Abstract
Rationale: Volume–outcome associations have been demonstrated in conditions with high morbidity and mortality; however, the existing literature regarding such associations in sepsis is not definitive.
Objectives: To test the hypothesis that annual hospital severe sepsis case volume is associated with mortality during admissions with severe sepsis in teaching and nonteaching hospitals.
Methods: This work was a retrospective cohort study of administrative data from the South Carolina State Inpatient Database using multivariate logistic regression and case mix adjustment.
Measurements and Main Results: In the calendar year 2010, 9,815 patients were admitted with severe sepsis or septic shock. Hospitals were stratified into low- (0–75 cases/yr, n = 26), intermediate- (76–300 cases/yr, n = 19), and high (>300 cases/yr, n = 12) -volume tertiles. Patients admitted to hospitals with a low annual case volume for sepsis had higher adjusted odds of dying before discharge (odds ratio, 1.56; 95% confidence interval, 1.25–1.94) compared with patients admitted to high-volume hospitals. Hospitalization at intermediate-volume hospitals was not associated with a difference in mortality (odds ratio, 0.99; 95% confidence interval, 0.90–1.09) compared with high-volume hospitals. There was no difference between the mortality rates of intermediate- and high-volume hospitals at different severity of illness quartiles. Hospital length of stay differed significantly by hospital case volume (low = 8.0, intermediate = 12.7, high = 14.9 [d]; P < 0.0001).
Conclusions: Hospitals with low annual sepsis case volume are associated with higher mortality rates, whereas hospitals with intermediate sepsis case volumes are associated with similar mortality rates compared with hospitals with high case volumes.
Keywords: critical illness, sepsis, high volume, low volume, risk adjustment
Current evidence suggests that outcomes for high-acuity conditions are influenced by the case volume of the treating hospital (1–6), with high-volume (HV) centers yielding improved outcomes. Trauma, low–birth weight neonatology, and acute myocardial infarction have all been shown to possess volume–outcome relationships (1–6) and share clinical features, including: significant morbidity and mortality; need for rapid interventions; and resource intensity. Severe sepsis is a leading cause of death in the United States (7, 8), which shares these same characteristics, leading investigators to examine whether it, too, exhibits a volume–outcome relationship. Although one large study in the United Kingdom showed no relationship between sepsis case volume and outcomes (9), several studies of U.S. hospitals have demonstrated that higher sepsis case volumes are associated with lower risk-adjusted mortality rates (10–12).
Recent data suggest that the mortality rate from severe sepsis has declined in recent years, despite a lack of new pharmacologic therapeutics (13). The gradual adoption of improved emergency department and intensive care unit (ICU) structure and care processes over time may partially explain declining mortality (14–18); however, rates of adoption vary considerably among different hospital types (19, 20). Thus, volume–outcome relationships may be a surrogate indicator of variation in the implementation of best practices, and may diminish over time as nontertiary hospitals more rapidly adopt validated interventions. In the context of the current debate over the regionalization of critical care (21–23), a better understanding of whether outcome gaps between larger and smaller hospitals are closing is crucial.
South Carolina is a predominantly rural state, with a population of about 4.7 million, and is located inside of the recently identified “sepsis belt” (24). As part of an initiative to address health disparities in critical illness in South Carolina, we sought to characterize the sepsis case volume–outcome relationships across all hospital types in our state to determine: (1) if they continue to exist across all hospital sizes; and (2) whether regionalizing sepsis care in South Carolina might be an important target for reducing health disparities. We hypothesized that care at higher-volume hospitals will be associated with improved risk-adjusted outcomes compared with care at lower-volume hospitals. Furthermore, we hypothesized that the relationship between sepsis case volume and outcomes will diminish with increasing volumes.
Methods
Study Design
We conducted a retrospective cohort analysis of the association between sepsis case volume and sepsis outcomes in the state of South Carolina. The South Carolina Office of Research Statistics maintains a State Inpatient Database containing patient and hospital demographics, diagnosis codes, procedure codes, a modifier code for ICU-level care, discharge status, and charges for all nonfederal hospital admissions annually. We obtained both patient-identified and hospital-identified data for the analysis.
Subject Selection
Data were obtained for all admissions, from the calendar year 2010, with the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes of severe sepsis (995.92) or septic shock (785.52). This approach provides a high positive predictive value (100%) for identifying patients with severe sepsis (25). In addition, as the use of 995.92 and 785.52 is increasing over time, historical limitations with sensitivity are improving (26). Admissions in which a patient was transferred from one acute care hospital to another were excluded due to the difficulty in determining the relative impact that each hospital had on outcomes. Interhospital transfers were identified by examining the discharge and admission dates for any patient who had more than one admission during the year. If a discharge date fell within the same calendar day of an admission date for the same patient to a different hospital, this was deemed to be a transfer between hospitals, and the entire episode was excluded from the analysis.
Hospital and Patient Variables
Annual hospital sepsis case volume was defined as the exposure variable and was categorized into three tertiles: low volume (LV; <75 cases of sepsis/yr), intermediate volume (IV; 75–300 cases of sepsis/yr), and HV (>300 cases of sepsis/yr). These tertile boundaries were selected to ensure adequate statistical power for intertertile comparison. A sample size estimate suggested that at least 500 patients would be required per group to detect a 7% change in mortality with 85% power, and a boundary of 75 sepsis cases per year ensured that the LV group would achieve this sample size. The upper boundary (300) was set at the median number of annual admissions for South Carolina hospitals. Thus, annual sepsis case rates in the IV group were below the median, whereas sepsis case rates in the HV group were above the median. The dependent outcome variable was hospital mortality. To control for potential confounders, demographics, including age, race, sex, and insurance status, as well as a sepsis mortality prediction score (27), were used as covariates. In the database, race was categorized as being: non-Hispanic white (white); non-Hispanic black (black); or other. The “other” race category represented less than 2% of patients and, therefore, was combined with the black cohort during analysis rather than being treated as an independent race category. Insurance status was categorized into four groups: commercial; Medicare; Medicaid; or other.
We used a sepsis mortality prediction score adapted from a previously published instrument (28) to adjust for illness severity. Briefly, the score uses the Elixhauser index (29) to estimate comorbidities, whereas the severity of the acute illness was measured through a composite of: (1) need for ICU care; (2) procedure coding for mechanical ventilation within 24 hours of admission; and (3) the presence of shock, as determined by ICD-9-CM coding (785.50, 785.52). Our adaptation of this instrument demonstrated good discriminatory ability (c statistic = 0.776) and calibration (Hosmer-Lemeshow, P = 0.2019) when predicting death in a validation cohort of patients with sepsis (27).
Sensitivity Analysis
As hospital size may influence coding practices, we performed a sensitivity analysis using a previously validated, sensitive approach for defining a severe sepsis cohort from administrative data. Angus and colleagues used a combination of ICD-9-CM codes for infection and organ failures and validated this definition against a clinically defined cohort (30). Compared with the “Explicit Diagnosis Code” approach used to define the current primary cohort, the Angus approach has a higher negative predictive value and sensitivity, but a lower positive predictive value (25). Thus, a larger cohort of patients with severe sepsis may be anticipated with the Angus approach; however, some patients in this cohort may not meet the clinical definition of severe sepsis. Sepsis case volume–outcome relationships were determined in each cohort. To evaluate whether the observed volume–outcome relationships were influenced by cases of nosocomial sepsis which occurred during admission for other conditions with volume–outcome relationships (trauma, myocardial infarction, etc.), we examined whether the presence of sepsis on admission influenced the volume–outcome relationship.
Statistical Analysis
Baseline demographics in each sepsis case volume tertile were compared using ANOVA for continuous variables and the Chi-square test for categorical variables. Multivariable logistic regression was used to measure the association between case volume and hospitalization outcomes while adjusting for potential confounders, such as age, race, sex, insurance status, Charlson score (31), need for mechanical ventilation, probability of mortality (as determined by the sepsis mortality prediction score), and hospital urban versus rural status. Hospital-level clustering was adjusted for using mixed-effects modeling. Model construction began with sociodemographics and proceeded with the sequential addition of Charlson score, need for mechanical ventilation, the probability of mortality, and hospital location. A variable denoting whether or not severe sepsis was present on admission was included in the model during sensitivity analysis. The mortality prediction score yielded a predicted in-hospital mortality expressed as a probability. For the purposes of the logistic regression, the predicted mortality was divided into risk deciles. Reported odds ratios (ORs) represent the change in odds with each increasing risk decile. To better explore for associations between severity of illness and volume–outcome relationships, the mortality prediction scores were also divided into quartiles and outcomes were compared between IV and HV hospitals for patients inside of each quartile. Hospital classification was compared between the primary cohort and the sensitivity cohort by κ statistic.
All statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC). This study was approved by the Institutional Review Board at the Medical University of South Carolina.
Results
Hospital and Patient Characteristics
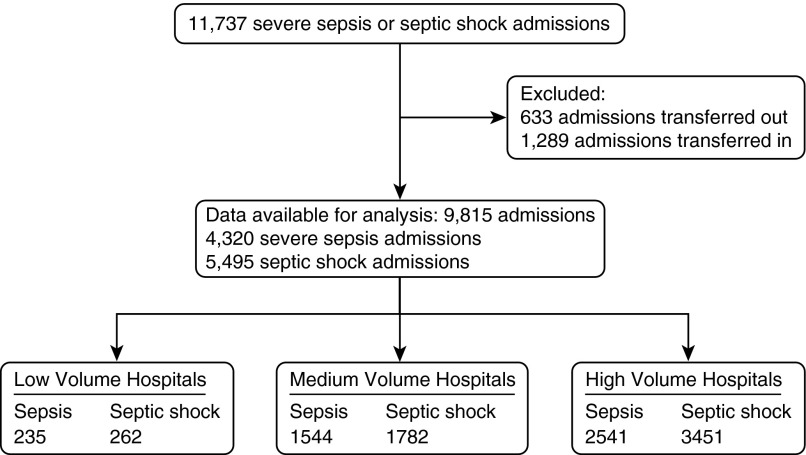
In 2010, there were 11,737 admissions in the state with severe sepsis (995.92) or septic shock (785.52) (Figure 1). After transfer-related exclusions, 9,815 admissions were included in the analyses. The baseline characteristics of the 1,922 admissions that were excluded for interhospital transfer were not significantly different than the characteristics of those included (data not shown).
Figure 1.
Flowchart of admissions included in the final analyses.
Baseline characteristics of hospitals are provided in Table 1. We found a wide distribution of sepsis case volume in each tertile, with the mean volume being 19, 175, and 499 cases/yr in the LV, IV, and HV tertiles, respectively. HV hospitals (50%) were more likely than IV or LV hospitals to be designated as “teaching” hospitals, as defined by the presence of resident physicians.
Table 1.
Baseline characteristics of hospitals stratified by sepsis case volume tertile
| Characteristics |
Low Volume (1–74) |
Intermediate Volume (75–299) |
High Volume (≥300) |
P Value |
|---|---|---|---|---|
| (n = 26) | (n = 19) | (n = 12) | ||
| Mean annual sepsis cases (SD) | 19 (18) | 175 (58) | 499 (134) | P < 0.0001 |
| Teaching hospitals, % | 0 (0) | 2 (11) | 6 (50) | P < 0.0001 |
| Rural location, % | 19 (73) | 8 (42) | 1 (8) | P < 0.0001 |
| Beds Size | P < 0.0001 | |||
| <100, % | 20 (77) | 0 (0) | 0 (0) | |
| 100–299, % | 6 (23) | 14 (74) | 6 (50) | |
| ≥300, % | 0 (0) | 5 (26) | 6 (50) |
Patient characteristics were also significantly different between each volume tertile (Table 2). There were statistically significant differences in age, sex, and race between tertiles such that patients with sepsis at HV hospitals were more likely to be younger, male, and of white race compared with patients at LV hospitals. HV hospital patients were also less likely to have Medicare coverage and more likely to have commercial insurance than patients cared for at LV centers. Patients cared for at LV hospitals required mechanical ventilation less frequently and had a lower predicted mortality than patients at IV or HV hospitals, as determined by the sepsis mortality prediction score. Despite this, there was a trend toward higher unadjusted mortality rates in LV hospitals compared with IV and HV hospitals. Length of stay was significantly longer at HV hospitals compared with LV hospitals.
Table 2.
Patient characteristics stratified by hospital volume tertile
| Characteristics |
Low Volume (1–74) |
Intermediate Volume (75–299) |
High Volume (300 + ) |
P Value |
|---|---|---|---|---|
| (n = 497) | (n = 3,326) | (n = 5,992) | ||
| Mean age (SD) | 71.6 (15.1) | 68.7 (14.6) | 65.7 (17.0) | P < 0.0001 |
| Female, % | 54 | 54 | 31 | P = 0.0053 |
| Race, % | P < 0.0001 | |||
| White | 58 | 64 | 64 | |
| Black | 36 | 35 | 34 | |
| Other | 6 | 1 | 2 | |
| Insurance, % | P < 0.0001 | |||
| Medicare | 70 | 71 | 65 | |
| Medicaid | 11 | 8 | 12 | |
| Commercial | 14 | 15 | 16 | |
| Other | 5 | 6 | 7 | |
| Mean Charlson score (SD) | 1.5 (1.8) | 1.8 (2.0) | 1.6 (1.8) | P = 0.2976 |
| Mechanical ventilation, % | 27 | 36 | 38 | P < 0.0001 |
| Mean mortality probability (SD) | 26.7 (12.6) | 28.8 (13.4) | 28.9 (13.6) | P = 0.0153 |
| In-hospital mortality, % | 32 | 29 | 29 | P = 0.1838 |
| Mean length of stay (SD) | 8.0 (7.3) | 12.7 (14.1) | 14.9 (17.9) | P = 0.0001 |
Case Volume Is Associated with Sepsis Outcomes
After adjustment for relevant covariates, patients with sepsis cared for at LV hospitals had a greater odds of death compared with patients cared for at HV hospitals (OR, 1.56; 95% confidence interval [CI], 1.25–1.94; Table 3). Patients cared for at IV hospitals had no difference in the odds of death compared with those cared for at HV hospitals (OR, 0.99; 95% CI, 0.90–1.09). Age, predicted mortality (risk decile), need for mechanical ventilation, and Charlson score were all associated with mortality in the multivariate model, whereas race, sex, insurance status, and hospital location did not have predictive capabilities.
Table 3.
Adjusted odds of in-hospital mortality during admission with severe sepsis
| Odds Ratio (95% CI) | |
|---|---|
| Low-volume hospital | 1.56 (1.25–1.94) |
| Intermediate volume hospital | 0.99 (0.90–1.09) |
| High-volume hospital (referent) | 1 |
| Need for mechanical ventilation | 2.76 (2.47–3.08) |
| Mortality risk (decile) | 1.31 (1.26–1.36) |
| Charlson score | 1.06 (1.03–1.09) |
| Age, yr | 1.02 (1.01–1.02) |
| Rural hospital location (referent = urban) | 1.05 (0.92–1.19) |
| Male sex | 1.08 (0.98–1.19) |
| Black race | 1.03 (0.93–1.14) |
| Medicaid | 0.95 (0.79–1.13) |
| Commercial insurance | 1.13 (0.99–1.29) |
Definition of abbreviation: CI = confidence interval.
Sensitivity Analysis
A separate severe sepsis cohort was derived using the Angus approach. Using this approach, 57,695 nontransferred cases of severe sepsis were identified from the same dataset, of which 55,779 cases were cared for at HV hospitals (see Table E1 in the online supplement). When the same boundary conditions used in the primary cohort were applied to this cohort, hospitals were classified into the same tertiles with high rates of agreement (LV, κ = 0.71; HV, κ = 0.8). The majority of cases with severe sepsis (68%) were predicted to have a mortality risk in the lowest-risk decile in contrast to the primary cohort, where only 12% of cases were designated in the lowest-risk decile. Although the mean age and Charlson scores were both higher in the Angus cohort for each volume tertile, the overall mortality rate for this cohort was 10.4%. Multivariable logistic regression performed on the Angus cohort confirmed the association between mortality and relevant covariates, such as need for mechanical ventilation, predicted mortality, and age; however, no association between case volume and outcome was identified in this cohort (Table E2).
Adjustment for the presence of severe sepsis on admission did not significantly alter the volume–mortality association. The presence of sepsis on admission was associated with reduced odds of mortality when compared with nosocomial severe sepsis (Table E3).
Severity of Illness Is Not Associated with the Volume–Outcome Relationship
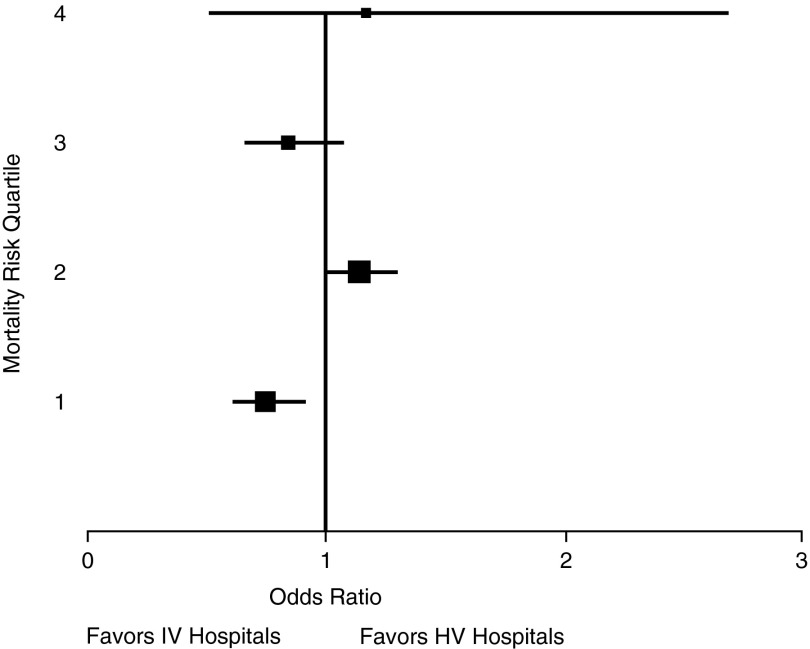
To further investigate whether outcomes at IV and HV hospitals are, indeed, equivalent, adjusted mortality was compared between each group across different strata of predicted mortality. To allow for sufficient sample sizes, the predicted mortality was restratified into risk quartiles. Multivariable logistic regression demonstrated no discernible pattern between predicted mortality and volume–outcome relationships (Figure 2). Odds of survival were slightly better in IV hospitals for patients in the lowest-risk quartile, but worse for patients in the second risk quartile. There was no association between case volume and mortality in those patients with the highest risk of death (quartiles 3 and 4).
Figure 2.
Adjusted odds of in-hospital mortality at intermediate-volume hospitals versus high-volume hospitals across different severity of illness levels. Box size is proportional to sample size in each risk quartile. Whiskers represent 95% confidence interval. HV = high-volume; IV = intermediate-volume.
Discussion
Using an administrative dataset from a broad range of hospital sizes and types, we have confirmed that patients admitted with sepsis to hospitals with low annual sepsis case volumes continue to have increased odds of mortality. In the current study, approximately 5.1% of the study population was cared for exclusively by hospitals with a low sepsis case volume. If this percentage is extrapolated to national sepsis incidence data (7), more than 33,000 people per year may receive care for sepsis at LV hospitals, representing a substantial target for improvement. However, unlike previous work in this area (10–12), our data suggest that, above a certain volume threshold, there is no association between mortality and increasing sepsis case volume.
There are several potential mechanisms that underline the volume–outcome relationship identified in this study and others. First, greater case volumes lead to more clinician experience, which, in turn, may lead to improved outcomes. Second, HV hospitals are, in general, better resourced and, by extension, have better capability to systematically implement best practices. By contrast, smaller, rural hospitals often lack the necessary resources to implement evidence-based interventions, such as early identification and resuscitation protocols or high-intensity physician staffing (32, 33). Thus, case volume may be a surrogate marker for ICU structures and processes, which may impact sepsis mortality risk. Finally, patient characteristics may also contribute to the observed volume–outcome pattern. Patients at smaller, rural hospitals have less access to primary care (34, 35), and, therefore, may have more poorly controlled comorbidities (36). Although adjustment for Charlson score did not mitigate the mortality gap seen at LV hospitals in this study, this score only controls for the presence of a comorbidity, and not for the adequacy of its treatment.
Volume–outcome relationships have previously been identified in trauma and high-risk neonatology (1–3, 6), leading to regionalization strategies to improve outcomes (3, 37–39). The current data suggest, however, that improved outcomes in sepsis are not confined to the highest-volume hospitals alone, as care at IV hospitals was associated with mortality odds similar to care at HV hospitals. This observation suggests that there may be a volume threshold above which additional case volume does not improve outcomes. Such findings would be important to the development of effective and safe regionalization plans for sepsis care, as they would: (1) increase the number of centers qualified to care for severe sepsis, thus decreasing the potential for capacity strain at specialized centers; (2) minimize prehospitalization transit times, thereby potentially avoiding adverse outcomes caused by delay to appropriate antibiotic therapy or early resuscitation (18, 40, 41); and (3) reduce patient and family burden associated with transfer of care to a geographically distant hospital. Due to the logistical challenges of regionalizing sepsis care, however, a more pragmatic approach to improving care at LV hospitals may involve the identification and dissemination of the ICU structures and processes, which are responsible for improved outcomes at larger hospitals. Although this is beyond the capabilities of the current dataset, these results suggest that IV hospitals should be included in future attempts to identify optimal practices.
Our findings differ from those of previous studies, which have either failed to show a volume–outcome relationship in sepsis (9) or have shown that increasing case volume is associated with improved mortality even at very high-volume levels (10–12). There are several possible reasons for the observed differences. First, the hospital types differ between studies. Shahin and colleagues (9) compared U.K. hospitals that participate in the Intensive Care National Audit and Research Centre Case Mix Program. The Case Mix Program provides audit and feedback on ICU outcomes, as well as education and outreach, which may influence U.K. hospitals’ care quality initiatives in a manner that narrows the gap between care delivered at high– and low–case volume hospitals (42). Walkey and Wiener examined the volume–outcome relationship of severe sepsis care at U.S. academic centers (11), which represent less than 8% of all U.S. hospitals (43, 44), and possess several characteristics that may influence volume–outcome relationships, including patient mix, provider experience/availability, and familiarity with/adherence to best practices.
Students and physicians in training play large roles in patient care at these hospitals, and case volume may impact these providers’ skill sets differently than board-certified physicians who provide care at community hospitals. In addition, hospital size may also impact the quality of physicians in training who work at an academic medical center (45, 46). Second, each study uses a different method for risk adjustment. Volume–outcome relationships can be confounded by selective referral practices, mandating the use of risk adjustment during analysis. The U.K. hospitals were analyzed using a clinical risk adjustment tool provided by Intensive Care National Audit and Research Centre, whereas Powell and colleagues (10) controlled only for sociodemographics and comorbidities. The current study and others each used different risk adjustment tools for administrative data (27, 47, 48). As such, differences in unmeasured confounders between the different approaches may also contribute to the disparate findings.
Finally, and perhaps optimistically, our observation, that care at IV hospitals is associated with a similar risk of mortality as care at HV hospitals, may be related to a temporal improvement in severe sepsis care at nontertiary centers. Implementation of best practices may be notoriously slow and is influenced by the characteristics and environment of the adopting organization (49, 50). Despite these limitations, hospitals with fewer resources are still likely to implement best practices over time, particularly if performance measures are publicly reported or tied to financial incentives. As previous studies that have examined volume–outcome relationships in severe sepsis used older data (9, 10, 12), our results may be reflective of more recent trends in severe sepsis care at nontertiary hospitals. However, we are unable to conclude this with certainty.
This study has limitations. Our decision to define the study population using the ICD-9-CM codes for severe sepsis and septic shock was deliberate, as this approach has been shown to have superior specificity and tends to identify a population with a greater severity of illness than approaches using combinations of codes for infection and organ dysfunction (51–53). As we were most interested in examining the relationship between case volume and mortality in patients at a higher risk of death, this choice of cohorts was appropriate for our study. However, this approach has limited sensitivity (25), and some cases of sepsis may not have been included in our analysis. A sensitivity analysis using a previously published, highly inclusive approach yielded a cohort with a very low risk of mortality, suggesting that this cohort was less representative of true severe sepsis. This may explain why a volume–outcome relationship was not seen using this approach and, furthermore, suggests that the explicit diagnosis coding approach used for the primary cohort was a more appropriate choice. Another limitation of this study was the inability to parse out which individual characteristics of IV and HV hospitals were most beneficial to survival in sepsis. For instance, as high-intensity ICU staffing models are more common in larger hospitals (54), it is possible that staffing intensity may contribute to the observed volume–outcome relationship (55). However, as none of the hospitals in the LV tertile meet “fully acceptable” Leapfrog standards for ICU physician staffing (56), we were unable to determine the relative importance of staffing intensity in this cohort of hospitals. Although the results are adjusted for hospital-level clustering, the database was not sufficiently granular to allow for adjustment for ICU-level clustering. These data come from a single U.S. state; therefore, generalizability may also be considered a limitation. Finally, the effect that interhospital transfer between different-sized hospitals has on outcomes in sepsis is not addressed by this study, but is an important area for future investigation.
Conclusions
Hospital case volume is associated with mortality during admission with severe sepsis in a broad mix of hospital types. Care at hospitals with low annual sepsis case volumes is associated with increased mortality, whereas care at hospitals with intermediate annual case volumes is not associated with worse mortality compared with hospitals with high annual case volumes. Future efforts toward the dissemination of best practices to LV hospitals (19), ICU outreach programs using telehealth technology (57), and/or regionalization of sepsis care (21, 22, 58) may all be important strategies to eliminate existing disparities. Important barriers exist, which could limit the effectiveness of each strategy, and, at this time, the optimal approach is not known. Further investigation regarding the merits and pitfalls of each is required before any approach is adopted in a widespread manner.
Footnotes
Supported by Telemedicine and Advanced Technology Research Center, Department of Defense grant W81XWH-10-2-0057 (D.W.F. and K.N.S.) and the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina, National Institutes of Health/National Center for Advancing Translational Sciences grants KL2 TR000060 and UL1 TR000062 (A.J.G.).
Author Contributions: A.J.G., D.W.F., and K.N.S. all provided substantial contributions to the conception and design of the study and the analysis and interpretation of data; A.J.G. authored the manuscript; D.W.F. and K.N.S. provided critical review of the manuscript.
The study sponsors provided no input to study design, data analysis, interpretation of results, or manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1513/AnnalsATS.201406-287OC on June 18, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.MacKenzie EJ, Rivara FP, Jurkovich GJ, Nathens AB, Frey KP, Egleston BL, Salkever DS, Scharfstein DO. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 2.Phibbs CS, Bronstein JM, Buxton E, Phibbs RH. The effects of patient volume and level of care at the hospital of birth on neonatal mortality. JAMA. 1996;276:1054–1059. [PubMed] [Google Scholar]
- 3.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. 2007;356:2165–2175. doi: 10.1056/NEJMsa065029. [DOI] [PubMed] [Google Scholar]
- 4.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 5.Magid DJ, Calonge BN, Rumsfeld JS, Canto JG, Frederick PD, Every NR, Barron HV National Registry of Myocardial Infarction 2 and 3 Investigators. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA. 2000;284:3131–3138. doi: 10.1001/jama.284.24.3131. [DOI] [PubMed] [Google Scholar]
- 6.Nathens AB, Jurkovich GJ, Maier RV, Grossman DC, MacKenzie EJ, Moore M, Rivara FP. Relationship between trauma center volume and outcomes. JAMA. 2001;285:1164–1171. doi: 10.1001/jama.285.9.1164. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Nat Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 9.Shahin J, Harrison DA, Rowan KM. Relation between volume and outcome for patients with severe sepsis in United Kingdom: retrospective cohort study. BMJ. 2012;344:e3394. doi: 10.1136/bmj.e3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell ES, Khare RK, Courtney DM, Feinglass J. Volume of emergency department admissions for sepsis is related to inpatient mortality: results of a nationwide cross-sectional analysis. Crit Care Med. 2010;38:2161–2168. doi: 10.1097/CCM.0b013e3181f3e09c. [DOI] [PubMed] [Google Scholar]
- 11.Walkey AJ, Wiener RS. Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med. 2014;189:548–555. doi: 10.1164/rccm.201311-1967OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaieski DF, Edwards JM, Kallan MJ, Mikkelsen ME, Goyal M, Carr BG. The relationship between hospital volume and mortality in severe sepsis. Am J Respir Crit Care Med. 2014;190:665–674. doi: 10.1164/rccm.201402-0289OC. [DOI] [PubMed] [Google Scholar]
- 13.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 14.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 15.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 16.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 17.Wallace DJ, Angus DC, Barnato AE, Kramer AA, Kahn JM. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012;366:2093–2101. doi: 10.1056/NEJMsa1201918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scales DC, Dainty K, Hales B, Pinto R, Fowler RA, Adhikari NK, Zwarenstein M. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA. 2011;305:363–372. doi: 10.1001/jama.2010.2000. [DOI] [PubMed] [Google Scholar]
- 20.Bach PB, Carson SS, Leff A. Outcomes and resource utilization for patients with prolonged critical illness managed by university-based or community-based subspecialists. Am J Respir Crit Care Med. 1998;158:1410–1415. doi: 10.1164/ajrccm.158.5.9804042. [DOI] [PubMed] [Google Scholar]
- 21.Kahn JM, Asch RJ, Iwashyna TJ, Haynes K, Rubenfeld GD, Angus DC, Asch DA. Physician attitudes toward regionalization of adult critical care: a national survey. Crit Care Med. 2009;37:2149–2154. doi: 10.1097/CCM.0b013e3181a009d0. [DOI] [PubMed] [Google Scholar]
- 22.Singh JM, MacDonald RD. Pro/con debate: do the benefits of regionalized critical care delivery outweigh the risks of interfacility patient transport? Crit Care. 2009;13:219–225. doi: 10.1186/cc7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwashyna TJ, Kahn JM. Regionalization of critical care. In: Scales DC, Rubenfeld GD, editors. The organizaton of critical care. New York: Humana Press; 2014. pp. 217–233. [Google Scholar]
- 24.Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G. National variation in United States sepsis mortality: a descriptive study. Int J Health Geogr. 2010;9:9. doi: 10.1186/1476-072X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, Chen L, Flanders S. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 27.Ford DW, Goodwin AJ, Simpson KN. A sepsis severity scoring tool for use with adminstrative data [abstract] Am J Respir Crit Care Med. 2013;187:A1561. [Google Scholar]
- 28.Lagu T, Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Higgins TL. Development and validation of a model that uses enhanced administrative data to predict mortality in patients with sepsis. Crit Care Med. 2011;39:2425–2430. doi: 10.1097/CCM.0b013e31822572e3. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Keeler EB, Rubenstein LV, Kahn KL, Draper D, Harrison ER, McGinty MJ, Rogers WH, Brook RH. Hospital characteristics and quality of care. JAMA. 1992;268:1709–1714. [PubMed] [Google Scholar]
- 33.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306:45–52. doi: 10.1001/jama.2011.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Professions BoH. Rockville, MD: Health Resources and Services Administration; 1992. Rural health professions facts: Supply and distribution of health professions in rural america. [Google Scholar]
- 35.Rosenblatt RA, Hart LG. Physicians and rural America. West J Med. 2000;173:348–351. doi: 10.1136/ewjm.173.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mainous AG, III, King DE, Garr DR, Pearson WS. Race, rural residence, and control of diabetes and hypertension. Ann Fam Med. 2004;2:563–568. doi: 10.1370/afm.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabbe BJ, Simpson PM, Sutherland AM, Wolfe R, Fitzgerald MC, Judson R, Cameron PA. Improved functional outcomes for major trauma patients in a regionalized, inclusive trauma system. Ann Surg. 2012;255:1009–1015. doi: 10.1097/SLA.0b013e31824c4b91. [DOI] [PubMed] [Google Scholar]
- 38.Sampalis JS, Denis R, Lavoie A, Fréchette P, Boukas S, Nikolis A, Benoit D, Fleiszer D, Brown R, Churchill-Smith M, et al. Trauma care regionalization: a process–outcome evaluation. J Trauma. 1999;46:565–579, discussion 579–581. doi: 10.1097/00005373-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Yeast JD, Poskin M, Stockbauer JW, Shaffer S. Changing patterns in regionalization of perinatal care and the impact on neonatal mortality. Am J Obstet Gynecol. 1998;178:131–135. doi: 10.1016/s0002-9378(98)70639-8. [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 41.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 42.Wallace DJ, Kahn JM. Severe sepsis in the UK and the case volume–outcome association. BMJ. 2012;344:e3494. doi: 10.1136/bmj.e3494. [DOI] [PubMed] [Google Scholar]
- 43.American Hospital AssociationFast facts on US hospitals. [Accessed 2014 May 27]. Available from: http://www.aha.org/research/rc/stat-studies/fast-facts.shtml
- 44.Council of teaching hospitals Association of American Medical Colleges[Accessed 2014 May 27]. Available from: https://members.aamc.org/eweb/dynamicpage.aspx?site=aamc&webcode=aamcorgsearchresult&orgtype=hospital/health%20system
- 45.Koike S, Kodama T, Matsumoto S, Ide H, Yasunaga H, Imamura T. Residency hospital type and career paths in Japan: an analysis of physician registration cohorts. Med Teach. 2010;32:e239–e247. doi: 10.3109/01421591003695311. [DOI] [PubMed] [Google Scholar]
- 46.Kawamura A. Determinant factors of residency faculties in newly graduated medical doctors. Byoin. 2009;68:1005–1009. [Google Scholar]
- 47.UHC risk adjustment methodology for 2011 data base. University HealthSystem Consortium[Accessed 2014 Jun 4]. Available from: https://www.uhc.edu/docs/45015425_2011_05risk_modelsummarymortality_2011models.pdf
- 48.Averill RF, Goldfield N, Steinbeck B, Grant T, Muldoon J, Brough J, Gay J. St. Paul, MN: 3M; 1997. Development of the all patient refined drgs (apr-drgs) [Google Scholar]
- 49.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 50.Berta WB, Baker R. Factors that impact the transfer and retention of best practices for reducing error in hospitals. Health Care Manage Rev. 2004;29:90–97. doi: 10.1097/00004010-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Poulose JT, Cartin-Ceba R, Shoja A, Trillo-Alvarez C, Paul A, Kashyap R, Cabello-Garza J, Poulose L, Shari G, Kojicic M, et al. Comparison of international classification of disease ninth revision (ICD-9) coding with retrospective case review for the diagnosis of septic shock [abstract] Am J Respir Crit Care Med. 2009;179:A4361. [Google Scholar]
- 52.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whittaker SA, Mikkelsen ME, Gaieski DF, Koshy S, Kean C, Fuchs BD. Severe sepsis cohorts derived from claims-based strategies appear to be biased toward a more severely ill patient population. Crit Care Med. 2013;41:945–953. doi: 10.1097/CCM.0b013e31827466f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angus DC, Shorr AF, White A, Dremsizov TT, Schmitz RJ, Kelley MA Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) Critical care delivery in the United States: distribution of services and compliance with Leapfrog recommendations. Crit Care Med. 2006;34:1016–1024. doi: 10.1097/01.CCM.0000206105.05626.15. [DOI] [PubMed] [Google Scholar]
- 55.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 56.The leapfrog group hospital survey results. The Leapfrog Group[Accessed 2014 Jan 15]. Available from: http://www.leapfroggroup.org/cp?frmbmd=cp_listings&find_by=state&city=&state=SC
- 57.Lilly CM, Cody S, Zhao H, Landry K, Baker SP, McIlwaine J, Chandler MW, Irwin RS University of Massachusetts Memorial Critical Care Operations Group. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305:2175–2183. doi: 10.1001/jama.2011.697. [DOI] [PubMed] [Google Scholar]
- 58.Kahn JM, Branas CC, Schwab CWADA, Asch DA. Regionalization of medical critical care: what can we learn from the trauma experience? Crit Care Med. 2008;36:3085–3088. doi: 10.1097/CCM.0b013e31818c37b2. [DOI] [PubMed] [Google Scholar]