Abstract
Rationale: There are limited objective measures of the severity of lung disease before children are able to routinely perform spirometry, generally at age 6 years. Identifying risk factors for reduced lung function at age 6 provides opportunities to intervene and slow the progression of cystic fibrosis (CF) lung disease.
Objectives: To evaluate early childhood predictors of lung function at age 6–7 in a large U.S. CF cohort in the current era of widespread early eradication therapy for Pseudomonas aeruginosa (P. aeruginosa).
Methods: Participants were children with CF enrolled before age 4 in the Early Pseudomonas Infection Control (EPIC) Observational Study, a multicenter, longitudinal study that enrolled P. aeruginosa–negative children not exceeding 12 years of age. Linear regression was used to estimate the association between potential early childhood risk factors and the best FEV1% predicted at age 6–7 years.
Measurements and Main Results: Four hundred and eighty-four children (of 1,797 enrolled in the EPIC Observational Study) met the eligibility criteria for this analysis. Mean (SD) age at enrollment was 2.0 (1.3) years. In a multivariable model adjusted for age at enrollment, the following risk factors were significantly associated with lower mean (95% confidence interval) FEV1% predicted at age 6–7: weight percentile less than 10% during the year of enrollment (–5.3 [–9.1, –1.5]), P. aeruginosa positive during the year of enrollment (–2.8 [–5.7, 0.0]), crackles or wheeze during the year of enrollment (–5.7 [–9.4, –1.9]), mother’s education of high school or less (–4.2 [–7.3, –1.2]), and mother smoked during pregnancy (–4.4 [–8.8, 0.1]).
Conclusions: In this large U.S. cohort, we identified several early childhood risk factors for lower FEV1 at age 6–7 years, most of which are modifiable.
Clinical trial registered with www.clinicaltrials.gov (NCT00097773).
Keywords: cystic fibrosis, lung function, microbiology, tobacco smoke pollution
Children with cystic fibrosis (CF) can begin to develop lung disease within the first few months of life (1). However, there are limited objective measurements available to determine the severity of lung disease before children are reliably able to perform spirometry, generally at age 6 years (2). The ability to identify risk factors for low FEV1% predicted (FEV1 expressed as a percentage of the predicted normal value) at age 6 could provide opportunities to intervene and slow the progression of CF lung disease. Previous studies have identified risk factors for low FEV1% predicted at age 6, including female sex, poor nutritional status, viral infections, persistent infection with Pseudomonas aeruginosa (P. aeruginosa), respiratory symptoms, pulmonary exacerbations, and low socioeconomic status (3–8). It is unclear whether these risk factors remain applicable in the current era, as children with CF born today are more likely to be detected by newborn screening (NBS) and treated more aggressively with antibiotics and CF-specific therapies, and to undergo eradication therapy when P. aeruginosa is isolated from respiratory cultures (9–11).
The Early Pseudomonas Infection Control Observational Study (EPIC OBS) is a multicenter, prospective observational cohort study of risk factors for the acquisition of P. aeruginosa and outcomes associated with P. aeruginosa in young U.S. children with CF enrolled between 2004 and 2006 (12). The study collected data on potentially modifiable risk factors for low FEV1% predicted at age 6–7 that have not previously been evaluated, including daycare attendance, breastfeeding, and environmental tobacco smoke exposure, as well as potentially modifiable risk factors that have been studied previously, for example, nutritional status, infection with P. aeruginosa, respiratory symptoms, and pulmonary exacerbations. The objective of the current analysis was to evaluate early childhood risk factors for low lung function at age 6–7 years in the EPIC OBS cohort. Our hypothesis was that children with lower lung function at age 6–7 would have potentially modifiable risk factors in the first few years of life. Portions of this work have been presented in abstract form.
Methods
Study Participants
The design of the EPIC OBS has been described previously (12). Children with an established diagnosis of CF (13) and not more than 12 years of age were enrolled at 59 accredited U.S. CF care centers between 2004 and 2006. Requirements for inclusion in the current analysis included the following: enrolled in EPIC OBS before age 4 years, no prior lifetime respiratory isolation of P. aeruginosa or P. aeruginosa negative for at least 2 years before enrollment, spirometry measurements reported during at least two clinic visits between age 6 and 7 years, and encounter and family survey data available during the first year enrolled in EPIC OBS. Written informed consent was obtained from the parent(s) or guardian of each participant, and the study was approved by the institutional review board at each participating site.
Data Collection
Study data were collected at each clinical encounter via the Cystic Fibrosis Foundation National Patient Registry (CFFNPR) and study-specific forms (12). CFFNPR data collected at each encounter included results of respiratory cultures obtained as part of routine clinical care, weight and height percentiles, and FEV1% predicted (beginning at age 6). Data collected on study-specific encounter forms included the following: physician report of crackles or wheezes on chest auscultation; and parent report of cough frequency, activity level, days with reduced levels of activity due to cough or shortness of breath, and antibiotic use. Potential risk factors reported by parents on the annual family survey included maternal education, household income, immunizations, any exposure to environmental tobacco smoke, breastfeeding, wood-burning stoves, and swimming pools or hot tubs in the previous year. FEV1 was expressed as a percentage of the predicted value for a healthy reference population based on the equations of Wang and colleagues (14). The data cutoff for the current analysis was December 31, 2013.
Statistical Analysis
The primary end point for all analyses was the single best value of FEV1% predicted at age 6–7 years. Univariate and multivariable linear regression models with robust variance estimates were used to evaluate the primary end point in relation to potential risk factors measured during the year of enrollment in EPIC OBS. Risk factors measured during the year of enrollment included exposures reported on the annual family survey; growth measurements (mean weight, height, or body mass index percentile during the year of enrollment), respiratory culture results, respiratory signs and symptoms, and oral and inhaled antibiotic treatments reported at clinical encounters; and episodes of pulmonary exacerbations treated with intravenous antibiotics and reported in the CFFNPR. The statistically significant predictors (defined as P < 0.05) from initial univariate screening were evaluated in multivariable linear regression models. Risk factors that remained statistically significant in the presence of other risk factors were retained in the final multivariable models. All models were adjusted for age at enrollment in EPIC OBS as a continuous variable. All statistical computations were performed with Stata software (version 12.1, 2011; StataCorp LP, College Station, TX).
Results
Participant Characteristics
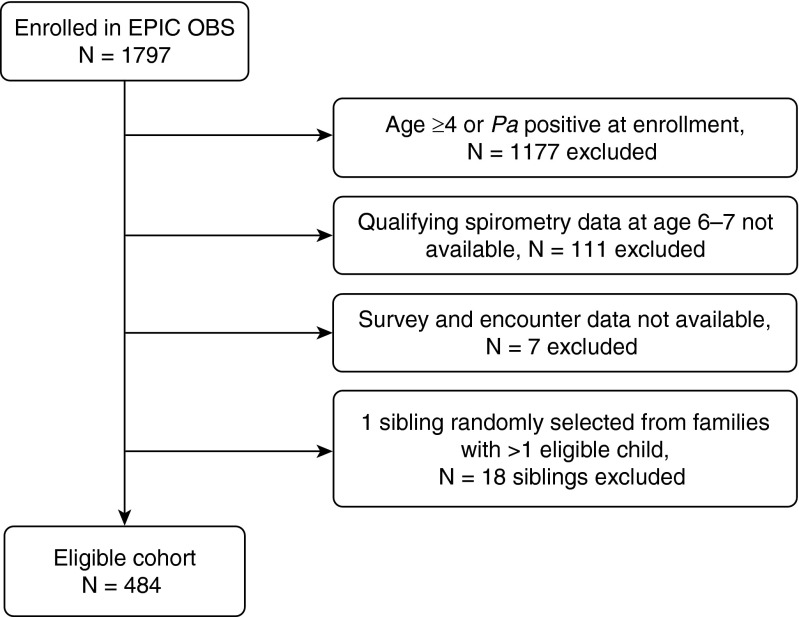
A total of 484 children enrolled in the EPIC OBS met the criteria for inclusion in the current analysis (Figure 1). Eligible children were born between 2001 and 2006 and diagnosed with CF at a mean (SD) age of 3.6 (0.6) months. Mean age at study enrollment was 2.0 (1.3) years and mean observation time in the study before age 6 was 4.0 (1.3) years. The majority of participants were white (95.2%), non-Hispanic (90.1%), and reported using pancreatic enzyme supplements (90.5%). In the enrollment year, mean (SD) percentiles were 40.6 (26.9) for weight and 38.0 (25.6) for height. A small percentage of subjects had poor nutrition (i.e., <10th percentile for age) as measured by weight percentile (14.5%) or height percentile (17.6%).
Figure 1.
Study flow chart of cohort for the current analysis. EPIC OBS = Early Pseudomonas Infection Control Observational Study; Pa = Pseudomonas aeruginosa.
Lung function data at age 6–7 years were available from a mean of 5.9 (1.8) quarterly clinic visits per child. There were 18 children with only two visits; the time range between these two visits was 60 days to 1.4 years. The mean best FEV1% predicted at age 6–7 years was 108.9 (15.3)% predicted. Table 1 describes the mean best FEV1% predicted at age 6–7 by baseline characteristics of the study cohort. Mean best FEV1% predicted at age 6–7 differed significantly by mode of diagnosis (diagnosed by newborn or prenatal screening vs. other presentations), mother’s education level, insurance status (Medicaid vs. non-Medicaid), and weight and height percentile in the enrollment year (<10th percentile vs. ≥10th percentile).
Table 1.
Demographic characteristics of participants and best FEV1% predicted at age 6–7 years
| Risk Factor | Baseline, n (%)* |
Best FEV1% predicted at age 6–7, Mean (SD) |
P Value | |||
|---|---|---|---|---|---|---|
| Participants with Risk Factor | Participants without Risk Factor | Participants with Risk Factor | Participants without Risk Factor | Mean Difference (95% Confidence Interval) | ||
| Age enrolled, 2 to <4 yr | 237 (49.0) | 247 (51.0) | 107.9 (16.0) | 109.9 (14.6) | −1.9 (–4.7, 0.8) | 0.17 |
| Female | 241 (49.8) | 243 (50.2) | 108.1 (15.6) | 109.7 (15.0) | −1.5 (–4.2, 1.2) | 0.28 |
| F508del homozygous† | 254 (52.5) | 219 (45.2) | 110.0 (15.0) | 107.9 (15.6) | 2.1 (–0.7, 4.9) | 0.14 |
| Diagnosed by screening‡ | 168 (34.7) | 300 (62.0) | 111.1 (15.0) | 107.8 (15.6) | 3.2 (0.4, 6.1) | 0.03 |
| Meconium ileus‡ | 127 (26.2) | 341 (70.4) | 109.0 (14.0) | 109.0 (15.9) | −0.0 (–3.0, 2.9) | 0.98 |
| Mother’s education high school or less§ | 133 (27.5) | 345 (71.3) | 105.4 (15.3) | 110.2 (15.1) | −4.8 (–7.9, –1.7) | 0.002 |
| Household income <$40,000§ | 150 (31.0) | 284 (58.7) | 107.1 (16.3) | 109.8 (14.7) | −2.7 (–5.8, 0.4) | 0.09 |
| Medicaid recipient§ | 211 (43.6) | 267 (55.2) | 107.2 (16.2) | 110.1 (14.5) | −2.9 (–5.7, –0.1) | 0.05 |
| Weight < 10th percentile|| | 70 (14.5) | 414 (85.5) | 104.3 (15.5) | 109.7 (15.1) | −5.4 (–9.3, –1.5) | 0.007 |
| Height < 10th percentile|| | 85 (17.6) | 399 (82.4) | 105.2 (17.0) | 109.7 (14.8) | −4.5 (–8.4, –0.6) | 0.02 |
| Body mass index < 10th percentile|| | 8 (3.4) | 229 (96.6) | 105.1 (17.1) | 108.0 (16.0) | −2.9 (–14.3, 8.5) | 0.62 |
All percentages reflect a denominator of 484 participants with the exception of body mass index (which was assessed only in 237 children ≥ 2 yr of age at enrollment). Mean differences and P values reflect simple linear regression models.
Genotype data were missing for 11 participants.
Diagnosis by screening includes prenatal and newborn screening. Data related to diagnosis by screening and meconium ileus were missing for 16 participants.
Education and income reflect family survey data reported during the year of enrollment. Medicaid status reflects data reported in the Cystic Fibrosis Foundation National Patient Registry during the year of enrollment. Items with missing data included education (n = 6), income (n = 50), and Medicaid status (n = 6).
Weight, height, and body mass index percentiles reflect the mean of best quarterly values during the year of enrollment.
In the year of enrollment, most participants reported frequent or productive cough at one or more encounters, but there were few children with decreased activity compared with their peers or activity limited by shortness of breath (Table 2). Despite having no prior lifetime respiratory isolation of P. aeruginosa or being P. aeruginosa negative for at least 2 years before enrollment, 30.8% of subjects had at least one culture positive for P. aeruginosa in the year of enrollment. P. aeruginosa was reported to be mucoid in 2.3% of subjects. The majority of participants received the influenza vaccine in the enrollment year and had at least one course of oral antibiotics recorded. Mean best FEV1% predicted at age 6–7 differed significantly by presence of wheeze or crackles on chest examination or isolation of P. aeruginosa from a respiratory culture at one or more encounters during the enrollment year, intravenous antibiotics prescribed during the enrollment year, and cigarette exposure during the enrollment year or a mother who smoked while pregnant (Table 2).
Table 2.
Risk factors reported at one or more cystic fibrosis clinic visits in the year of enrollment and best FEV1% predicted at age 6–7 years
| Risk Factor | Risk Factor Status as Reported during Baseline Year, n (%)* |
Best FEV1% Predicted at Age 6–7, Mean (SD) |
P Value | |||
|---|---|---|---|---|---|---|
| Participants with Risk Factor | Participants without Risk Factor | Participants with Risk Factor | Participants without Risk Factor | Mean Difference (95% Confidence Interval) | ||
| Frequent or productive cough | 282 (58.3) | 202 (41.7) | 108.4 (16.0) | 109.6 (14.3) | −1.3 (–4.0, 1.5) | 0.36 |
| Less active relative to peers | 73 (15.1) | 393 (81.2) | 108.6 (14.5) | 108.9 (15.4) | −0.3 (–3.9, 3.4) | 0.88 |
| Activity limited by shortness of breath† | 139 (28.7) | 345 (71.3) | 109.0 (15.8) | 108.9 (15.1) | 0.1 (–2.9, 3.2) | 0.93 |
| Wheeze on chest examination | 71 (14.7) | 413 (85.3) | 103.8 (18.7) | 109.8 (14.5) | −5.9 (–10.5, –1.4) | 0.01 |
| Crackles on chest examination | 61 (12.6) | 423 (87.4) | 103.4 (21.2) | 109.7 (14.1) | −6.3 (–11.8, –0.9) | 0.02 |
| Pa-positive culture | 149 (30.8) | 335 (69.2) | 106.5 (16.3) | 110.0 (14.8) | −3.4 (–6.5, –0.4) | 0.03 |
| Methicillin-resistant Staphylococcus aureus–positive culture | 42 (8.7) | 442 (91.3) | 106.0 (16.5) | 109.2 (15.2) | −3.2 (–8.4, 1.9) | 0.22 |
| Oral antibiotics prescribed‡ | 411 (84.9) | 73 (15.1) | 109.1 (15.4) | 107.8 (14.9) | 1.3 (–2.4, 5.0) | 0.49 |
| Inhaled antibiotics prescribed‡ | 146 (30.2) | 338 (69.8) | 108.0 (16.7) | 109.3 (14.7) | −1.3 (–4.4, 1.9) | 0.43 |
| intravenous antibiotics prescribed‡ | 156 (32.2) | 328 (67.8) | 106.0 (16.5) | 110.3 (14.5) | −4.2 (–7.3, –1.2) | 0.006 |
| Dornase alfa prescribed | 225 (46.5) | 259 (53.5) | 109.1 (16.3) | 108.7 (14.4) | 0.4 (–2.3, 3.2) | 0.75 |
| Influenza vaccine received | 408 (84.3) | 67 (13.8) | 109.3 (15.3) | 107.2 (15.6) | 2.0 (–2.0, 6.1) | 0.32 |
| Household member smoked | 159 (32.8) | 323 (66.7) | 107.8 (15.4) | 109.4 (15.3) | −1.6 (–4.6, 1.3) | 0.27 |
| Any smoking exposure | 230 (47.5) | 247 (51.0) | 107.3 (15.0) | 110.6 (15.3) | −3.3 (–6.1, –0.6) | 0.02 |
| Mother smoked while pregnant | 52 (10.7) | 414 (85.5) | 103.3 (15.2) | 109.5 (15.0) | −6.1 (–10.5, –1.8) | 0.006 |
| Wood-burning stove used in home | 44 (9.1) | 437 (90.3) | 107.0 (18.3) | 109.2 (15.0) | −2.3 (–7.8, 3.3) | 0.42 |
| Swimming pool used | 359 (74.2) | 125 (25.8) | 108.4 (15.7) | 110.3 (14.2) | −1.9 (–4.9, 1.1) | 0.21 |
| Hot tub used | 60 (12.4) | 423 (87.4) | 107.9 (13.3) | 109.0 (15.6) | −1.1 (–4.7, 2.6) | 0.56 |
| Others with CF in same home | 77 (15.9) | 406 (83.9) | 106.1 (16.9) | 109.4 (15.0) | −3.3 (–7.3, 0.7) | 0.11 |
| Daycare > 9 h/wk | 221 (45.7) | 253 (52.3) | 107.7 (15.1) | 109.7 (15.4) | −1.9 (–4.7, 0.8) | 0.17 |
| Breastfed§ | 88 (35.6) | 154 (62.3) | 111.2 (13.5) | 109.2 (15.1) | 2.0 (–1.8, 5.7) | 0.30 |
| Palivizumab received§ | 111 (44.9) | 113 (45.7) | 111.6 (13.5) | 108.2 (15.8) | 3.4 (–0.4, 7.3) | 0.08 |
Definition of abbreviations: CF = cystic fibrosis; Pa = Pseudomonas aeruginosa.
Respiratory symptoms, chest examination findings, and associated treatments reflect study-specific encounter data. Culture findings reflect Cystic Fibrosis Foundation National Patient Registry (CFFNPR) culture data. Intravenous antibiotics reflect CFFNPR data on episodes of hospitalization or home intravenous antibiotics for a pulmonary exacerbation. Vaccines, smoking exposures, other environmental exposures, and breastfeeding reflect the most recent family survey collected during the year of enrollment. Participants were considered as having the risk factor if reported at one or more clinic visits or at the most recent family survey during the first year enrolled. All percentages reflect a denominator of 484 participants, with the exception of breastfeeding and palivizumab (which were assessed only in participants < 2 yr of age). Participant numbers (percentages) that sum to less than 484 (100%) indicate that there were missing data. Mean differences and P values reflect simple linear regression models.
Activity limited by at least 1 day/month by shortness of breath or cough.
At least one course reported during the year of enrollment.
Breastfeeding and palivizumab are reported for participants enrolled at age less than 2 years (n = 247). Participant numbers (percentages) that sum to less than 247 (100%) indicate that there were missing data.
Impact of Early Childhood Risk Factors on Lung Function at Age 6–7 Years
In simple regression models adjusted only for age at enrollment, the following risk factors from the enrollment year were significantly associated with mean best FEV1% predicted at ages 6–7 years: mean weight less than 10th percentile, any clinic visit with wheezes or crackles reported on examination, a culture positive for P. aeruginosa, treatment with intravenous antibiotics, maternal education, any smoking exposure, or a mother who smoked during pregnancy. In a multivariable regression model, age at enrollment, poor nutrition, P. aeruginosa–positive respiratory cultures, mother’s education, and having wheezes or crackles reported on examination remained statistically significant; having a mother who smoked during pregnancy was borderline significant and was retained in the final model (Table 3). There were no statistically significant interactions between age at enrollment and any of the other risk factors in the final model.
Table 3.
Multivariable linear regression model of baseline risk factors associated with best FEV1% predicted at age 6–7 years
| Risk Factor* | Mean Difference in Best FEV1% Predicted† | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age at enrollment on study | −1.3 | −2.4, –0.3 | 0.01 |
| Weight percentile < 10% during year of enrollment | −5.3 | −9.1, –1.5 | 0.006 |
| P. aeruginosa positive during year of enrollment | −2.8 | −5.7, 0.0 | 0.05 |
| Crackles or wheeze during year of enrollment | −5.7 | −9.4, –1.9 | 0.003 |
| Mother’s education high school or less | −4.2 | −7.3, –1.2 | 0.007 |
| Mother smoked during pregnancy | −4.4 | −8.8, 0.1 | 0.055 |
The multivariable model was based on observations for 461 participants.
For age at enrollment, the mean difference in best FEV1% predicted reflects the mean difference per 1-year increase in age. For categorical covariates, the mean difference in best FEV1% predicted reflects the mean difference relative to the reference category. Reference categories included weight percentile equal to or greater than 10%, P. aeruginosa negative, no sounds on chest examination, mother’s education beyond high school, and no smoking during pregnancy.
An additional analysis was performed with the 413 participants who had encounter data collected at enrollment and during at least four quarterly clinic visits between enrollment and age 6. Among these participants, the mean (SD) number of quarterly clinic visits was 12.5 (4.9). Oral and inhaled antibiotics were recorded at a mean (SD) of 53.1 (25.1)% and 16.8 (22.2)% of quarterly clinic visits. Neither the percentage of quarterly clinic visits with oral antibiotics nor the percentage of quarterly clinic visits with inhaled antibiotics was statistically significant when added to the multivariable model (data not shown).
Discussion
The EPIC Observational Study is a U.S. national prospective study designed to define risk factors for age at initial acquisition of P. aeruginosa (12). Participants were enrolled between 2004 and 2006, when P. aeruginosa eradication therapy was already in widespread use: in 2006, 97% of the EPIC OBS sites reported prescribing antibiotics for P. aeruginosa acquisition “often” or “always” on the annual site survey of clinical practices. In addition, data were collected on potential risk factors that have not been previously evaluated. Thus, this study provides an important opportunity to evaluate new risk factors and corroborate previously identified risk factors for reduced lung function at early school age in a large, contemporary U.S. cohort. We found that poor nutrition, the presence of abnormal chest sounds, and acquisition of P. aeruginosa in the year of enrollment, as well as mother’s educational status and maternal smoking during pregnancy, were risk factors for low FEV1% predicted at ages 6–7 years. These results may enable clinicians to target young patients at risk for low lung function and address risk factors that (with the exception of mother’s educational status) are potentially modifiable.
Our analysis also supports previous reports that have described associations between low FEV1% predicted at age 6 years and low socioeconomic status (7, 15). Schechter and colleagues used data from the CFFNPR from 1994 and reported that low socioeconomic status, as indicated by being a recipient of Medicaid, was associated with lower FEV1% predicted, including at age 6 years. In our cohort, the relationship between Medicaid status and FEV1% predicted at age 6 years was similar to the relationship between maternal education and FEV1% predicted at age 6 years, although Medicaid status did not reach statistical significance in our multivariable model. The mechanism(s) for this association are unclear, but may involve differences in disease self-management, tobacco smoke exposure, psychological stress, and exposure to environmental pollutants (16–19). Access to care does not appear to explain these associations in the United States, as prescriptions for chronic CF therapies, treatment of pulmonary exacerbations, and rates of hospitalization are not reduced among patients with lower socioeconomic status (20, 21).
We have previously reported that maternal smoking was associated with lower FEV1% predicted in the year of enrollment for EPIC OBS participants (22). Others have described cross-sectional (23–27) and longitudinal associations (18) between lower lung function and second-hand smoke exposure, but the effect of maternal smoking during pregnancy on lung function in children with CF has not to our knowledge been examined elsewhere. The effect of maternal smoking during pregnancy has been shown to be associated with lower lung function in healthy infants and adolescents and more symptoms in young children with asthma (28–30). Our results will need to be corroborated in other cohorts, but encouraging mothers known to be expecting a child with CF, for example, with positive results on prenatal screening, to stop smoking may provide benefit to later lung function.
Our analysis supports previous reports that have described associations between low FEV1% predicted at age 6 years and poor nutrition (4, 8). Using data from the Epidemiologic Study of Cystic Fibrosis (ESCF) from 1994 to 1996, Konstan and colleagues found that children with weight below the fifth percentile at age 3 years had an FEV1% predicted at age 6 years that was a mean 5–16% lower than that of children with weight above the fifth percentile (4). VanDevanter and colleagues used ESCF data from 1994 to 2005 to develop a prediction score for low FEV1% predicted at age 6 years (8). They reported that children with weight below the 10th percentile at a stable clinic visit between ages 2 and 5 were likely to have FEV1% predicted at age 6 years 4–10% lower than for children with weight above the 10th percentile. We found a similar magnitude of association between children with weight below the 10th percentile during the year of enrollment and FEV1% predicted at age 6 years (mean 6% predicted lower than for children with weight above the 10th percentile), even though the mean FEV1% predicted at age 6 years in our cohort (108% predicted) was substantially higher than in either ESCF cohort (97–100% predicted). We did not evaluate the fifth percentile as a cutoff point, as only 31 children had weight below the fifth percentile during the year of enrollment.
Several studies have demonstrated that young age at initial P. aeruginosa acquisition is an important risk factor for the development of more severe CF lung disease (6, 31–33). It is likely that the earlier acquisition of P. aeruginosa allows more time for phenotypes to develop that are associated with pulmonary exacerbations and/or lower FEV1% predicted, for example, mucoidy (34, 35). Our prior primary analysis of the entire EPIC OBS cohort found no modifiable risk factors for P. aeruginosa acquisition (12). In contrast to the findings of the current analysis, Zemanick and colleagues found, among EPIC OBS participants at least 6 years of age, no significant change in the slope of FEV1% predicted after initial acquisition of P. aeruginosa (36). Reasons for the difference in findings from the same overall cohort include the different age ranges of the two analyses, the fact that Zemanick and colleagues evaluated rate of FEV1 decline whereas the current analysis evaluated best value of FEV1% predicted in a 1-year period, and the fact that we had a longer mean observation period (mean, 4.0 vs. 2.6 yr). They did find that acquisition of P. aeruginosa was associated with an increased rate of pulmonary exacerbations and the presence of crackles or wheeze on physical examination, both of which are risk factors for FEV1 decline (37, 38).
Previous analyses have also described associations between FEV1% predicted at age 6 years and the presence of respiratory signs or symptoms (e.g., daily cough or crackles or wheeze on auscultation), the frequency of oral antibiotics, and the frequency of pulmonary exacerbations treated with intravenous antibiotics (4, 5, 8, 39). In our study, the presence of crackles or wheeze on auscultation in the year of enrollment was associated with FEV1% predicted at age 6 years. A retrospective analysis of data from the Australasian Cystic Fibrosis Bronchoalveolar Lavage (ACFBAL) study described an association between the frequency of new respiratory symptoms in the first 2 years of life and lower FEV1 z scores at age 6 years (5). A more recent analysis from the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) cohort did not find an association between respiratory symptoms or the number of days of hospitalization in the first 2 years of life and lung function at ages 4–8 years, although their study had only 56 participants (6). Neither the frequency of treatment with oral or inhaled antibiotics in the enrollment year, or between enrollment and age 6 years, was associated with FEV1% predicted in our study. Unfortunately, we did not collect data on either the indication or duration of antibiotics, so our analysis of antibiotic exposure is somewhat limited.
Our study made use of data collected prospectively as part of EPIC OBS, a large study conducted at multiple U.S. CF centers. None of the other environmental risk factors we were able to investigate (including receiving the influenza vaccine or palivizumab, daycare attendance, breastfeeding, and environmental tobacco smoke exposure) were associated with statistically significant differences in FEV1% predicted. These exposures were all self-reported, and thus open to misclassification. Given our sample size, we were also limited in our ability to compare degrees of exposure (e.g., duration of breastfeeding). This limitation was amplified for the first 2 years of life, because we could assess risk factors only among the subjects enrolled before age 2 years. Limitations of our data include varying degrees of missingness and the potential for misclassification. Given the observational nature of the study, we can describe only associations between environmental exposures and lung function, and cannot determine causality. The generalizability of our results may be limited in that at the time of enrollment in the EPIC OBS, newborn screening for CF was not yet universal in the United States. The diagnosis of CF via NBS allows more opportunities to intervene to avoid malnutrition, but without proper infection control NBS can be a risk factor for infection with P. aeruginosa (40).
In conclusion, we have reported on risk factors for low FEV1% predicted at age 6–7 years. Our results reinforce the importance of delaying acquisition of P. aeruginosa and avoidance of poor nutrition and demonstrate that low socioeconomic status, presence of abnormal chest sounds, and in utero smoke exposure are also associated with low FEV1% predicted at age 6–7 years. Although the ability to modify smoke exposure or low socioeconomic status may be limited, our results may assist clinicians in targeting these young patients who are at risk for low lung function with more aggressive therapy, closer monitoring, and by supporting their families’ empowerment and disease self-management—factors associated with improved outcomes in children with CF (41).
Acknowledgments
Acknowledgment
The authors thank Bruce Marshall, M.D., and the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. In addition, the authors thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry. The authors also thank all the site investigators and research coordinators, as well as all the participants in the EPIC Observational Study and their families.
Footnotes
Supported by Cystic Fibrosis Foundation grants OBSERV04K0 and EPIC0K0 to M.R. (Seattle Children’s Hospital, Seattle, WA); Nemours Children’s Clinic Research Program (Jacksonville, FL); D.B.S. was supported by the Cystic Fibrosis Foundation (SANDERS11A0) and the Institute for Clinical and Translational Research (ICTR) through NIH National Center for Advancing Translational Sciences (NCATS) grants UL1TR000427 and KL2TR000428.
Prior presentation: Portions of this article were presented as an oral and poster presentation at the 2014 North American Cystic Fibrosis Conference in Atlanta, Georgia.
Author Contributions: Conception and design: D.B.S., J.E., M.R.; acquisition of data: J.E.; analysis and interpretation: D.B.S., J.E., C.L.R., M.S.S., W.M., M.R.; drafting the manuscript for important intellectual content: D.B.S., J.E., C.L.R., M.S.S., W.M., R.L.G., M.R.; final approval of the manuscript: D.B.S., J.E., C.L.R., M.S.S., W.M., M.R.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld M, Allen J, Arets BH, Aurora P, Beydon N, Calogero C, Castile RG, Davis SD, Fuchs S, Gappa M, et al. American Thoracic Society Assembly on Pediatrics Working Group on Infant and Preschool Lung Function Testing. An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc. 2013;10:S1–S11. doi: 10.1513/AnnalsATS.201301-017ST. [DOI] [PubMed] [Google Scholar]
- 3.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, Johnson CA, Morgan WJ Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142:624–630. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 5.Byrnes CA, Vidmar S, Cheney JL, Carlin JB, Armstrong DS, Cooper PJ, Grimwood K, Moodie M, Robertson CF, Rosenfeld M, et al. ACFBAL Study Investigators. Prospective evaluation of respiratory exacerbations in children with cystic fibrosis from newborn screening to 5 years of age. Thorax. 2013;68:643–651. doi: 10.1136/thoraxjnl-2012-202342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey KA, Ranganathan S, Park J, Skoric B, Adams AM, Simpson SJ, Robins-Browne RM, Franklin PJ, de Klerk NH, Sly PD, et al. AREST CF. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med. 2014;190:1111–1116. doi: 10.1164/rccm.201407-1277OC. [DOI] [PubMed] [Google Scholar]
- 7.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163:1331–1337. doi: 10.1164/ajrccm.163.6.9912100. [DOI] [PubMed] [Google Scholar]
- 8.VanDevanter DR, Wagener JS, Pasta DJ, Elkin E, Jacobs JR, Morgan WJ, Konstan MW. Pulmonary outcome prediction (POP) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45:1156–1166. doi: 10.1002/ppul.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstan MW, VanDevanter DR, Rasouliyan L, Pasta DJ, Yegin A, Morgan WJ, Wagener JS Scientific Advisory Group and Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Trends in the use of routine therapies in cystic fibrosis: 1995–2005. Pediatr Pulmonol. 2010;45:1167–1172. doi: 10.1002/ppul.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanDevanter DR, Elkin EP, Pasta DJ, Morgan WJ, Konstan MW Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Changing thresholds and incidence of antibiotic treatment of cystic fibrosis pulmonary exacerbations, 1995–2005. J Cyst Fibros. 2013;12:332–337. doi: 10.1016/j.jcf.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Döring G, Flume P, Heijerman H, Elborn JS Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros. 2012;11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld M, Emerson J, McNamara S, Thompson V, Ramsey BW, Morgan W, Gibson RL EPIC Study Group. Risk factors for age at initial Pseudomonas acquisition in the cystic fibrosis epic observational cohort. J Cyst Fibros. 2012;11:446–453. doi: 10.1016/j.jcf.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 15.Quittner AL, Schechter MS, Rasouliyan L, Haselkorn T, Pasta DJ, Wagener JS. Impact of socioeconomic status, race, and ethnicity on quality of life in patients with cystic fibrosis in the United States. Chest. 2010;137:642–650. doi: 10.1378/chest.09-0345. [DOI] [PubMed] [Google Scholar]
- 16.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998;129:406–411. doi: 10.7326/0003-4819-129-5-199809010-00011. [DOI] [PubMed] [Google Scholar]
- 17.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Arch Pediatr Adolesc Med. 2002;156:1094–1100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 18.Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, Naughton K, Jennings J, Ellen J, Cutting GR. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299:417–424. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169:816–821. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 20.Schechter MS, McColley SA, Regelmann W, Millar SJ, Pasta DJ, Wagener JS, Konstan MW, Morgan WJ. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Socioeconomic status and the likelihood of antibiotic treatment for signs and symptoms of pulmonary exacerbation in children with cystic fibrosis. J Pediatr. 2011;159:819–824.e1. doi: 10.1016/j.jpeds.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis; North American Scientific Advisory Group for ESCF. Association of socioeconomic status with the use of chronic therapies and healthcare utilization in children with cystic fibrosis. J Pediatr. 2009;155:634–639.e1–4. doi: 10.1016/j.jpeds.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld M, Emerson J, McNamara S, Joubran K, Retsch-Bogart G, Graff GR, Gutierrez HH, Kanga JF, Lahiri T, Noyes B, et al. EPIC Study Group Participating Clinical Sites. Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis EPIC observational cohort. Pediatr Pulmonol. 2010;45:934–944. doi: 10.1002/ppul.21279. [DOI] [PubMed] [Google Scholar]
- 23.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med. 1990;323:782–788. doi: 10.1056/NEJM199009203231203. [DOI] [PubMed] [Google Scholar]
- 24.Campbell PW, III, Parker RA, Roberts BT, Krishnamani MR, Phillips JA., III Association of poor clinical status and heavy exposure to tobacco smoke in patients with cystic fibrosis who are homozygous for the F508 deletion. J Pediatr. 1992;120:261–264. doi: 10.1016/s0022-3476(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 25.Smyth A, O’Hea U, Williams G, Smyth R, Heaf D. Passive smoking and impaired lung function in cystic fibrosis. Arch Dis Child. 1994;71:353–354. doi: 10.1136/adc.71.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beydon N, Amsallem F, Bellet M, Boulé M, Chaussain M, Denjean A, Matran R, Pin I, Alberti C, Gaultier C. Pulmonary function tests in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:1099–1104. doi: 10.1164/rccm.200205-421OC. [DOI] [PubMed] [Google Scholar]
- 27.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet. 2001;358:1964–1965. doi: 10.1016/s0140-6736(01)06970-7. [DOI] [PubMed] [Google Scholar]
- 28.Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189:401–407. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- 29.Tepper RS, Williams-Nkomo T, Martinez T, Kisling J, Coates C, Daggy J. Parental smoking and airway reactivity in healthy infants. Am J Respir Crit Care Med. 2005;171:78–82. doi: 10.1164/rccm.200406-711OC. [DOI] [PubMed] [Google Scholar]
- 30.Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, Martinez FD. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol. 1999;149:1030–1037. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 31.Pittman JE, Calloway EH, Kiser M, Yeatts J, Davis SD, Drumm ML, Schechter MS, Leigh MW, Emond M, Van Rie A, et al. Age of Pseudomonas aeruginosa acquisition and subsequent severity of cystic fibrosis lung disease. Pediatr Pulmonol. 2011;46:497–504. doi: 10.1002/ppul.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, Green CG, Collins J, Farrell PM. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol. 2001;32:277–287. doi: 10.1002/ppul.2009.abs. [DOI] [PubMed] [Google Scholar]
- 33.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Kosorok MR, Farrell PM, Laxova A, West SE, Green CG, Collins J, Rock MJ, Splaingard ML. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293:581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 35.Mayer-Hamblett N, Rosenfeld M, Gibson RL, Ramsey BW, Kulasekara HD, Retsch-Bogart GZ, Morgan W, Wolter DJ, Pope CE, Houston LS, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med. 2014;190:289–297. doi: 10.1164/rccm.201404-0681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zemanick ET, Emerson J, Thompson V, McNamara S, Morgan W, Gibson RL, Rosenfeld M EPIC Study Group. Clinical outcomes after initial Pseudomonas acquisition in cystic fibrosis. Pediatr Pulmonol. 2015;50:42–48. doi: 10.1002/ppul.23036. [DOI] [PubMed] [Google Scholar]
- 37.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, Stokes D, Wohl M, Wagener J, Regelmann W, Johnson C. Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139, 139.e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46:393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 39.Ren CL, Konstan MW, Rosenfeld M, Pasta DJ, Millar SJ, Morgan WJ Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Early childhood wheezing is associated with lower lung function in cystic fibrosis. Pediatr Pulmonol. 2014;49:745–750. doi: 10.1002/ppul.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, Rock MJ, Splaingard M. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26:81–88. doi: 10.1002/(sici)1099-0496(199808)26:2<81::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.Boyle MP, Sabadosa KA, Quinton HB, Marshall BC, Schechter MS. Key findings of the US Cystic Fibrosis Foundation’s clinical practice benchmarking project. BMJ Qual Saf. 2014;23:i15–i22. doi: 10.1136/bmjqs-2013-002369. [DOI] [PubMed] [Google Scholar]