Abstract
Purpose
To determine the source(s) of vitamin D in tear fluid and examine the expression of the endocytic proteins and putative vitamin D transporters megalin and cubilin in lacrimal and Harderian glands.
Methods
Wild-type, heterozygous, and vitamin D receptor (VDR) knockout C57BL/6 mice were used, with a subset of knockout mice fed a replenishment diet for some studies. Mouse lacrimal and Harderian glands from each group were used to measure megalin and cubilin by RT-PCR, Western blot, and immunohistochemistry. New Zealand white rabbits were used to collect lacrimal and accessory gland fluid for vitamin D mass spectroscopy measurements.
Results
Ten-week-old knockout mice were significantly (P < 0.05) smaller than wild-type mice. Real-time PCR and Western blot showed decreased expression of megalin and cubilin in select VDR knockout mouse groups. Immunohistochemistry showed apical duct cell megalin staining and weaker megalin staining in VDR knockout mice compared with controls. Vitamin D2 was more prevalent in rabbit lacrimal and accessory gland fluid than vitamin D3, and greater amounts of Vitamin D2 were found in in tear fluid obtained directly from lacrimal and accessory glands as compared with plasma concentrations.
Conclusions
This is the first study to demonstrate the presence of megalin and cubilin in lacrimal and accessory glands responsible for producing tear fluid. The results strengthen the hypothesis that megalin and cubilin are likely involved in the secretory pathway of vitamin D into tear fluid by the duct cells.
Keywords: tears, vitamin D, megalin, cubilin, lacrimal gland
This study demonstrates the presence of megalin and cubilin in lacrimal and accessory glands responsible for producing tear fluid. The results strengthen the hypothesis that megalin and cubilin are likely involved in the secretory pathway of vitamin D into tear fluid by the duct cells.
Numerous studies have been published describing the nonclassical actions of vitamin D. Our lab discovered that the cornea is capable of activating and metabolizing vitamin D (Fig. 1).1 This ability of the cornea to metabolize vitamin D was recently confirmed.2 We have also shown that vitamin D metabolites are present in tear fluid, probably serving a role in corneal maintenance, although the source of tear fluid vitamin D is unknown.1
Figure 1.
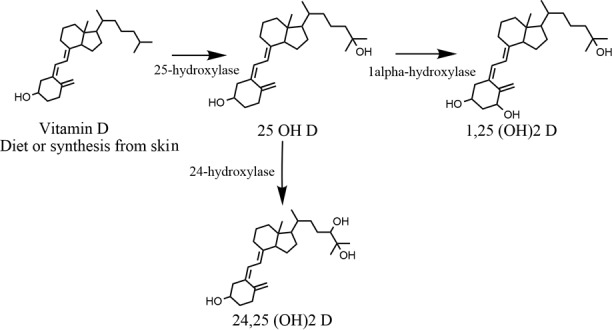
Vitamin D metabolism pathway. Inactive vitamin D is either synthesized from the skin or obtained through the diet. The inactive vitamin D is converted in the anterior segment of the eye to 25(OH)D by 25-hydroxylase, and then either to 1,25(OH)2D or 24,25(OH)2D by 1α-hydroxylase or 24-hydroxylase, respectively. This pathway is identical for both vitamin D2 and vitamin D3.
The classic pathway of vitamin D synthesis begins with UV light exposure of 7-dehydrocholesterol in the skin to produce previtamin D. This previtamin D3 is hydroxylated to 25 hydroxyvitamin D3 (25(OH)D3) in the liver and finally converted 1α, 25 dihydroxyvitamin D3 (1α,25(OH)D3) in the kidney. In order for this last step to take place, 25(OH)D3 needs to be reabsorbed at the proximal tubule of the kidney by the endocytic dual receptor complex megalin and cubilin.3
Megalin is a member of the low lipoprotein receptor superfamily4 and it is expressed in the plasma membrane and endocytic apparatus of many epithelial tissues including RPE and nonpigmented ciliary body epithelium,5 small intestine,6,7 renal proximal tubule,8,9 visceral yolk sac,10,11 thyroid gland,12 lungs,12 and parathyroid gland,13 among others. This receptor is known as a scavenger receptor because of its multiligand properties, which allows for nonspecific cellular uptake of proteins. Vitamin D binding protein (DBP) is one of the ligands for megalin.14 Cubilin is also an endocytic receptor that has been shown to be coexpressed with megalin in several absorptive epithelia and also has the potential for binding DBP.3
The purpose of this study was to determine the source(s) of vitamin D in tear fluid. This is particularly relevant given a recent study demonstrating that high serum vitamin D levels have a small but favorable effect on dry eye syndrome.15 In addition, because megalin and cubilin are involved in vitamin D transport and because megalin expression can be stimulated by vitamin D, we examined the expression of these proteins in the lacrimal and Harderian gland of wild-type and Vitamin D Receptor (VDR) knockout mice.16 We hypothesized that the lacrimal gland excretes vitamin D metabolites into the lacrimal fluid and that megalin and cubilin are involved in the lacrimal gland vitamin D secretory pathway. We also hypothesized that feeding the VDR knockout mice a special diet rich in calcium, phosphate, and lactose that has been shown to reverse some of the phenotypical features in these mice through normalization of their calcium and mineral metabolism,17–19 would also reverse phenotypical changes observed in the mice fed a normal diet.
Methods
Animals
Wild-type (+/+), heterozygous (+/−), and VDR knockout (−/−) C57BL/6 mice were obtained and bred from the Jackson Labs, Bar Harbor, ME, USA (Strain: B6.129S4-Vdr<tm1Mbd>/J). A subset of −/− mice were fed a replenishment diet (20% lactose, 2.0% Ca2+, 1.5% PO4−) that has been previously shown to partially alleviate the VDR (−/−) mouse phenotype.17–19 Lacrimal glands and Harderian glands were collected from mice of each genotype (n = 5 per group). Three New Zealand white rabbits were used to collect serum along with lacrimal and accessory gland fluid. All animal studies were approved by the University institutional animal care and use committee, and animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Lacrimal Cannulation
Three white New Zealand rabbits (2.2 kg) were used to collect lacrimal and accessory gland fluid. Before any manipulation, the rabbits were weighed to adjust anesthetic dosage with ketamine and xylazine. Vascular access was secured at the marginal ear vein and blood samples were taken for vitamin D metabolite analysis. Lacrimal cannulation was performed as described previously.20,21 Briefly, microcapillary tubes were fire polished and used to cannulate the lacrimal duct. After successful access to the lacrimal duct, 0.2 mL of 2 mg/mL pilocarpine was injected every 20 minutes for up to 3 hours to stimulate lacrimal fluid production. Pilocarpine injection results in an immediate increase in lacrimal secretion. A total of 100 to 200 μL was collected directly from the microcapillary tube with a micropipette. At the same time, fluid was collected from the combined accessory glands (Harderian and Wolfing) by collecting fluid from the fornix that pooled while the lacrimal gland was cannulated. Rabbits were euthanized with pentobarbital after the cannulation procedure.
Mass Spectroscopy
Vitamin D metabolites were analyzed by mass spectroscopy. The samples were extracted, derivatized and analyzed as described previously22 with modification. In brief, approximately 500 μL of samples spiked with internal standards (d6 vitamin D3, d6 25(OH) vitamin D3, and d6 1,25 (OH)2 vitamin D3) were extracted with 500 μL of methyl t- butyl ether (MTBE). The supernatants were transferred to a new Eppendorf tube and dried using an RC 1022 vacuum concentrator (Jouan, Winchester, VA, USA) before addition of 100 μL of 0.75 mg/mL PTAD (4-phenyl-1,2,4-triazole-3,5-dione) in acetonitrile for derivatization. Samples were left overnight at 4°C to allow complete derivatization. These samples were injected onto a liquid chromatography-tandem mass spectrometry system consisting with Agilent 1200 SL system (Agilent, Santa Clara, CA, USA) and ABSciex 4000 Qtrap system (Redwood City, CA, USA). The gradient and the MS/MS methods are provided in in Supplementary Tables S1 and S2. Vitamin D3 and its metabolites were quantified according to the calibration curve with deuterated internal standards.
Real-Time PCR
Tissue from 4- and 10-week-old VDR +/+, +/−, −/−, and −/− mice on the replenishment diet was used for mRNA quantification. Total RNA was obtained from mouse lacrimal and Harderian glands. Real-time PCR was used to quantify megalin and cubilin mRNA levels. The RT-PCR primers for megalin, cubilin, and Rps19 (used as the internal control) were designed through Roche Applied Science Universal Probe Library using the National Center for Biothechnology Information (NCBI) sequence identification numbers (NM_001081088.1, NM_001081084.2, NM_023133.1 for megalin, cubilin, and Rps19, respectively). The primers for megalin were as follows: forward primer, 5′-GAT GGA TTA GCC GTG GAC TG-3′; reverse primer, 5′-TCC GTT GAC TCT TAG CAT CTG A-3′. The primers for cubilin were as follows: forward primer, 5′-CAC TGC TTC CTA CAC CTC CAG-3′; reverse primer, 5′-TCC ACC AGA GAC ACT TGG AA-3′. The primers for Rps19 were as follows: forward primer, 5′-GTG GAA AAG GAC CAA GAT GG-3′; reverse primer, 5′-CTT TGT TCT AAT GCT TCT TGT TGG-3′. The mRNA was isolated and cDNA was synthesized using the ThermoScript RT-PCR system. First-strand synthesis was done at 55°C for 60 minutes, and inactivated at 85°C for 5 minutes. Equal amounts of cDNA were applied for PCR amplification in triplicates using a LightCycler 480 system and UPL probes (Roche Diagnostics Corporation, Indianapolis, IN, USA). Amplification was performed at 95°C for 10 minutes, followed by 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds. Quantitative values were obtained from the threshold cycle value (Ct), which is the point where a significant increase of fluorescence is first detected. S19 was used as an internal RNA control, and each sample was normalized on the basis of its S19 gene content (ΔCt). The formula 2^−(ΔCT) was used to analyze the results.
Western Blotting and Immunohistochemistry
Mice were used to examine the presence and location of megalin and cubilin in tear producing glands. For Western blots, lacrimal and Harderian gland tissue was dissected from periorbital and orbital structures, and weighed to calculate tissue to mouse weight ratios. Lacrimal and Harderian gland tissue was collected from each genotype and −/− mice on the special diet to measure expression of megalin and cubilin at 4 and 10 weeks of age. Dissected tissue was stored in a protease inhibitor solution at −80°C. The glands were freeze-dried with liquid nitrogen and crushed. Lysis buffer D at 95°C (0.3% sodium dodecyl sulfate, 10 mM tris/HCL, 10 μM sodium orthovanadate, 100 μM sodium fluoride, and protease inhibitor) was added to the tissue, which was collected for further processing. The mixture was sonicated five times in ice water followed by incubation at 95°C for 10 minutes, centrifuged at 14,000g (4°C) for 10 minutes followed by supernatant recollection. Sample protein concentration was measured using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA). Equal amounts of protein (20 μg) were loaded onto an 8% gel and separated by SDS-PAGE. Rabbit anti-megalin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and goat anti-cubilin (Santa Cruz Biotechnology) were used as primary antibodies. Horseradish peroxidase conjugated goat and rabbit anti-goat and anti-rabbit secondary antibodies were used to enhance detection. For loading controls, membranes were stripped and reprobed with β-actin antibody (CP01; Calbiochem, San Diego, CA, USA). Western blots were digitally photographed and blot density was determined by Fiji (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA).
For megalin immunohistochemistry, paraffin sections (5 μm) were cut from 10-week-old mice on a Leica microtome (Wetzlar, Germany). Sections were dried at 60°C in an oven for 1 hour, placed in xylene overnight, rehydrated in graded alcohols, and blocked for endogenous peroxidase. Demasking was achieved by heating the sections in TEG buffer (Tris-EGTA buffer, pH 9) at approximately 100°C for 10 minutes, after which the sections were cooled at room temperature for 30 minutes and incubated for 30 minutes in 50 nM NH4Cl in 0.01 M PBS. Permeabilization was obtained with 0.05% saponin (1% BSA, 0.2% gelatine, 0.05% saponin in 0.01 M PBS) and sections were incubated with 1:500 of Protein A purified sheep anti-megalin (kindly provided by Pierre Verroust, MD) or Protein A purified sheep Ig (DAKO, Glostrup, Denmark) in 0.01 M PBS, 0.1% BSA and 0.3% Triton X-100 followed by washing and incubation with HRP-conjugated secondary antibody. Labelling was visualized by incubation with diaminobenzidine and 0.03% H2O2 for 10 minutes. Sections were counterstained with Meier's hematoxylin stain and images were acquired with a Leica DMR microscope equipped with a Leica DFC320 camera. Images were transferred by a Leica TFC Twain 6.1.0 program and processed by using Corel Photopaint version 12.0.0.536 (Corel Cooperation, Ottawa, ON, Canada).
Statistical Analysis
The Kolmogorov-Smirnov test was run to test for data normality. ANOVA with Tukey's post hoc multiple comparison test was used to compare most experimental groups. A Kruskal-Wallis test was run on the one data set that failed the normality test. All analyses were performed using InStat software (GraphPad Software, Inc., La Jolla, CA, USA). A P value of less than 0.05 was considered significant.
Results
Macroscopic Features
Four-week-old mice had no significant difference in body weight or gland/body weight ratios among genotypes. Significant differences (P < 0.05) were observed when comparing the 10-week-old VDR −/− mice (mean +/− SE body weight = 17.52 ± 2.09 g) with the wild-type (21.98 ± 1.83 g), heterozygous (17.52 ± 2.094 g), and special diet knockout mice (24.21 ± 2.52 g). No other weight comparisons were significant. ANOVA of lacrimal gland/body weight ratios (%) showed a significantly increased ratio (P < 0.05) of VDR −/− mice on the special diet when compared to +/+, +/−, and −/− mice (Fig. 2A). Harderian gland/body weight ratios in 10-week-old mice showed a significant difference in −/− mice when compared with +/+, +/−, and −/− mice on the special diet (Fig. 2B). Interestingly, this ratio was higher in the −/− mice than all other genotypes.
Figure 2.
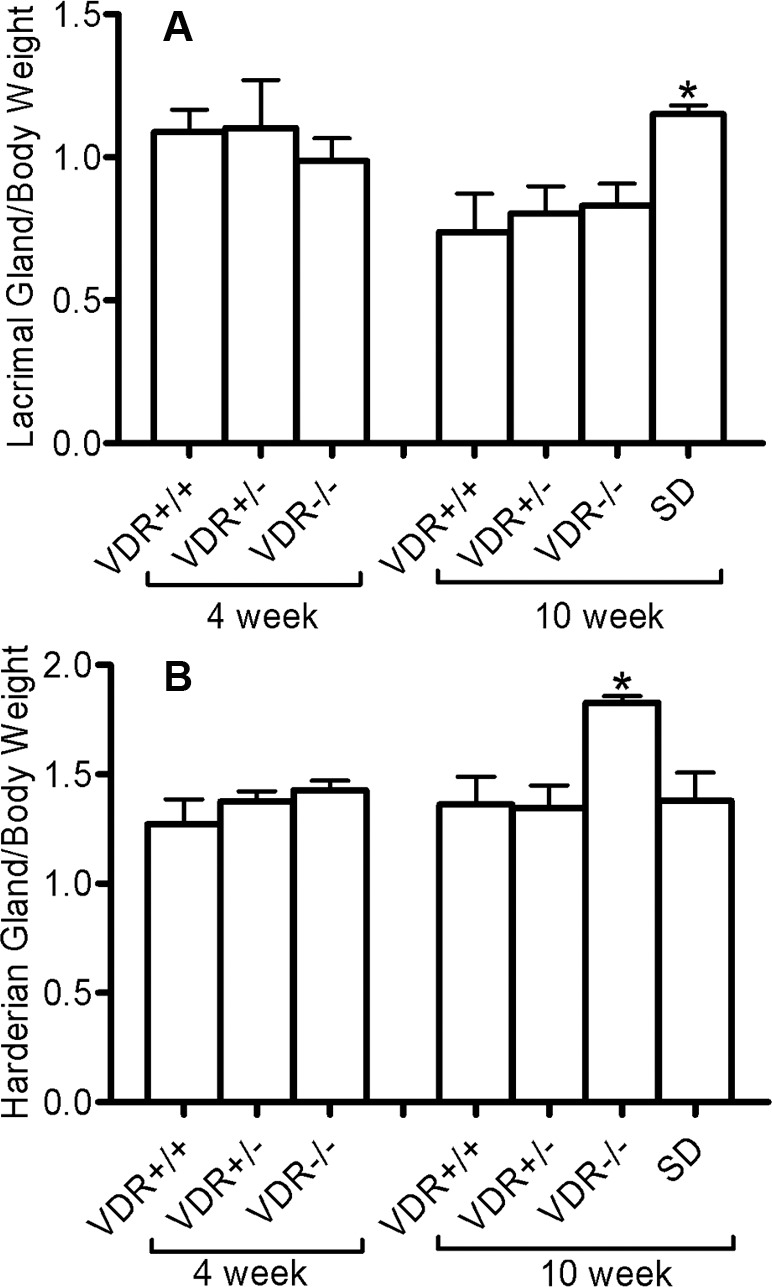
Ratio of lacrimal (A) and Harderian gland (B) weight to mouse body weight. There were no differences in the ratios of 4-week-old mice (n = 5). There was a significant difference (*P < 0.05, n = 5) in the lacrimal gland ratio comparing VDR +/+ and VDR −/− mice on the special diet (SD), and in the Harderian gland ratio between 10-week-old VDR −/− mice and VDR +/+ mice (*P < 0.01, n = 5). Bars indicate mean ± standard error (SE).
Level of Vitamin D Metabolites in Plasma and Gland Fluids
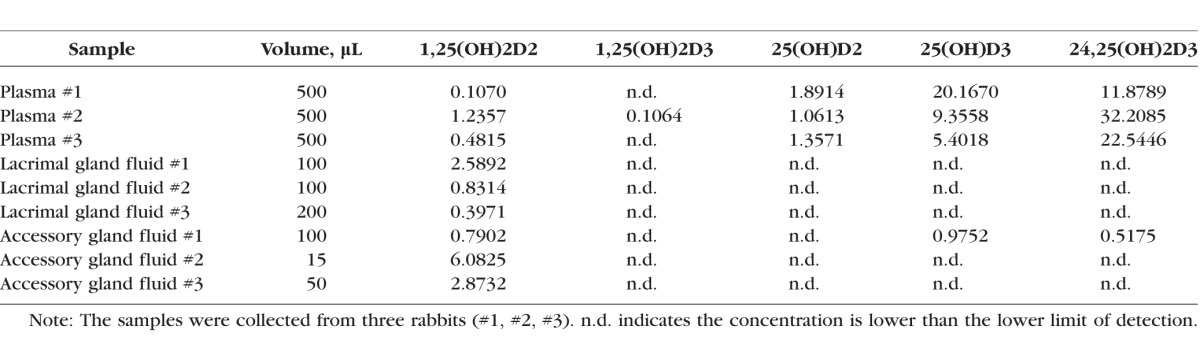
To identify the source(s) of tear fluid vitamin D, we analyzed secretions from lacrimal and Harderian glands for vitamin D metabolites by mass spectrometry. The Table shows the concentrations of 25-hydroxyvitamin D2 (25(OH)D2), 25-hydroxyvitamin D3 (25(OH)D3), 1,25(OH)D2, 1,25(OH)D3, 24,25(OH)D3, and 24,25(OH)2D in the plasma, lacrimal gland fluid, and accessory gland fluid in each of the individual rabbits examined. The abbreviation n.d. indicates concentrations that were below the limit of detection. The measureable levels were quite variable but indicate several trends, thus results from all samples are shown. Plasma analysis showed the presence of all vitamin D metabolites, although only one plasma sample had measureable 1α,25(OH)D3. In addition, 1α,25(OH)D2 levels were generally higher in lacrimal and accessory gland fluid than in plasma, indicative of a secretory pathway into tear. In addition, vitamin D2 species were much more prevalent in lacrimal and accessory gland fluid than vitamin D3 species.
Table.
Vitamin D Metabolites in Plasma and Fluid Collected From Lacrimal and Accessory Glands
Megalin and Cubilin mRNA VDR Levels in Knockout and Wild-Type Mouse Lacrimal and Harderian Glands
To begin to examine whether megalin and cubilin could influence the final content of vitamin D in tear fluid, we analyzed their expression in lacrimal and Harderian glands by RT-qPCR. Mouse lacrimal gland megalin mRNA levels were significantly lower in 4-week-old −/− mice compared with +/+ and +/− mice (P < 0.05). There was also a significant difference (P < 0.01) in megalin mRNA levels in −/− mice with and without the special diet when compared with +/+ and +/− in 10-week-old mouse lacrimal glands. In addition, megalin mRNA levels were significantly lower in −/− (P < 0.01) and +/− mice (P < 0.05) when compared with +/+, and −/− mice on the special diet (P < 0.001) in Harderian glands of 10-week-old mice (Fig. 3A).
Figure 3.
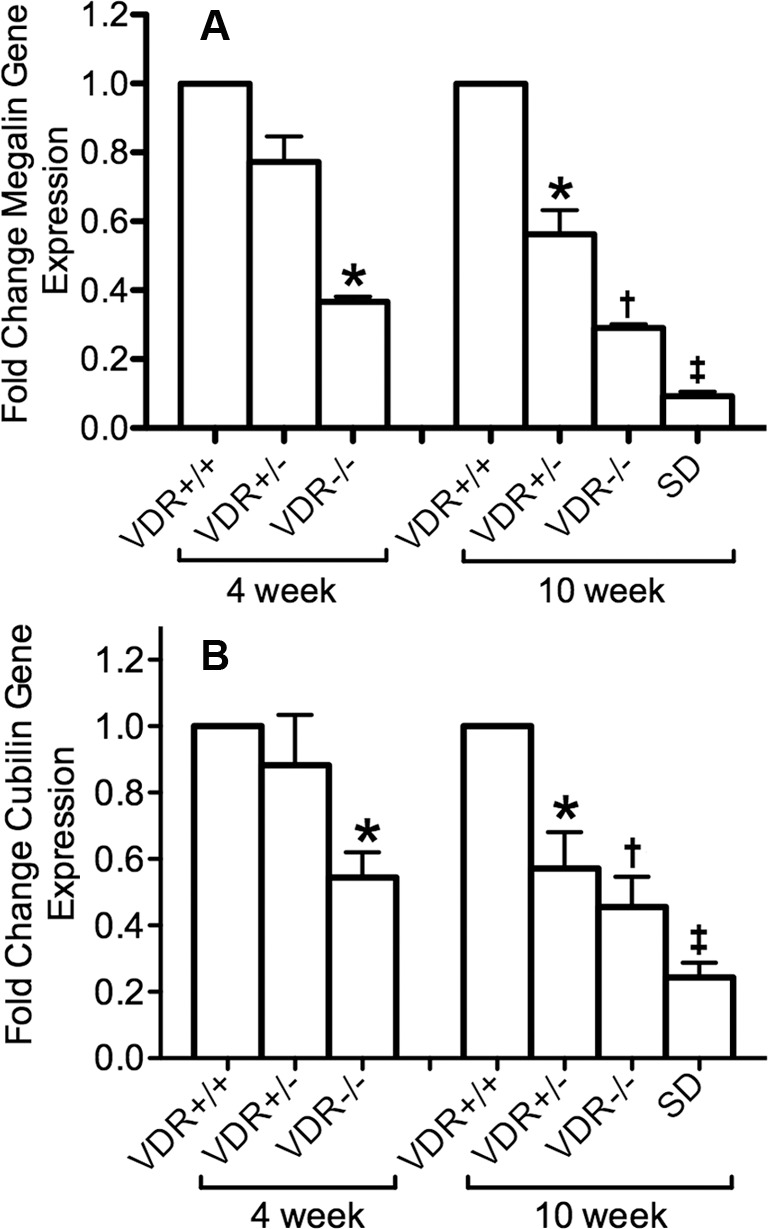
Lacrimal gland megalin (A) and cubilin (B) gene expression. The formula 2−(ΔΔCt) was used to graph the results. There was a significant decrease in megalin and cubilin mRNA expression in 4-week-old VDR −/−mice (*P < 0.05, n = 3). There was also a significant decrease in megalin and cubilin mRNA expression in 10-week-old VDR +/− mice (*P < 0.05, n = 3), VDR −/− mice (†P < 0.01, n = 3) and VDR −/− mice administrated the special diet (SD) (‡P < 0.001, n = 3). Bars indicate mean ± SE.
There was a decreased expression of cubilin in lacrimal glands of knockout mice as well. We found a significant difference (P < 0.05) in −/− mice when compared to +/+ and +/− mice at 4 weeks of age. There was also a significant difference (P < 0.05) when −/− mice were compared with +/+ and +/− mice at 10 weeks of age as well as a significant difference (P < 0.01) in −/− mice on the special diet compared with +/+, +/−, and −/− mice (Fig. 3B).
For Harderian glands, there was no difference in megalin or cubilin mRNA expression among genotypes of 4-week-old mice. There was also no difference in the cubilin mRNA expression among genotypes in 10-week-old mice, however a significant decrease was measured in megalin mRNA expression in 10-week-old VDR −/− mice compared with VDR +/+ mice (P < 0.05; Figs. 4A, 4B).
Figure 4.
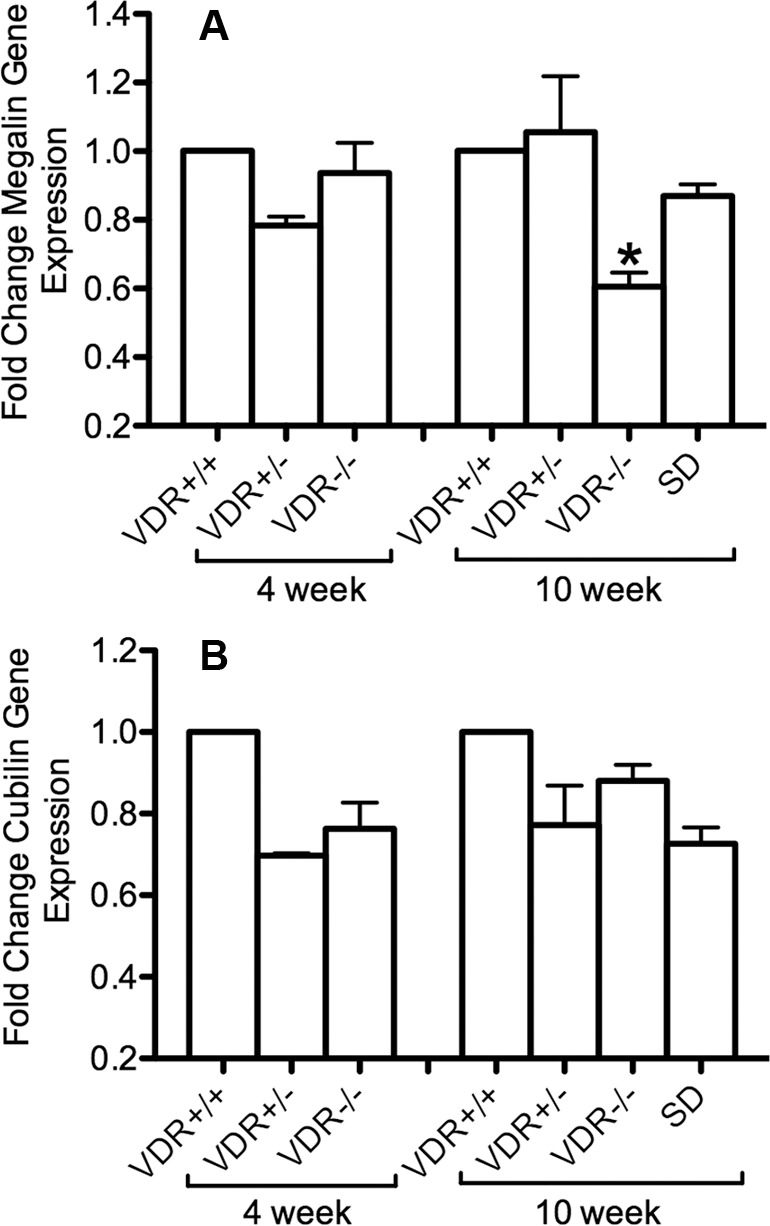
Harderian megalin (A) and cubilin (B) gene expression. The formula 2−(ΔΔCt) was used to graph the results. For 4-week-old mice, there was no difference in megalin and cubilin mRNA expression among genotypes. There was also no difference in the cubilin mRNA expression among genotypes in 10-week-old mice, but a significant decrease was showed in megalin mRNA expression in 10-week-old VDR −/− mice compared with VDR +/+ mice (*P < 0.05, n = 3). Bars indicate mean ± SE.
Megalin and Cubilin Protein Levels in VDR Knockout and Wild-Type Mouse Lacrimal and Harderian Glands
We extended our megalin and cubilin analyses by measuring protein levels using Western blotting. As shown in Figures 5B and 5D, in the lacrimal gland there was a decreased expression (P < 0.01) of cubilin in 4-week-old VDR −/− versus +/+ mice. There was also a small but significant decrease in protein expression (P < 0.05) of megalin and cubilin in lacrimal gland of 10-week-old VDR −/− versus +/+ mice (Figs. 5A–D). There was a significant recovery (P < 0.05) of cubilin expression when VDR −/− mice were fed the special diet. There was no significant recovery of megalin protein expression in 10-week-old mice fed the special diet. In Harderian gland (Fig. 6) there was a significant decrease (P < 0.05) in expression of megalin in 10-week-old VDR −/− versus +/+ mice. There were no significant differences in cubilin expression. The 4-week-old Harderian gland protein expression was the only data set that failed the normality test, and showed no significant differences between groups.
Figure 5.
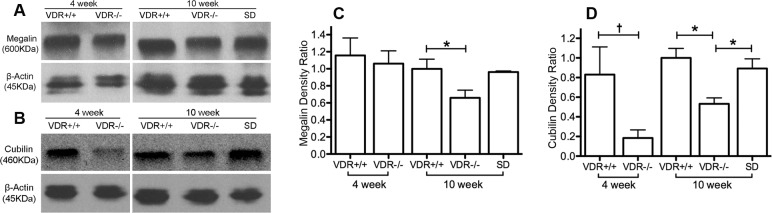
Lacrimal gland megalin (A, C) and cubilin (B, D) protein and protein intensity changes. Note the significantly decreased expression of megalin and cubilin in 10-week-old VDR −/− mice (*P < 0.05, n = 3), and the recovery of cubilin protein expression in VDR −/− mice on the special diet (SD). There was a significant decrease in cubilin protein expression in 4-week old VDR −/− mice (†P < 0.01, n = 3) compared with 4-week-old VDR +/+ mice. Bars indicate mean ± SE.
Figure 6.
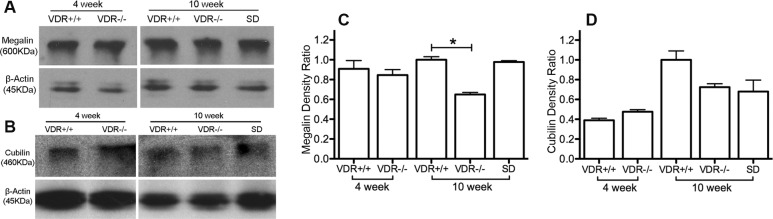
Harderian gland megalin (A, C) and cubilin (B, D) protein and protein intensity changes. Note the significantly decreased megalin expression in 10-week-old VDR −/− mice (*P < 0.05, n = 3). There was no difference in megalin or cubilin protein expression comparing 4-week-old VDR +/+ and VDR −/− mice. Bars indicate mean ± SE.
Immunohistochemistry
Results from lacrimal gland immunohistochemistry show staining of megalin on the apical membrane of the ducts. Megalin staining in VDR −/− mice appears weaker than in VDR +/+ mice (Fig. 7).
Figure 7.
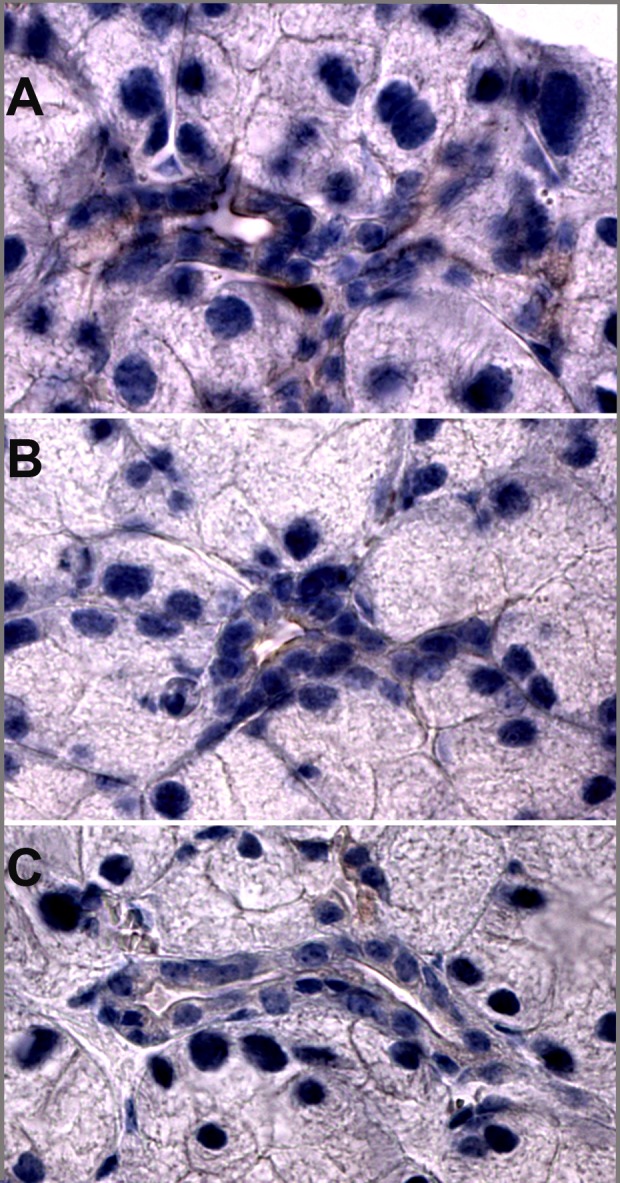
Megalin immunostaining in the lacrimal glands of 10-week-old mice. Megalin staining in VDR +/+ mice (A) appears stronger than in VDR −/− mice (B). (C) Secondary antibody control.
Discussion
Li et al.17 have previously described the vitamin D receptor knockout phenotype and one of its characteristics is growth retardation. As expected, we found that 10-week-old VDR −/− mice are significantly smaller than +/+ mice. Also, VDR −/− mice fed the special diet recovered their weight and are comparable with +/+ mice. Interestingly, when comparing gland/body weight ratios, we determined that 10-week-old VDR −/− mice had higher ratios than any phenotype indicating that vitamin D deficiency did not affect gland size as much as it did body weight, with the ratio normalizing following the replenishment diet, which helped mice to recover their weight.
The current study demonstrates the presence of megalin and cubilin in the lacrimal and Harderian glands, and provides evidence that these proteins may be involved in the secretion scheme of vitamin D into tear fluid. A recent study by Alsalem et al.2 confirmed previous results from our group demonstrating the presence of the metabolic machinery to process vitamin D and its metabolites within cells of the cornea, and importantly it also demonstrated that megalin and cubilin are present in several ocular barrier cell types. Megalin has also been detected in RPE and nonpigmented ciliary body epithelium, and a severe myopia phenotype as well as enlarged RPE melanosomes and abnormal ciliary body development were described in megalin-deficient mice.5 Previous work examining renal vitamin D uptake in megalin knockout mice demonstrated an increased urine concentration of 25(OH)D3 and decreased plasma levels of 25(OH)D3 and 1α,25(OH)D3, both resulting from decreased cellular uptake of the vitamin D-binding protein (DBP) complex from glomerular filtrate. In addition, loss of cubilin function resulted in a similar decrease in plasma concentrations of 25(OH)D3 and 1α,25(OH)D3 secondary to impaired renal uptake of 25(OH)D3-DBP.14,23 In the current study, mass spectroscopy examining vitamin D metabolites present in lacrimal and accessory gland fluid of New Zealand white rabbits detected primarily concentrations of the metabolite 1,25(OH)D2, with low or minimal concentrations of the other vitamin D metabolites being found at measureable levels. These results correspond to our previous study measuring vitamin D metabolite levels in rabbit tear fluid in which we found predominantly vitamin D2 species1; thus, the current study confirms that D2 species predominate in the rabbit. In humans, D2 species are only available through the diet. The higher concentration of 1,25(OH)D2 in rabbit glandular fluid compared with plasma indicates a likely active secretion of 1,25(OH)D2 from plasma to glandular fluid. A second possibility is increased conversion of 25(OH)D2 to 1,25(OH)D2 within the gland. This is unlikely because of the undetectable gland fluid 25(OH)D2 levels which were relatively high in the serum. The presence of the endocytosis-associated protein megalin in the apical membranes of lacrimal duct epithelium may be the reason for the low 25(OH)D2 glandular fluid levels (lower than 1,25(OH)D2), which, along with the associated protein cubilin, would possibly remove 25(OH)D2 prior to glandular secretion of the final tear fluid. This could potentially also lead to lower glandular fluid 1,25(OH)D2 levels than would otherwise be present without the presence and activity of apically-located megalin assuming 25(OH)D2 can be hydroxylated to 1,25(OH)D2 within or after leaving the gland ducts. Because DBP binds to 25(OH)D2 in a similar fashion but with lower affinity as compared with 25(OH)D3, and DBP is the vitamin D ligand associated with megalin, we hypothesize that the same process would hold true for 25(OH)D3.24,25 This is the process that occurs for 25(OH)D3 in the proximal tubule.11 Thus, the presence of megalin and cubilin in the duct system might modify the content of vitamin D metabolites, although the presence of remnant vitamin D metabolites in gland secretions suggests that the source of vitamin D in tear fluid could be from the lacrimal and Harderian glands.
Due to the small volume of the tear samples, the detection and quantification of vitamin D metabolites by mass spectroscopy are still challenging. In fact, for some of the vitamin D metabolites in our tear sample measurements, we did see clear peaks although they were below the limit of quantification. We list them as not detectable (n.d.) because large variations are an issue with such a low concentration measurement. Thus, in the future, an improved derivatization reagent with better sensitivity will be important for the accurate measurement of limited availability, low-volume samples such as the tear samples in this study.
This study demonstrated expression of megalin and cubilin in mouse lacrimal and accessory glands by RT-PCR, Western blot, and immunohistochemistry (megalin). In VDR knockout mice, there was a decreased expression of megalin in lacrimal and accessory glands and a decrease in cubilin expression in lacrimal gland by RT-PCR and Western blot. Immunohistochemistry showed megalin staining on the apical borders of the lacrimal gland in (+/+) mice with weaker staining in (−/−) mice. Attempts at cubilin immunohistochemical staining were unsuccessful due to the high background staining observed using available antibodies. Previous studies14,26,27 have shown that megalin has Ca2+ sensing properties and that Ca2+ is required for the uptake of proteins. Studies have also demonstrated that vitamin D increases expression of megalin, most likely through VDR activation.16 In the case of the current study, lack of VDR apparently leads to decreased megalin and cubilin expression. Interestingly, while mRNA levels of megalin and cubilin in mice on the special diet were lower than all other groups, protein levels rebounded in these mice. The increased megalin and cubilin protein levels in the −/− special diet mice corresponds to the rescued phenotype in these mice. It is possible that this increased protein expression with low mRNA levels represents more efficient protein synthesis or recovered ability for protein synthesis in mice with corrected calcium and magnesium levels.28,29
Previous studies on chronic kidney disease (CKD) have shown decreased renal megalin expression due to decreased levels of 25(OH) D3 and defective autocrine VDR activation.30 This leads to the development of secondary hyperparathyroidism and hypocalcaemia. In megalin knockout mice, the increased urinary excretion of vitamin D metabolites leads to secondary hyperparathyroidism and hypocalcaemia.14 Like CKD and megalin knockout mice, VDR knockout mice also have hypocalcaemia and hyperparathyroidism, among other phenotypical features. Our results demonstrate that VDR knockdown decreases megalin and cubilin expression in the lacrimal and Harderian gland. As noted above, reduced systemic megalin and cubilin have been shown to limit vitamin D availability in the blood. If megalin and cubilin do participate in vitamin D resorption from tear gland fluid prior to its final secretion onto the anterior surface of the eye, then reduced megalin and cubilin would help to compensate for the decreased systemic vitamin D availability by limiting ductal resorption.
This is the first study to demonstrate the presence of megalin and cubilin in the glands responsible for producing tear fluid. In addition, it was found that VDR knockout results in decreased expression of both megalin and cubilin in lacrimal and Harderian glands. It was also shown that vitamin D2 is found in greater amounts in tear fluid directly obtained from lacrimal and the accessory glands versus plasma, indicating secretion into tear fluid by the glands. The decreased production of megalin and cubilin in VDR knockout mice coupled with the apparent secretion of vitamin D into tear fluid along with the localization of megalin to the apical border of lacrimal duct cells strengthens the hypothesis that megalin and cubilin are likely involved in the secretory pathway of vitamin D into tear fluid by the duct cells.
Supplementary Material
Acknowledgments
The authors thank the Danish Council of Independent Research, Pierre Verroust, MD, for kindly providing the antibody, and John Ubels, PhD, for assisting with the lacrimal cannulation procedure.
Supported by National Institutes of Health (NIH) National Eye Institute (NEI) Grant R01EY021747 (MW; Bethesda, MD, USA), National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (BH; Research Triangle Park, NC, USA), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant U24 DK097154 (BH; Bethesda, MD, USA); the Danish Medical Research Council (RN; Copenhagen, Denmark); the Lundbeck Foundation (RN; Copenhagen, Denmark); the Velux Foundation (RN; Copenhagen, Denmark); the Novo Nordic Foundation (RN; Copenhagen, Denmark); and the Superfund Research Program P42 ES004699 (BH; Research Triangle Park, NC, USA).
Disclosure: X. Lu, None; R.A. Elizondo, None; R. Nielsen, None; E.I. Christensen, None; J. Yang, None; B.D. Hammock, None; M.A. Watsky, None
References
- 1. Lin Y,, Ubels JL,, Schotanus MP,, et al. Enhancement of vitamin D metabolites in the eye following vitamin D3 supplementation and UV-B irradiation. Curr Eye Res. 2012; 37: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsalem JA,, Patel D,, Susarla R,, et al. Characterization of vitamin D production by human ocular barrier cells. Invest Ophthalmol Vis Sci. 2014; 55: 2140–2147. [DOI] [PubMed] [Google Scholar]
- 3. Christensen EI,, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002; 3: 256–266. [DOI] [PubMed] [Google Scholar]
- 4. Raychowdhury R,, Niles JL,, McCluskey RT,, Smith JA. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989; 244: 1163–1165. [DOI] [PubMed] [Google Scholar]
- 5. Storm T,, Heegaard S,, Christensen EI,, Nielsen R. Megalin-deficiency causes high myopia, retinal pigment epithelium-macromelanosomes and abnormal development of the ciliary body in mice. Cell Tissue Res. 2014; 358: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birn H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. Am J Physiol Renal Physiol. 2006; 291: F22–F36. [DOI] [PubMed] [Google Scholar]
- 7. Birn H,, Christensen EI,, Nexo E,, et al. Characterization of binding and uptake of B12-binding proteins by megalin and intrinsic factor-receptor/GP280 in kidney proximal tubule. J Am Soc Nephrol. 1996; 7: A0310–A0310. [Google Scholar]
- 8. Seetharam B,, Levine JS,, Ramasamy M,, Alpers DH. Purification properties, and immunochemical localization of a receptor for intrinsic factor-cobalamin complex in the rat kidney. J Biol Chem. 1988; 263: 4443–4449. [PubMed] [Google Scholar]
- 9. Sahali D,, Mulliez N,, Chatelet F,, Dupuis R,, Ronco P,, Verroust P. Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med. 1988; 167: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doxsey S,, Kerjaschki D,, Farquhar MG. A large membrane glycoprotein (GP330) is a resident of coated pits of several absorbtive epithelia. J Cell Biol. 1983; 97: A178–A178. [Google Scholar]
- 11. Burke KA,, Jauniaux E,, Burton GJ,, Cindrova-Davies T. Expression and immunolocalisation of the endocytic receptors megalin and cubilin in the human yolk sac and placenta across gestation. Placenta. 2013; 34: 1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng G,, Bachinsky DR,, Stamenkovic I,, et al. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J Histochem Cytochem. 1994; 42: 531–542. [DOI] [PubMed] [Google Scholar]
- 13. Juhlin C,, Holmdahl R,, Johansson H,, Rastad J,, Akerstrom G,, Klareskog L. Monoclonal antibodies with exclusive reactivity against parathyroid cells and tubule cells of the kidney. Proc Natl Acad Sci U S A. 1987; 84: 2990–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nykjaer A,, Dragun D,, Walther D,, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999; 96: 507–515. [DOI] [PubMed] [Google Scholar]
- 15. Galor A,, Gardener H,, Pouyeh B,, Feuer W,, Florez H. Effect of a Mediterranean dietary pattern and vitamin D levels on Dry Eye syndrome. Cornea. 2014; 33: 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu W,, Yu WR,, Carling T,, et al. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest. 1998; 28: 100–107. [DOI] [PubMed] [Google Scholar]
- 17. Li YC,, Amling M,, Pirro AE,, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998; 139: 4391–4396. [DOI] [PubMed] [Google Scholar]
- 18. Paz HB,, Tisdale AS,, Danjo Y,, Spurr-Michaud SJ,, Argueso P,, Gipson IK. The role of calcium in mucin packaging within goblet cells. Exp Eye Res. 2003; 77: 69–75. [DOI] [PubMed] [Google Scholar]
- 19. Elizondo RA,, Yin Z,, Lu X,, Watsky MA. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Ophthalmol Vis Sci. 2014; 55: 5245–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ubels JL,, Foley KM,, Rismondo V. Retinol secretion by the lacrimal gland. Invest Ophthalmol Vis Sci. 1986; 27: 1261–1268. [PubMed] [Google Scholar]
- 21. Liliom K,, Guan Z,, Tseng JL,, Desiderio DM,, Tigyi G,, Watsky MA. Growth factor-like phospholipids generated after corneal injury. Am J Physiol. 1998; 274: C1065–C1074. [DOI] [PubMed] [Google Scholar]
- 22. Aronov PA,, Hall LM,, Dettmer K,, Stephensen CB,, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008; 391: 1917–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nykjaer A,, Fyfe JC,, Kozyraki R,, et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A. 2001; 98: 13895–13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984; 21: 81–86. [DOI] [PubMed] [Google Scholar]
- 25. Houghton LA,, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006; 84: 694–697. [DOI] [PubMed] [Google Scholar]
- 26. Christensen EI,, Gliemann J,, Moestrup SK. Renal tubule gp330 is a calcium binding receptor for endocytic uptake of protein. J Histochem Cytochem. 1992; 40: 1481–1490. [DOI] [PubMed] [Google Scholar]
- 27. Lundgren S,, Carling T,, Hjalm G,, et al. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem. 1997; 45: 383–392. [DOI] [PubMed] [Google Scholar]
- 28. Brostrom CO,, Bocckino SB,, Brostrom MA. Identification of a Ca2+ requirement for protein synthesis in eukaryotic cells. J Biol Chem. 1983; 258: 14390–14399. [PubMed] [Google Scholar]
- 29. Rubin H. Central roles of Mg2+ and MgATP2- in the regulation of protein synthesis and cell proliferation: significance for neoplastic transformation. Adv Cancer Res. 2005; 93: 1–58. [DOI] [PubMed] [Google Scholar]
- 30. Parikh A,, Chase HS,, Vernocchi L,, Stern L. Vitamin D resistance in chronic kidney disease (CKD). BMC Nephrol. 2014; 15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


