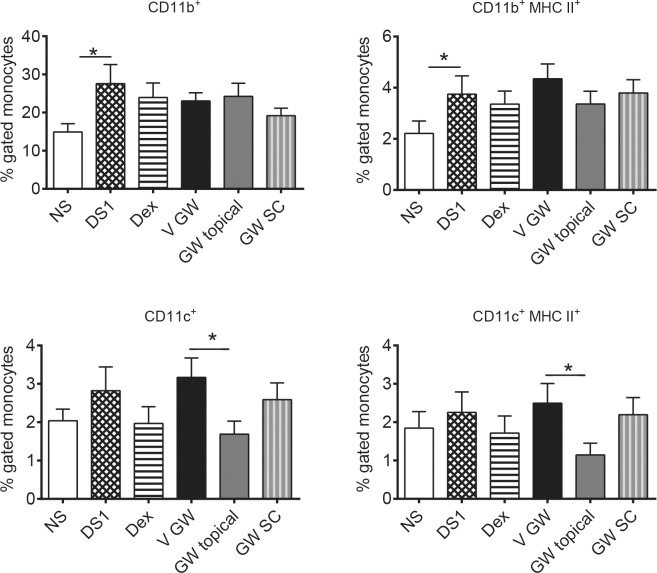
Figure 4.
Topical treatment with GW559090 decreases CD11c+ and CD11c+/MHC II+ dendritic cells in draining cervical lymph nodes. Percent gated cells (FACS) isolated from draining CLN, which were pooled from each mouse, were stained for CD11c, CD11b, and MHC II. N = 16 mice per group. *P < 0.05; compared to control (DS5 versus NS; GW versus GW vehicle; 2-way ANOVA with fixed treatment and random experiment effects followed by Dunnett's multiple comparison procedure). DS1, desiccating stress for 1 day without treatment; all other groups are DS1 with treatment; GW topical, topical bilateral GW559090 (30 mg/mL; 60 μg per eye); GW SC, GW559090 SC twice daily (30 mg/mL; 120 μg).
There was a significant decrease in IL-1α, MMP-9, TGF-β1, and CXCL9 transcripts in corneal epithelium of mice treated with GW559090 compared to vehicle control (Fig. 3A). In conjunctiva, there was a significant reduction in TGF-β1, expression but no change regarding IL-1α, MMP-9, and CXCL9 (Fig. 3B). These results indicated that GW559090 can decrease some inflammatory biomarkers on the ocular surface.
Topical Treatment With GW559090 Decreases Dendritic Cell Activation
The draining CLN are an integral part of the immune cycle in DED.21,22 To determine the impact of GW559090, if any, on the CLN, mice were subjected to DS for 1 day and treated either topically or systemically (SC; identical 120 μg dose as topical) twice-daily with GW559090. Mice receiving subcutaneous drug were concomitantly administered vehicle topically to both eyes to mimic the wetting of the ocular surface that occurs with a topical eye drop. The 1-day time point was selected since the number of DCs migrating to the draining CLN had been shown to peak after 1 day of DS in a previous investigation.22 Draining CLN were excised and prepared for flow cytometry analysis of T cells (CD4, CD8), monocytes (CD11b), dendritic cells (CD11c), and MHC II.
One day of DS led to a significant increase in CD11b+ monocytes in CLN compared to normal mice. None of the other cell populations studied was significantly altered by DS in draining CLN. None of the treatments had an impact on either of the T-cell populations or CD11b+ monocytes. While CD11c+ and CD11c+/MHC II+ cells tended to be increased by DS, albeit not significantly, topical treatment with GW559090 reduced both, activated and nonactivated, forms of DCs compared to the vehicle control group. Systemic treatment with GW559090 did not produce the same effect.
Discussion
Dry eye is an inflammatory disease of the ocular surface mediated by autoreactive T cells and is associated with corneal barrier dysfunction, increased expression, and levels of inflammatory cytokines and chemokines, tear film instability, and discomfort. This study demonstrated that an integrin α4 antagonist, when administered locally to the eye, improves the corneal epithelial barrier function, decreases inflammatory markers in the cornea, and inhibits the migration and activation of antigen-presenting cells in a murine model of DED.
Primed and activated T lymphocytes traffic from the bloodstream to sites of inflammation with the help of adhesion receptors expressed at their cell surface that interact with corresponding adhesion molecules on the vascular endothelium. Lymphocytic integrins α4β1 (VLA-4) and αLβ2 (LFA-1) bind to endothelial VCAM-1 and ICAM-1, respectively. At sites of inflammation, lymphocytic integrin receptors can interact with certain tissue components, fibronectin in the case of α4β1, which further aids in homing and lymphocyte activation. GW559090 has high-affinity for α4β1. In cell adhesion assays, it potently blocked cell adhesion of α4β1 to VCAM-1 and fibronectin (CS-1 domain), as well as α4β7 to MAdCAM. The latter interaction is of relevance in the gut environment, but not known to have a role in the eye. In Sjögren's patients, who have xerostomia and DED, integrin α4β1 has been detected in T lymphocytic infiltrates in labial tissue and VCAM-1 on vascular and dendritic cells.23
Increased uptake of fluorescent dyes by the corneal epithelium is a hallmark of DED. We have reported previously that corneal staining intensity with OGD in mice positively correlates with a reduction in corneal barrier function after experimental DS.3,8 This mimics what is observed clinically in dry eye patients in whom it is demonstrated by fluorescein staining of the cornea. Low staining scores are indicative of dye exclusion, that is corneal barrier integrity. In contrast, high staining scores are reflective of barrier dysfunction. Corneal fluorescein staining scores have been used as important endpoints for diagnosis of DED and as an efficacy parameter in clinical trials. Over 5 days in a controlled DS environment mice developed corneal barrier dysfunction, evident as punctate corneal OGD staining, as had been reported previously.3,8 In this murine model, topical GW559090 dose-dependently reduced corneal OGD staining similar to a topical corticosteroid, dexamethasone phosphate. Topical steroids are effective medications for DED if lubricants and nonsteroidal immunomodulators are not effective, but they are typically used short-term because of the potential to develop steroid-related ocular adverse events. A similar improvement of corneal staining in this animal model with a different integrin α4β1 antagonist, BIO-8809, had been reported previously.13 Relative to body weight, topical application of a drug to the mouse eye far exceeds the dose given to a human by 2 to 3 orders of magnitude. It is conceivable that topical treatment in the mouse achieves relevant systemic exposure. Since integrins have a role in leukocyte trafficking it could be argued that systemic exposure may be the prerequisite for an integrin antagonist to treat ocular disease. Thus, it was important to determine whether systemic treatment was more effective. Interestingly, when comparing systemic with topical treatment of the same dose side-by-side GW559090 improved corneal staining significantly only when administered topically. This suggested that there is a local component to GW559090 therapy in this disease.
It has been suggested that DED is the consequence of an immune cycle that involves the migration of antigen-presenting dendritic cells (DC) from the ocular surface to the draining cervical lymph nodes (CLN) where the priming of autoreactive T cells takes place.21 These autoreactive CD4+ T cells then migrate back to the ocular surface propagating the disease.9,24,25 With integrin receptors present on CD4+ T cells the question arose whether treatment with an integrin α4 antagonist affected T cells in the draining lymph nodes. One day of DS neither increased CD4+ or CD8+ T cells in CLN, nor did treatment with GW559090 or dexamethasone decrease the number of T cells. As previously reported, 1 day of DS significantly elevated CD11b+ monocytes in CLN.22,26 However, neither of the drug treatments was able to reduce the number of monocytes.
It has been shown previously that DC are important for the immune-mediated pathology induced by DS, as DC-depleted mice do not develop dry eye disease.22 Cells CD11c+ DC appeared elevated, albeit not significantly, by short-term DS. In contrast to topical dexamethasone or systemic GW559090, which had no significant effect on these cells, activated (MHC-II+) and nonactivated CD11c+ cells were decreased by topical GW559090. This interesting finding suggested that GW559090 prevents the migration of antigen-presenting cells to the draining lymph nodes and that this effect requires drug present at the ocular surface. Similarly, as discussed earlier only topical GW559090 improved corneal staining. Taken together, these results implicated that GW559090 acts locally at the level of the ocular surface to treat ocular inflammation related to DED by preventing the migration of antigen-presenting DC to the draining CLN, thus interrupting the immune cycle.
The proinflammatory milieu at the ocular surface in DED and this murine model is well described in the literature.3,16,18,27–29 The expression of many cytokines and chemokines is increased in the cornea and conjunctiva, resulting in elevated levels in the tear film. In this study, topical GW559090 inhibited the expression of IL-1α, MMP-9, CXCL9, and TGFβ1 in the corneal epithelium, and of TGFβ1 in the conjunctiva. Interleukin-1α is a proinflammatory cytokine that is released by epithelium and inflammatory cells. Its potential relevance for the disease is highlighted by the clinical development of an IL-1 receptor antagonist for ocular surface inflammation, EBI-005.30,31 Matrix metalloproteinase-9 is a protease that has been implicated in the breakage of tight junctions of corneal epithelium and in the corneal barrier disruption in desiccating stress.8,32,33 Tear levels of MMP-9 have been shown to correlate with corneal staining intensity and other clinical parameters in dry eye patients.34 Together with CXCL10, −11, CXCL9 attracts IFN-γ producing Th1 cells, while other chemokines, such as CCL5, attract innate immune cells. Transforming growth factor-β1 is involved in Th-17 priming together with IL-6 and IL-23, and it is found elevated in tears of dry eye patients.35–37 Thus, GW559090 reduces some inflammatory markers in this animal model that are associated with ocular surface inflammation in DED.
In conclusion, this study demonstrated an improvement in objective signs of dry eye by GW559090 in the murine DS model. The potent integrin α4 antagonist acted locally at the level of the ocular surface, presumably by preventing the migration of antigen-presenting cells to the draining lymph nodes with a resulting interruption of the immune cycle of dry eye. Integrin α4 potentially represents a novel target for the treatment of dry eye disease.
Acknowledgments
The authors thank Joel Sederstrom, who provided expert assistance with flow cytometry experiments; the former GSK Receptor Pharmacology Unit, Stevenage/UK for generating the receptor binding and cell adhesion data; and Edit Kurali (GSK Statistical Consulting Group, Quantitative Sciences) for expert assistance with the statistical analysis.
Supported by GSK and by the Cytometry and Cell Sorting Core at Baylor College of Medicine, which is funded by the National Institutes of Health (NIH; Bethesda, MD, USA) NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574.
Disclosure: A.H. Krauss, GSK (E); R.M. Corrales, None; F.S.A. Pelegrino, None; J. Tukler-Henriksson, None; S.C. Pflugfelder, GSK (F, C); C.S. de Paiva, GSK (F, C)
References
- 1. Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007; 5: 179–193. [DOI] [PubMed] [Google Scholar]
- 2. Pflugfelder SC,, Jones D,, Ji Z,, Afonso A,, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999. ; 19: 201–211. [DOI] [PubMed] [Google Scholar]
- 3. de Paiva CS,, Chotikavanich S,, Pangelinan SB,, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009; 2: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Massingale ML,, Li X,, Vallabhajosyula M,, Chen D,, Wei Y,, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009. ; 28: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 5. Stern ME,, Schaumburg CS,, Dana R,, Calonge M,, Niederkorn JY,, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010; 3: 425–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stern ME,, Schaumburg CS,, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013. ; 32: 19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dursun D,, Wang M,, Monroy D,, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002. ; 43: 632–638. [PubMed] [Google Scholar]
- 8. de Paiva CS,, Corrales RM,, Villarreal AL,, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006. ; 47: 2847–2856. [DOI] [PubMed] [Google Scholar]
- 9. Niederkorn JY,, Stern ME,, Pflugfelder SC,, et al. Desiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176: 3950–3957. [DOI] [PubMed] [Google Scholar]
- 10. Cross AH,, Naismith RT. Established and novel disease-modifying treatments in multiple sclerosis. J Intern Med. 2014. ; 275: 350–363. [DOI] [PubMed] [Google Scholar]
- 11. Cohen LB,, Nanau RM,, Delzor F,, Neuman MG. Biologic therapies in inflammatory bowel disease. Trans Res. 2014; 163: 533–556. [DOI] [PubMed] [Google Scholar]
- 12. Sheppard JD,, Torkildsen GL,, Lonsdale JD,, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014. ; 121: 475–483. [DOI] [PubMed] [Google Scholar]
- 13. Ecoiffier T,, El Annan J,, Rashid S,, Schaumberg D,, Dana R. Modulation of integrin alpha4beta1 (VLA-4) in dry eye disease. Arch Ophthalmol. 2008. ; 126: 1695–1699. [DOI] [PubMed] [Google Scholar]
- 14. Ravensberg AJ,, Luijk B,, Westers P,, et al. The effect of a single inhaled dose of a VLA-4 antagonist on allergen-induced airway responses and airway inflammation in patients with asthma. Allergy. 2006. ; 61: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 15. Pulido R,, Elices MJ,, Campanero MR,, et al. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. J Biol Chem. 1991; 266: 10241–10245. [PubMed] [Google Scholar]
- 16. de Paiva CS,, Corrales RM,, Villarreal AL,, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006. ; 83: 526–535. [DOI] [PubMed] [Google Scholar]
- 17. Marsh P,, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999. ; 106: 811–816. [DOI] [PubMed] [Google Scholar]
- 18. Coursey TG,, Bohat R,, Barbosa FL,, Pflugfelder SC,, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014. ; 193: 5264–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon KC,, Park CS,, You IC,, et al. Expression of CXCL9, −10, −11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010. ; 51: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoon KC,, de Paiva CS,, Qi H,, et al. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007. ; 48: 2561–2569. [DOI] [PubMed] [Google Scholar]
- 21. Pflugfelder SC,, de Paiva CS,, Li DQ,, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008; 27 (suppl 1): S9–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaumburg CS,, Siemasko KF,, de Paiva CS,, Pflugfelder SC,, Stern ME. Ocular surface antigen presenting cells are necessary for activation of autoreactive T cells and development of autoimmune lacrimal keratoconjunctivtis. J Immunol. 2011; 187: 3653–3662. [DOI] [PubMed] [Google Scholar]
- 23. Edwards JC,, Wilkinson LS,, Speight P,, Isenberg DA. Vascular cell adhesion molecule 1 and alpha 4 and beta 1 integrins in lymphocyte aggregates in Sjogren's syndrome and rheumatoid arthritis. Ann Rheum Dis. 1993. ; 52: 806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coursey TG,, Gandhi NB,, Volpe EA,, Pflugfelder SC,, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013. ; 8: e78508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X,, Volpe EA,, Gandhi NB,, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012. ; 7: e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X,, Schaumburg CS,, Coursey TG,, et al. CD8(+) cells regulate the T helper-17 response in an experimental murine model of Sjogren syndrome. Mucosal Immunol. 2014. ; 7: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corrales RM,, Stern ME,, de Paiva CS,, Welch J,, Li DQ,, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006. ; 47: 3293–3302. [DOI] [PubMed] [Google Scholar]
- 28. de Paiva CS,, Pangelinan SB,, Chang E,, et al. Essential role for c-Jun N-terminal kinase 2 in corneal epithelial response to desiccating stress. Arch Ophthalmol. 2009. ; 127: 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Paiva CS,, Volpe EA,, Gandhi NB,, et al. Disruption of TGF-beta signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS One. 2011; 6: e29017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hou J,, Townson SA,, Kovalchin JT,, et al. Design of a superior cytokine antagonist for topical ophthalmic use. Proc Natl Acad Sci U S A. 2013; 110: 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldstein MH,, Tubridy KL,, Agahigian J,, et al. A phase 2 exploratory study of a novel interleukin-1 receptor inhibitor (EBI-005) in the treatment of moderate-to-severe allergic conjunctivitis. Eye Contact Lens. 2015; 41: 145–155. [DOI] [PubMed] [Google Scholar]
- 32. Luo L,, Li DQ,, Doshi A,, Farley W,, Corrales RM,, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004. ; 45: 4293–4301. [DOI] [PubMed] [Google Scholar]
- 33. Pflugfelder SC,, Farley W,, Luo L,, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005. ; 166: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chotikavanich S,, de Paiva CS,, Li DQ,, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009; 50: 3203–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gutcher I,, Donkor MK,, Ma Q,, Rudensky AY,, Flavell RA,, Li MO. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 2011. ; 34: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stockinger B,, Veldhoen M,, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007. ; 19: 353–361. [DOI] [PubMed] [Google Scholar]
- 37. Zheng X,, de Paiva CS,, Rao K,, et al. Evaluation of the transforming growth factor-beta activity in normal and dry eye human tears by CCL-185 cell bioassay. Cornea. 2010. ; 29: 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]