Abstract
Rationale: Chronic obstructive pulmonary disease is associated with a worse overall survival in non–small cell lung cancer. Lung emphysema is one component of chronic obstructive pulmonary disease. We hypothesized that emphysema of the tumor region may result in larger tumors and a poorer overall survival.
Methods: We evaluated 304 cases of non–small cell lung cancer from a prospectively enrolled cohort. The lung was divided into equal volumetric thirds (upper, middle, or lower region). Emphysema was defined as percentage of low-attenuation areas less than −950 Hounsfield units (%LAA−950) and measured for each region. Whole-lung %LAA−950 was defined as the emphysema score of the entire lung parenchyma, whereas regional %LAA−950 was the score within that particular region (upper, middle, or lower). The emphysema score of the region in which the tumor occurred was defined as the tumor %LAA−950. Tumor diameter was measured while blinded to characteristics of the lung parenchyma. A proportional hazards model was used to control for multiple factors associated with survival.
Measurements and Main Results: Increasing tumor %LAA−950 was associated with larger tumors (P = 0.024). Survival, stratified by stage, was significantly worse in those with tumor %LAA−950 greater than or equal to the 50th percentile versus less than the 50th percentile (P = 0.046). Whole-lung %LAA−950 and regional %LAA−950 (e.g., regional emphysema without tumor occurring in the region) were not significantly associated with survival. There were no differences in presenting symptoms or locations of mediastinal or distant metastasis by emphysema score. Increasing tumor %LAA−950 was associated with an increased risk of death (adjusted hazard ratio, 1.36; confidence interval, 1.09–1.68; P = 0.006) after adjustment for age, sex, smoking status, histology, stage, performance status, chemotherapy, radiation, and surgery. Sensitivity analyses revealed no significant difference in the effect size or test of significance for each of the following conditions: (1) exclusion of cases with central tumor location, (2) exclusion of cases where surgery was performed, (3) exclusion of cases where radiation therapy was performed, (4) exclusion of cases where epidermal growth factor receptor tyrosine kinase inhibitors were administered, and (5) inclusion of only stage IV disease.
Conclusions: Increasing emphysema of the region in which a non–small cell lung cancer tumor occurs is associated with increasing tumor size and worse overall survival.
Keywords: non–small cell lung cancer, emphysema
Chronic obstructive pulmonary disease is associated with a worse overall prognosis in early-stage lung cancer (1). Lung emphysema results in tissue destruction, directly contributing to airflow obstruction and chronic obstructive pulmonary disease. These tissue changes could potentially affect the growth or other characteristics of lung tumors that occur in the region (2).
Recently it was demonstrated that alveolar hypoxia promotes lung tumor growth in a murine model (3). Emphysema is associated with marked ventilation–perfusion mismatch and results in worsening alveolar hypoxia (4, 5). We hypothesized that emphysema in the region (not merely lobe) where a tumor occurs may result in larger non–small cell lung cancer tumors and worse overall survival. To address this hypothesis we performed a detailed regional analysis of tumors and emphysema from computed tomography scans of cases enrolled in a large, prospectively enrolled lung cancer cohort.
Methods
Patients
This study was approved by the Partners Human Research Committee (1999-P-004935/118). Details of enrollment and follow up of this cohort have been described previously (6, 7). Briefly, patients (>18 yr old) with pathologically confirmed, newly diagnosed non–small cell lung cancer were consecutively recruited and followed at Massachusetts General Hospital between 2002 and 2006. More than 85% of eligible patients were enrolled. Detailed demographic, past medical history, and exposure history were collected via a dedicated questionnaire, which included a question regarding emphysema. Specifically, participants were asked if they had ever been diagnosed “by a physician” as having emphysema.
Computed Tomographic Scans
Included cases had to have a chest computed tomographic scan with intravenous contrast performed before initiation of surgery, radiation, or chemotherapy, and within 3 months of enrollment in the study. Cases with lobar collapse or infiltrate, the presence of significant lymphangitic spread, isolated mediastinal disease, or multiple tumors were excluded due to the inability to identify the primary tumor.
Tumors were classified as parenchymal or central. Location was considered parenchymal if it did not involve a lobar (or more proximal) airway and the margin of the tumor was within 2 cm of the costal pleura. This definition was modified from that of Lindell and colleagues to more accurately classify tumors larger than those identified in a screening population (8). Given the lack of a standard definition for central versus parenchymal location, we used a second definition of “surrounded completely by lung parenchyma” to classify a tumor as parenchymal in location.
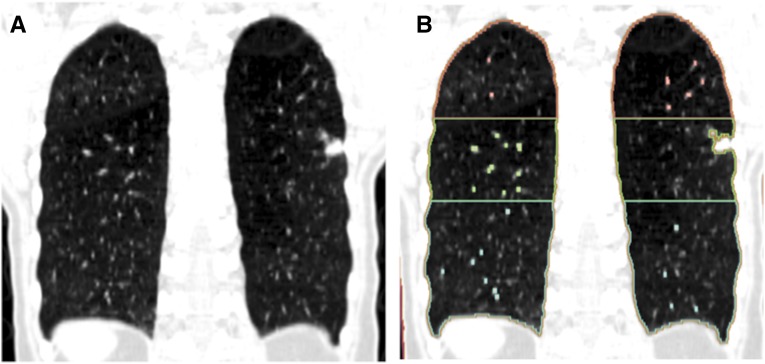
Automated densitometric analysis for measurement of emphysema was performed using Airway Inspector Software (www.airwayinspector.org) as described previously (6, 9–12). Briefly, automated densitometric analysis relies on using the density value acquired within each voxel of the image to segment the lung parenchyma from other structures such as the chest wall, mediastinum, and vasculature. Extraction of the lung parenchyma from the higher-density structures was performed via automated analysis of the density histogram (13). The volume of the remaining area was then measured. Emphysema was defined as the percentage of voxels with attenuation less than −950 Hounsfield units. “Whole-lung %LAA−950” is calculated by applying this measure over the entirety of the lung parenchyma. The volume of the lung was also divided into equal volumetric thirds (upper, middle, lower) in a superior-inferior fashion. “Regional %LAA−950” was calculated as the percentage of voxels with attenuation less than −950 Hounsfield units within a region (upper, middle, or lower). Tumors were objectively given a regional location (upper, middle, or lower) based on the predominant region of the volume of tumor. Emphysema score of the region where the tumor occurred is hereafter referred to as “tumor %LAA−950” (Figure 1).
Figure 1.
(A) Coronal computed tomography image from a non–small cell lung cancer case. (B) Automated densitometric analysis was used to segment out high-attenuation structures (mediastinum, vascular structures, etc.), and the remaining lung regions broken into three equal volumes (upper, middle, and lower). A 1.2-cm lesion is seen in the middle region (although anatomically it exists in the superior segment of the left lower lobe). The tumor percentage of low-attenuation areas less than −950 Hounsfield units (%LAA−950) in this case would be equal to the middle region %LAA−950.
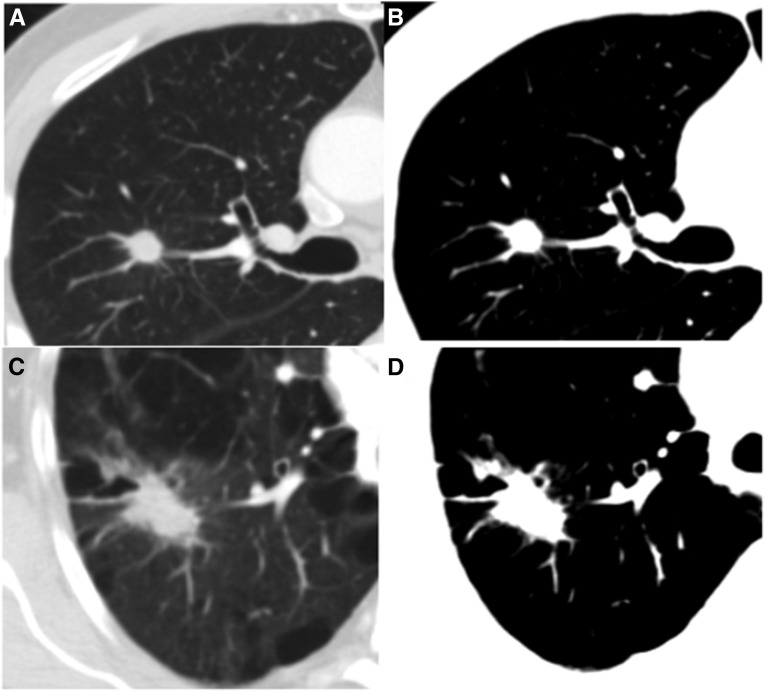
Tumor size was determined by measurement of the largest solid component identified on axial section (3D Slicer; www.slicer.org). Each study was opened into a window set between −680 and 90 Hounsfield units (Figure 2). These settings obscure the parenchyma while still allowing good visualization of the lesion. Tumor diameter was then measured while blinded to the characteristics of the surrounding lung parenchyma.
Figure 2.
Axial computed tomography images from two cases with non–small cell lung cancer. (A) A 1.1-cm lesion in a region of 1.6% low-attenuation areas less than −950 Hounsfield units (%LAA−950). Each case was opened into a “blinded” window setting (B) before measurement of the tumor. (C) A 3.9-cm right upper lobe lesion. The tumor %LAA−950 in this region was 24.6%. The “blinded” window setting (D) effectively obscures even large amounts of emphysema, allowing measurement of tumor diameter in an objective manner.
Lymph nodes were considered to be positive if they were enlarged (≥1 cm in short axis) on computed tomography, were positive on positron emission tomography or positron emission tomography–computed tomography report, or positive based on pathology report from mediastinoscopy or surgical lymph node sampling. Lymph node sites were classified according to the seventh edition of tumor-node-metastasis (14). Distant metastatic sites were recorded as documented in the chart or by imaging.
Statistical Analysis
All statistical analyses were performed using STATA (Version 12; College Station, TX). P values less than or equal to 0.05 were considered significant, and all statistical tests were two-sided. t tests for comparison of %LAA−950 were performed after log transformation. For time-to-event analyses, the results of the Wilcoxon (Breslow) test for equality of the survivor function were performed to minimize the effect of the small risk sets in the tails of the survival distributions. The stratified log rank test was used to compare dichotomized tumor %LAA−950 among strata of stage.
Tumor %LAA−950 was included as a continuous variable for linear regression for tumor size. The validity of linear regression models was tested via assessment of the distribution of the kernel density plot of the residuals for normality. A Cox proportional hazards model was created and includes tumor %LAA−950 (continuous), age, sex, smoking status, histology, stage, performance status, chemotherapy, radiation, and surgery. Sensitivity analyses were performed by running the base model while limiting the model to particular groups. Specifically, each of the following limitations was placed on the proportional hazards model and the analysis performed again: (1) exclusion of cases with central tumor location, (2) exclusion of cases where surgery was performed, (3) exclusion of cases where radiation therapy was performed, (4) exclusion of cases where epidermal growth factor receptor tyrosine kinase inhibitors were administered, and (5) inclusion of only stage IV disease. The proportional hazards assumption was tested via the non-zero slope method of Grambsch and Therneau (15).
Results
Cohort
There were a total of 236 analyzed cases. The demographics of the cohort are shown in Table 1. Sixty-eight cases were excluded. There were no significant differences in demographic data between those with computed tomographic scans that were analyzed and those that were excluded. The lobar distribution of tumors by percentage was: right upper, 30.7%; right middle, 5.1%; right lower, 19.6%; left upper, 31.1%; and left lower, 13.6%.
Table 1.
Characteristics of the cohort
| Characteristic | Distribution/ Frequency | N |
|---|---|---|
| Age, yr |
66.1 ± 11.0 |
236 |
| Sex, n (%) | ||
| Female | 124 (52.5) | |
| Male | 112 (47.5) | |
| Smoking, n (%) | ||
| Never | 28 (11.9) | |
| Former | 132 (55.9) | |
| Current | 76 (33.2) | 236 |
| Histology, n (%) | ||
| Adenocarcinoma | 131 (55.5) | |
| Squamous cell | 30 (12.7) | |
| Large cell | 14 (5.9) | |
| Non–small cell lung cancer (not otherwise specified/mixed) | 61 (25.9) | 236 |
| Tumor size | 3.2 ± 1.6 | 236 |
| Tumor location, n (%) | ||
| Central | 78 (33.1) | |
| Parenchymal | 158 (66.9) | 236 |
| Stage, n (%) | ||
| I | 49 (20.7) | |
| II | 9 (3.8) | |
| III | 66 (28.0) | |
| VI | 112 (47.5) | 236 |
| Eastern Cooperative Oncology Group performance, n (%) | ||
| Status 0–2 | 225 (96.5) | |
| Status 3–4 | 8 (3.5) | 236 |
| Therapy, n (%) | ||
| Surgery | 101 (42.8) | 236 |
| Chemotherapy | 177 (75.0) | 236 |
| Radiation | 73 (31.0) | 236 |
| Epidermal growth factor receptor tyrosine kinase inhibitors* | 48 (20.3) | (131) |
Data presented as n (%) or mean ± SD.
Epidermal growth factor receptor tyrosine kinase inhibitors prescribed as salvage therapy for adenocarcinoma (without genetic testing) during this time.
Emphysema Scores
All computed tomographic scans were acquired with GE scanners (GE Healthcare, Waukesha, WI). Approximately half (50.3%) were obtained at a 5-mm slice thickness, with an additional 41% obtained at a 2.5-mm slice thickness. Sixty-seven percent of individuals who self-reported having “emphysema” had whole-lung quantitative emphysema (whole-lung %LAA−950) scores in the top quartile (P < 0.001). The median whole-lung %LAA−950 with range was 0.5% (0.001–53.8%). Based on reference equations to predict normal lung attenuation, 56% of cases in this cohort have whole-lung %LAA−950 greater than the predicted score (16).
To assess if the tumor itself may exert local effects on the %LAA−950 score, we compared each regional %LAA−950 score with the same region when cases with tumor in that region were excluded. For instance, the upper region %LAA−950 was compared between cases with tumor in that region versus those without tumor. There were no differences in regional %LAA−950 between cases with and without tumor in any given region.
Tumor Diameter
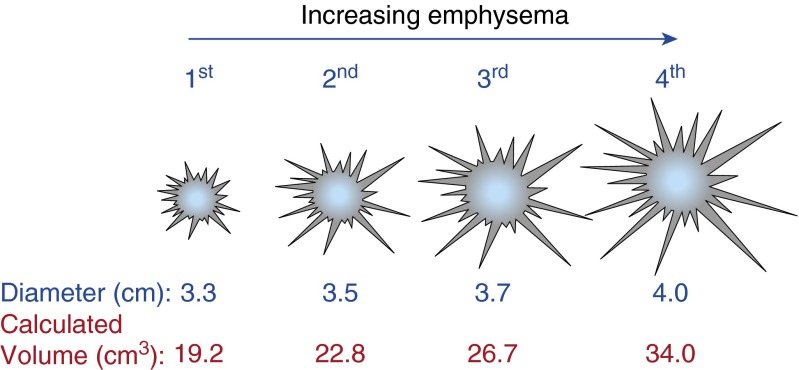
Increasing tumor %LAA−950 approached statistical significance (P = 0.058) as a predictor of greater tumor diameter via linear regression. Limiting the analysis to tumors occurring in a parenchymal location (modified Lindell definition), tumor %LAA−950 was significantly associated with increasing tumor diameter (P = 0.024). We also evaluated this relationship after defining parenchymal location as tumor “not completely surrounded by lung parenchyma.” Tumor %LAA−950 was also a significant predictor of tumor diameter when including cases completely surrounded by lung parenchyma (P = 0.017). Adjusting for differences in computed tomography slice thickness did not significantly alter the relationship of increasing emphysema associated with increasing tumor diameter (P = 0.020). Tumor diameter was treated as a continuous variable for statistical analysis, but for the purposes of illustrating the size difference it has been broken into quartiles in Figure 3. Tumors in the upper quartile are on average 21% larger versus those in the lowest quartile of tumor %LAA−950. None of the regional %LAA−950 scores alone (when tumor was not present in that region) were statistically significant predictors of tumor size. Thus, emphysema in nontumor regions was not predictive of larger tumors.
Figure 3.
Illustration of the increase in tumor size by quartile of tumor percentage of low-attenuation areas less than −950 Hounsfield units. Tumors in the upper quartile are 21% larger by diameter (P = 0.024, linear regression for the continuous variable) than those in the lowest quartile. Assuming a spherical configuration, these tumors would be 44% larger by calculated volume.
Survival Estimates
Presenting symptoms or asymptomatic detection (via chest X-ray or computed tomographic scan) were documented to assess for possible identification bias (see Table E1 in the online supplement). There was no significant difference in the distribution of presenting symptoms between subjects with tumor %LAA−950 less than the 50th percentile and greater than or equal to the 50th percentile (dichotomized). There was also no difference in tumor %LAA−950 at any lymph node station or metastatic site (Table E2).
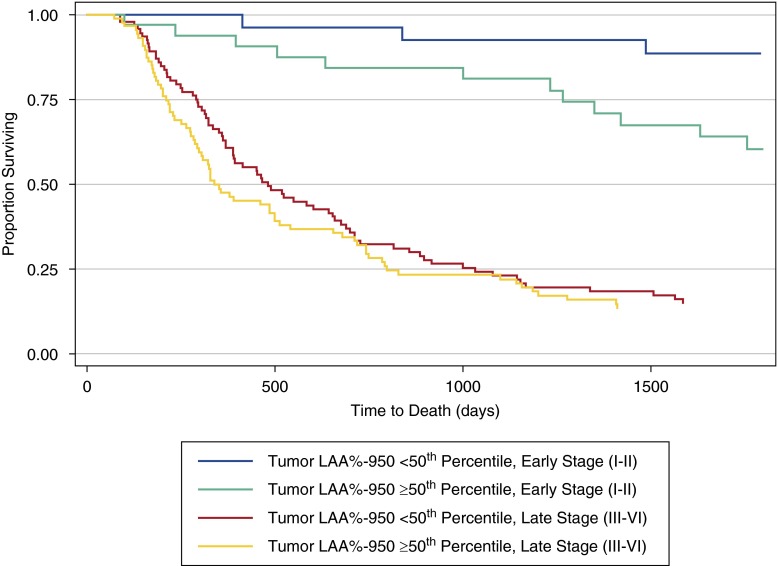
Mean follow-up time was 1,051 (±958) days. Initial unadjusted analysis was performed through time-to-event analysis using dichotomized emphysema scores. Given the dramatic differences in survival by stage for non–small cell lung cancer, Kaplan-Meier curves are presented stratified by early-stage (I or II) and late-stage (III–VI) disease. There was a greater overall survival for subjects with a tumor %LAA−950 score less than the 50th percentile, stratified by stage (Figure 4, P = 0.046). This remained significant regardless of whether all cases were included (above, P = 0.046) or limited to a parenchymal tumor location (P = 0.048).
Figure 4.
Kaplan-Meier survival curves by dichotomized tumor emphysema score (tumor percentage of low-attenuation areas less than −950 Hounsfield units [%LAA−950]), stratified by early- or late-stage disease. Emphysema scores of the region of the tumor greater than or equal to the 50th percentile were associated with a worse overall prognosis than tumors that occurred in regions of lower emphysema (<50th percentile) among both early- and late-stage non–small cell lung cancer (stratified log rank, P = 0.046). Greater emphysema was associated with a poorer prognosis in both strata of non–small cell lung cancer.
Proportional Hazards Model
In univariate analysis, tumor %LAA−950 for parenchymal lesions (regardless of which definition was used) was associated with a worse overall survival (modified Lindell definition, hazard ratio [HR], 1.30; confidence interval [CI], 1.01–1.68; P = 0.038). Thus, for a 10% increase in tumor %LAA−950 there is a 30% increase in the hazard of death. Regional %LAA−950 for any specific region was not significantly associated with survival when excluding cases with tumor that occurred within that region (e.g., upper lung region emphysema score is not a significant predictor of death when a tumor is not present in that region). Increasing tumor diameter is a component of the tumor-node-metastasis staging system and associated with a worse overall survival (17). As expected, increasing tumor diameter was significantly associated with an increased hazard of death (HR, 1.01; CI, 1.01–1.02 for every 1-mm increase in tumor diameter; P < 0.001).
To account for multiple possible confounders, a Cox proportional hazards model was constructed. Age, sex, smoking status, histology, performance score, parenchymal tumor location, stage, radiation therapy, chemotherapy, and surgery were included as part of the model with regional tumor emphysema (Table 2). Tumor %LAA−950 remained a statistically significant predictor of overall survival when included as part of this model (adjusted HR [HRadj], 1.36; CI, 1.09–1.68; P = 0.006). This HR is nearly identical to the unadjusted estimate. Tumor diameter is accounted for in the model, as it is included in the stage classification. Additional adjustment of the Cox proportional hazards model for computed tomography slice thickness did not result in alteration of the result (HRadj = 1.37; 1.10–1.71; P = 0.005)
Table 2.
Base proportional hazards model for risk of death
| Adjusted Estimates |
|||
|---|---|---|---|
| HRadj | Confidence Interval | P Value | |
| Tumor %LAA−950* | 1.36 | 1.09–1.68 | 0.006 |
| Age | 1.02 | 1.00–1.03 | 0.03 |
| Sex | |||
| Male | 1.33 | 1.05–2.15 | 0.03 |
| Female | Ref | Ref | |
| Smoking | |||
| Never | Ref | Ref | |
| Former | 0.96 | 0.57–1.64 | 0.89 |
| Current | 0.89 | 0.51–1.53 | 0.67 |
| Histology | |||
| Adenocarcinoma | 1.16 | 0.76–1.78 | 0.48 |
| Squamous cell | 0.97 | 0.53–1.78 | 0.93 |
| Large cell | 0.72 | 0.34–1.50 | 0.39 |
| Non–small cell lung cancer (not otherwise specified/mixed) | Ref | Ref | |
| Eastern Cooperative Oncology Group performance | |||
| Status 0–2 | Ref | Ref | |
| Status 3–4 | 1.53 | 0.73–3.2 | 0.26 |
| Tumor location | |||
| Central | 1.21 | 0.86–1.70 | 0.27 |
| Parenchymal | Ref | Ref | |
| Stage | |||
| I | Ref | Ref | |
| II | 1.75 | 0.47–6.59 | 0.41 |
| III | 6.45 | 2.52–16.5 | <0.000 |
| IV | 11.9 | 4.91–28.8 | <0.000 |
| Radiation | 0.93 | 0.54–1.57 | 0.77 |
| Chemotherapy | 0.49 | 0.24–1.03 | 0.06 |
| Surgery | 0.67 | 0.44–1.04 | 0.07 |
Definition of abbreviations: %LAA−950 = percentage of low-attenuation areas less than −950 Hounsfield units; HRadj = adjusted hazard ratio.
For a 10% change in tumor %LAA−950.
We performed several sensitivity analyses to assess for potential bias (Table 3). First, we evaluated for a possible bias induced by our definition of central versus parenchymal location. There was no effect on the hazard of tumor emphysema by omitting the covariate for central location from the model (HRadj, 1.33; CI, 1.08–1.66; P = 0.008). We also performed a sensitivity analysis by excluding all cases with a central tumor (modified Lindell definition). Omission of these cases did not alter the HR for tumor %LAA−950 (HRadj, 1.34; CI, 1.08–1.65; P = 0.008; Table 4). Second, we assessed for the effect of surgery on the model. Excluding patients who underwent curative intent, surgical resection did not alter our results (HRadj, 1.30; CI, 1.01–1.68; P = 0.040). Third, excluding all patients who underwent radiation therapy did not significantly alter the result. During the study period, tyrosine kinase inhibitorss directed at the epidermal growth factor receptor were prescribed as salvage therapy for adenocarcinomas. Excluding cases where epidermal growth factor receptor tyrosine kinase inhibitors were prescribed did not significantly change the HRadj for tumor emphysema score (HRadj, 1.38; CI, 1.06–1.64; P = 0.015). Finally, to ensure that there was not an effect secondary to staging procedures or practices, we limited the evaluation to those with stage IV disease. Excluding earlier stages had no significant effect on the increased hazard of death associated with increasing tumor %LAA−950.
Table 3.
Sensitivity analysis for hazard of death associated with increasing tumor emphysema (tumor %LAA−950)
| Estimate for Tumor %LAA−950 |
|||
|---|---|---|---|
| HRadj | Confidence Interval | P Value | |
| Base model (Table 2)* | 1.36 | 1.09–1.68 | 0.006 |
| Excluding patients with central tumors | 1.34 | 1.08–1.65 | 0.008 |
| Excluding patients who underwent surgery† | 1.30 | 1.01–1.68 | 0.040 |
| Excluding patients who underwent radiation | 1.37 | 1.09–1.72 | 0.006 |
| Excluding patients who received epidermal growth factor receptor tyrosine kinase inhibitors | 1.32 | 1.06–1.64 | 0.015 |
| Limited to stage IV disease | 1.38 | 1.09–1.75 | 0.008 |
Definition of abbreviations: %LAA−950 = percentage of low-attenuation areas less than −950 Hounsfield units; HRadj = adjusted hazard ratio.
For a 10% change in tumor %LAA−950.
Surgery in stage I–II disease was excluded. The benefit of surgery in stage III disease is debated.
Discussion
In this investigation we found that tumors occurring in regions of greater emphysema (as measured by %LAA−950) are associated with a worse overall survival than tumors occurring in regions of less emphysema. This epidemiologic association is further bolstered by the discovery that larger tumors are found in regions of greater emphysema versus less emphysema.
Among the many studies of the relationship of lung cancer and emphysema, this is the first to provide data supporting a biological relationship. In this cohort, the difference in tumor size by region of emphysema is significant. The mean difference in tumor diameter between the lower and upper quartiles of emphysema was 0.7 cm (21%). Tumor size is a strong predictor of non–small cell lung cancer survival and adds an important biologic link to the described association between emphysema of the tumor region and survival (17). The magnitude of effect of this latter relationship is also nontrivial, with a 10% increase in emphysema resulting in an estimated 30% increase in the HR for death.
We chose to use automated densitometric analysis to alleviate bias associated with visual scoring systems. We also set the image window settings to obscure the nature of the lung parenchyma around the lesion and allow measurement of tumor diameter in a blinded fashion. The use of regional quantitative emphysema scores (e.g., in a superior-inferior and not lobar fashion) has been well validated from multiple analyses, including from the National Emphysema Treatment Trial (16, 18–20). In addition to eligibility criteria for lung volume reduction, regional differences (apical-basal) are used to phenotype α1-antitrypsin deficiency (21). Apical-basal distribution of smoking-related emphysema has also proven to be a robust phenotype, and less susceptible to artifact than lobar differences (16). Importantly, histologies of non–small cell lung cancer seem to occur preferentially based on lung regions, not lobes (6).
The differences in emphysema scores between regions that were reported here are similar to that seen in the National Emphysema Treatment Trial cohort. Benchmarking emphysema scores (%LAA−950) against normal values adjusted from the Multiethnic Study of Atherosclerosis (MESA) equations revealed that 56% of the whole-lung %LAA−950 measurements in this cohort were above the predicted normal values (16). This provided the basis for our initial evaluation of tumor emphysema by dichotomizing at the 50th percentile.
The role of emphysema as a risk factor for lung cancer occurrence has been extensively studied. Although associated with an increased risk of lung cancer in some studies, the overall role of emphysema as a risk factor for lung cancer is still under investigation (22–29). However, risk factors for incident lung cancer and those related to prognosis are not necessarily the same (e.g., radon exposure).
Only a few studies have evaluated the relationship between emphysema and prognosis in non–small cell lung cancer (30–32). Two of these investigations reported a significant association between emphysema and non–small cell lung cancer prognosis but included only patients undergoing surgical resection for early-stage disease (30, 32). Emphysema dramatically affects lung function and may determine surgical candidacy, the therapy most consistently associated with survival in non–small cell lung cancer, as well as rates of surgical complication (33, 34).
The third study retrospectively evaluated survival of patients with all stages of lung cancer at 2 years (31). Emphysema, in the absence of cancer, is a significant competing risk for death and may be a particularly important contributor in the setting of short follow-up times. In patients without lung cancer, emphysema is associated with an increase in the relative risk of death at 5 years of approximately 30% (between the highest and lowest tertile of emphysema) (19). Thus, emphysema may be associated with differences in non–small cell lung cancer survival even in the absence of a true pathophysiologic relationship with the tumor.
This report is the first to evaluate the relationship of emphysema and non–small cell lung cancer prognosis in a cohort with prolonged follow-up time that included all stages of lung cancer. This cohort has the additional advantage of being prospectively enrolled and one of the largest studied to date. We evaluated the effect of emphysema in the region of a tumor (e.g., tumor LAA%-950) to assess for an association between local emphysema and the tumor. Defining regions to evaluate emphysema allowed us to use regional emphysema scores (in the absence of tumor) as a comparator group to emphysema scores with a tumor in the region and eliminate a survival difference associated with the presence of emphysema alone.
Importantly, increasing emphysema of the tumor region was associated with a significantly worse overall survival, whereas regional emphysema score (in the absence of tumor in that region) was not associated with significant differences in survival. Regional emphysema was also associated with larger tumors, further bolstering the epidemiologic association.
One possible explanation for the findings of larger tumors in regions of emphysema is that the tumor itself is exerting a force on the surrounding lung parenchyma resulting in an artificially high emphysema measurement. There are several reasons that this would be a less likely explanation. First, there were no significant differences between regional emphysema scores, with and without the tumor. If the measure was affected by the presence of the tumor, the tumor emphysema scores (tumor %LAA−950) should generally be larger than regional (regional %LAA−950) emphysema scores (e.g., regional emphysema scores in the absence of a tumor). This was not the case. Second, the measurement of regional tumor emphysema encompasses a total volume of the third of the lungs, and would be unlikely to be significantly affected by distention of alveoli over a small area at the tumor border. Third, tumor growth would generally be predicted to compress the lung resulting in crowding of the parenchyma, driving the emphysema score down and biasing toward the null hypothesis.
We took additional steps to assess for the effects of bias and residual confounding. We first evaluated the effect of the definition of central tumor. This definition was used during the univariate analysis to assess for tumors that were more likely to have initiated in the lung parenchyma versus a central airway. We used two separate definitions of “central” while evaluating the relationship between tumor size and regional tumor emphysema and removed the variable completely from the multivariate proportional hazards model. In neither case did this alter the result. We also used sensitivity analysis to exclude cases that underwent surgery or radiation therapy or received epidermal growth factor receptor–targeted tyrosine kinase inhibitors. In no case did the association between increasing regional tumor emphysema and worse overall survival change.
To have appropriate follow-up time, we evaluated a cohort enrolled before the updated tumor-node-metastasis system and the most recent staging guidelines for non–small cell lung cancer (17, 35). Clinical staging (e.g., positron emission tomography scanning) was performed as indicated by recommendations at the time of enrollment (36). To further evaluate for possible confounding related to the staging evaluation, we captured lymph node and distant metastatic locations from computed tomographic scans, positron emission tomography–computed tomography reports, pathology reports, and the chart, and classified these based on the current lung cancer staging recommendations (14, 35). There were no differences in tumor emphysema scores by location of lymph node or distant metastases. To further rule out a staging effect we also limited the proportional hazards model to cases with stage IV disease. Increasing emphysema of the tumor region continued to be associated with an increased HR for death.
The finding of larger tumors associated with regions of emphysema is an important link in explaining the worse overall survival associated with tumors occurring in emphysematous regions. Increasing tumor diameter is a component of the tumor-node-metastasis staging system and is a well-established variable associated with a poorer prognosis (17).
There is biological plausibility for the finding of larger tumors in emphysematous regions. In mice, alveolar hypoxia results in preferential stabilization of hypoxia-inducible factor-2α; increased expression of vascular endothelial growth factor-A, fibroblast growth factor 2, and their receptors; and larger tumors (3). In human non–small cell lung cancer tumors, hypoxia-inducible factor-2α overexpression is associated with a worse overall prognosis (37). Vascular endothelial growth factor polymorphisms have also been associated with differential survival in early-stage non–small cell lung cancer (7).
Emphysematous regions may also provide an environment that results in more genetically heterogeneous and unstable tumors, leading to the development of larger and/or more aggressive tumor phenotypes. Emphysema is an inflammatory process, postulated to result in production of reactive oxygen species, DNA adduct formation, and increasing genetic mutation (38). In some cases, increasing genetic instability has been associated with a worse overall prognosis (39). We believe that the finding of larger tumors associated with emphysematous regions provides groundwork for further translational research.
This study has several limitations. We used data from a prospectively enrolled cohort of patients presenting with suspected or newly diagnosed lung cancer. Survival analysis in this setting could be prone to identification bias. However, we captured presenting symptoms (or asymptomatic detection by chest X-ray or computed tomography) and found no differences related to emphysema. There was no control group without lung cancer, so we cannot comment specifically on the risk of developing lung cancer related to emphysema. This is also a single-center study. However, the distribution of histology and anatomic location of tumors among the lung is nearly identical to that noted from more than 200,000 cases evaluated in the Surveillance, Epidemiology, and End Results (SEER) database, so it would be expected to be representative (40). Computed tomographic scans obtained in this cohort were not specifically protocoled for research purposes. However, computed tomographic scans from the National Emphysema Treatment Trial cohort have greater variation in slice thickness than that seen here and have been extensively analyzed for emphysema using quantitative densitometry (18, 41). We also adjusted for slice thickness, which did not alter our results.
In this report we demonstrated an association of regional tumor emphysema with larger tumors and a poorer overall survival in non–small cell lung cancer. Further studies may clarify the biological mechanisms that underlie this relationship.
Footnotes
Supported by grants RO1 CA092824 (D.C.C.), RO1 CA074386 (D.C.C.), RO1 CA090578 (D.C.C.), K25 HL104085 (R.S.J.E.), and R01HL116931 (R.S.J.E.).
Author Contributions: All authors have contributed in the three required areas, including substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; drafting the submitted article or revising it critically for important intellectual content; and providing final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145:346–353. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 3.Karoor V, Le M, Merrick D, Fagan KA, Dempsey EC, Miller YE. Alveolar hypoxia promotes murine lung tumor growth through a VEGFR-2/EGFR-dependent mechanism. Cancer Prev Res (Phila) 2012;5:1061–1071. doi: 10.1158/1940-6207.CAPR-12-0069-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finley TN. The determination of uneven pulmonary blood flow from the arterial oxygen tension during nitrogen washout. J Clin Invest. 1961;40:1727–1734. doi: 10.1172/JCI104395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsey CM, Estépar RSJ, Zhao Y, Yu X, Diao N, Heist RS, Wain JC, Mark EJ, Washko G, Christiani DC. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer. 2014;84:145–150. doi: 10.1016/j.lungcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heist RS, Zhai R, Liu G, Zhou W, Lin X, Su L, Asomaning K, Lynch TJ, Wain JC, Christiani DC. VEGF polymorphisms and survival in early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:856–862. doi: 10.1200/JCO.2007.13.5947. [DOI] [PubMed] [Google Scholar]
- 8.Lindell RM, Hartman TE, Swensen SJ, Jett JR, Midthun DE, Tazelaar HD, Mandrekar JN. Five-year lung cancer screening experience: CT appearance, growth rate, location, and histologic features of 61 lung cancers. Radiology. 2007;242:555–562. doi: 10.1148/radiol.2422052090. [DOI] [PubMed] [Google Scholar]
- 9.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RSJ, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz AA, Valim C, Yamashiro T, Estépar RSJ, Ross JC, Matsuoka S, Bartholmai B, Hatabu H, Silverman EK, Washko GR. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138:880–887. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz AA, Come CE, Ross JC, San José Estépar R, Han MK, Loring SH, Silverman EK, Washko GR COPDGene Investigators. Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest. 2012;141:736–744. doi: 10.1378/chest.11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK COPDGene Investigators. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest. 1988;94:782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 14.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 15.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 16.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11:898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 18.DeMeo DL, Hersh CP, Hoffman EA, Litonjua AA, Lazarus R, Sparrow D, Benditt JO, Criner G, Make B, Martinez FJ, et al. Genetic determinants of emphysema distribution in the national emphysema treatment trial. Am J Respir Crit Care Med. 2007;176:42–48. doi: 10.1164/rccm.200612-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, Dirksen A, Omenaas E, Gulsvik A, Bakke P. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 20.Gurney JW, Jones KK, Robbins RA, Gossman GL, Nelson KJ, Daughton D, Spurzem JR, Rennard SI. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183:457–463. doi: 10.1148/radiology.183.2.1561350. [DOI] [PubMed] [Google Scholar]
- 21.Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170:1172–1178. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 24.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138:1295–1302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 26.Smith B. The relationship between emphysema on CT scan and lung cancer. Chest. 2011;139:1259–, author reply 1259–1260. doi: 10.1378/chest.10-3229. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DO, Leader JK, Fuhrman CR, Reilly JJ, Sciurba FC, Weissfeld JL. Quantitative computed tomography analysis, airflow obstruction, and lung cancer in the Pittsburgh Lung Screening Study. J Thorac Oncol. 2011;6:1200–1205. doi: 10.1097/JTO.0b013e318219aa93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gierada DS, Guniganti P, Newman BJ, Dransfield MT, Kvale PA, Lynch DA, Pilgram TK. Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology. 2011;261:950–959. doi: 10.1148/radiol.11110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swensen SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J. 2002;19:1093–1098. doi: 10.1183/09031936.02.00264202. [DOI] [PubMed] [Google Scholar]
- 30.Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res. 2013;184:1–5. doi: 10.1016/j.jss.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 31.Gullón JA, Suárez I, Medina A, Rubinos G, Fernández R, González I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71:182–185. doi: 10.1016/j.lungcan.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Ueda K, Jinbo M, Li T-S, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12:6730–6736. doi: 10.1158/1078-0432.CCR-06-1196. [DOI] [PubMed] [Google Scholar]
- 33.Castaldi PJ, San José Estépar R, Mendoza CS, Hersh CP, Laird N, Crapo JD, Lynch DA, Silverman EK, Washko GR. Distinct quantitative computed tomography emphysema patterns are associated with physiology and function in smokers. Am J Respir Crit Care Med. 2013;188:1083–1090. doi: 10.1164/rccm.201305-0873OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licker MJ, Widikker I, Robert J, Frey J-G, Spiliopoulos A, Ellenberger C, Schweizer A, Tschopp J-M. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81:1830–1837. doi: 10.1016/j.athoracsur.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 35.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, Harris LJ, Detterbeck FC. Methods for staging non-small cell lung cancer. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 36.Silvestri GA, Tanoue LT, Margolis ML, Barker J, Detterbeck F American College of Chest Physicians. The noninvasive staging of non-small cell lung cancer: the guidelines. Chest. 2003;123:147S–156S. doi: 10.1378/chest.123.1_suppl.147s. [DOI] [PubMed] [Google Scholar]
- 37.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Kemp BL, Khuri FR, Liu D, Lee JJ, Wu W, Hong WK, Mao L. Prognostic implication of microsatellite alteration profiles in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6:559–565. [PubMed] [Google Scholar]
- 40.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner MJ.editors. SEER Survival Monograph: cancer survival among adults: US SEER Program, 1988–2001, patient and tumor characteristics. National Cancer Institute, SEER Program. Bethesda, MD: National Institutes of Health; 2007. NIH publication 07-6215 [Google Scholar]
- 41.Kong X, Cho MH, Anderson W, Coxson HO, Muller N, Washko G, Hoffman EA, Bakke P, Gulsvik A, Lomas DA, et al. ECLIPSE Study NETT Investigators. Genome-wide association study identifies BICD1 as a susceptibility gene for emphysema. Am J Respir Crit Care Med. 2011;183:43–49. doi: 10.1164/rccm.201004-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]