Abstract
Part 1 of this review discussed the connection between the human gut microbiota and health. Manipulation of the intestinal microbiota holds promise as a prospective therapy for gut dysbiosis, ameliorating symptoms of gastrointestinal and systemic diseases and restoring health. The concept of probiotics has existed for more than 100 y, and modern research methods have established sound scientific support for the perceived benefits of probiotic bacteria, which mainly include Lactobacillus and Bifidobacterium genera. On the basis of these evidence-based functional approaches, dietary interventions that supplement the normal diet with probiotics or prebiotics are now considered as potentially viable alternatives or adjuncts to the use of steroids, immunosuppressants, and/or surgical interventions. Studies investigating the impact on gastrointestinal disorders, such as diarrhea, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS); and systemic metabolic diseases, such as type 2 diabetes and obesity, in response to the use of probiotics and prebiotics are reviewed. Further, fecal microbial transplantation (FMT) is discussed as an exciting development in the treatment of gut dysbiosis using microbes.
The possibility of modifying the gut microbiota to replace harmful bacteria with more favorable microbiota has been widely explored since observations in 1907 that consumption of fermented products containing Lactobacillus bulgaricus was associated with longevity and good health.1 The advent of modern molecular techniques has provided evidence that the gut microbiota plays a pivotal role in both health and gastrointestinal disease and has further impacts on diseases beyond the gut. In Part 1 of this article, the authors discussed the development, composition, and functions of the gut microbiome in healthy adults in healthy states and the ways in which those characteristics change in dysbiotic disease states. Although it is unclear whether gut dysbiosis causes systemic and gastrointestinal disease or whether the diseases manifest with symptomatic gut dysbiosis, the maintenance of a healthy gut microbiota may be integral to managing the causes or symptoms of both chronic and acute diseases.
Part 2 of this article investigates methods that have been shown to modulate and stabilize the gut microbiota and also to restore it to its healthy composition from dysbiotic states as seen in irritable bowel syndrome (IBS); inflammatory bowel disease (IBD); systemic metabolic diseases, such as obesity and type 2 diabetes; and allergic diseases, such as atopic eczema. The authors discuss treatments using probiotics, prebiotics, antibiotics, and/or fecal microbiota transformation (FMT) as alternatives to steroids, immunesuppressants, and surgical interventions.
Probiotics
The Food and Agriculture Organization (FAO) of the United Nations and the World Health Organization (WHO) have defined probiotics as “live microorganisms that confer a beneficial effect on the health of the host when administered in adequate amounts.”2 This definition has been expanded to require that probiotic organisms used in food must be capable of surviving passage through the gut and tolerant of gastric juices and exposure to bile. In addition, probiotic organisms must be safe and effective and maintain their effectiveness and potency for the duration of the shelf-life of the product.3
Delivery of Probiotics
A range of manufacturing processes enables the delivery of probiotics to the consumer in numerous ways, from dairy foods, such as fermented milks and cheeses, to nondairy foods, such as cereals to freeze-dried powders. Delivery matrices may influence probiotic functionality in numerous ways, including induction of changes in the composition and physiology of the probiotic cells, provision of bioactive compounds within the matrix, and delivery of the end products of fermentation, such as organic acids and secondary metabolites such as antimicrobials. The palatability of the delivery matrix can also alter the frequency at which probiotic products are consumed and incorporated into the diet.4 An important consideration is the effect that these factors may have, not only on a product’s shelf-life and stability but also on the fitness of the probiotic cells. These results directly impact the quantity of active probiotic delivered to the consumer, which forms a vital part of the manufacturer’s label claims.5
History of Probiotics
In 1907, Metchnikoff6 hypothesized that the consumption of large quantities of fermented milk products containing bacteria contributed to the long and healthy lives of Bulgarian peasants. The term probiotic was originally proposed in 1965 by Lilly and Stillwell7 as an alternative to the term antibiotic to describe substances secreted from microorganisms that promote the growth of other microorganisms rather than retard it. The meaning of probiotic was redefined in 1974 by R. B. Parker as “organisms and substances that contribute to intestinal microbial balance,” a definition more closely aligned with its meaning today.8 Further changes were proposed by various researchers in 1989,9 1992,10 and 1996.11 The most recent definition was agreed upon at a FAO and WHO committee in 2002 and is “Live microorganisms, which when administered in adequate amounts, confer a health benefit on the host.”3
Selection Criteria
The historical identification of an organism as a probiotic is largely based on years of administration to humans with no harmful side effects. Sanders5 has reviewed the definition, sources, selection, and uses of probiotics, and any probiotic product should meet the following guidelines, which were established jointly by the FAO and WHO for product manufacturers, who should3: (1) properly identify, to the level of strain, all probiotics in the product and deposit all strains in an international culture collection; (2) characterize each strain for traits important to its safety and function; (3) validate the probiotic’s health benefits in human studies, including identifying the quantity of the microorganism required to provide the benefit; and (4) provide truthful and not misleading labeling of efficacy claims and content to the end of shelf life.
The most widely used probiotic organisms belong to the lactic acid bacteria (LAB) and Bifidobacteria genera, with Lactobacillus and Bifidobacterium being the most extensively studied.
Scientific Rationale for Probiotics
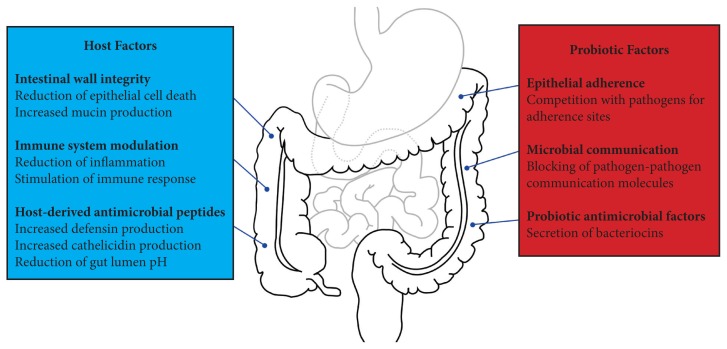
The literature on the use of probiotics is becoming extensive, and the impact of probiotics on gastrointestinal disorders was recently reviewed by Ringel et al.12 Studies have shown a diversity of outcomes and evidence for symptom and disease management, showing that probiotics can greatly improve prognosis in some cases but have little to no effect in others. A rapidly growing body of evidence supports the use of probiotics to ameliorate intestinal dysbiosis, and probiotics have been shown to improve the intestinal barrier, stimulate the immune system, and produce antibacterial effects, alongside modulating intestinal motility and reducing visceral pain, which may contribute to probiotics’ effectiveness in various diseases. Figure 1 provides an overview of the mechanisms of action of probiotics.
Figure 1.
Mechanisms of Action of Probiotics
In vitro evidence suggests that probiotics improve intestinal-wall integrity and barrier function by preventing epithelial cell death (apoptosis)13 and increasing mucin production by the host.14 Epithelial integrity is important in preventing the transmission of pathogens from the gastrointestinal tract (GIT) to other parts of the body. In addition, probiotic bacteria directly and indirectly, through production of specific proteins, compete for binding sites on epithelial cells in vitro, preventing invading pathogens from adhering to the gut wall.15 In vitro studies also indicate that probiotic bacteria suppress the growth of microbial pathogens by (1) directly producing antimicrobial factors16; (2) stimulating the host’s cells to produce their own antimicrobial factors, defensins, and cathelicidins17,18; and (3) lowering the intestinal pH via release of short chain fatty acids (SFCAs) from epithelial cells.17 Probiotic bacteria are also able to interfere with cell-to-cell signaling molecules that allow bacteria to communicate.19 These molecules are particularly important in the early stages of invasion by enteric pathogens because they are used to regulate several traits that allow pathogens to establish and maintain infection in their hosts, including decreasing motility, inhibiting biofilm formation, and reducing expression of virulence-specific genes.20 From the perspective of inflammation reduction, probiotics have been shown both to stimulate host production of anti-inflammatory cytokines21 and suppress production of proinflammatory cytokines.22 Many studies have focused on the use of probiotic supplements as treatments or adjuvants to current therapies for disorders of the GIT. In the current article, the authors discuss studies that have detailed the effect of probiotic supplementation for some of the major dysbiotic states of the gut.
Probiotics and Antibiotic-associated Diarrhea
Antibiotic treatment, even for conditions unrelated to gut health, may disturb the gastrointestinal microbiota, resulting in a range of symptoms, including antibiotic-associated diarrhea (AAD). Administration of aminopenicillins, cephalosporin, and clindamycin, which act particularly on anaerobes, is most commonly associated with diarrhea.23,24 Symptoms of AAD include frequent watery bowel movements, urgency, and cramp-like abdominal pain, and AAD is associated with altered intestinal microbiota, reduced intestinal-wall integrity, and diminished vitamin and mineral metabolism.24 Different studies have shown that the incidence of AAD is between 5% and 62%, depending on the specific type of antibiotic, the health of the host and exposure to pathogens, and it may occur at any point from the start of antibiotic therapy to up to 2 months after the end of treatment.24 As the integrity of the normal gastrointestinal microbiota is compromised, the overgrowth of a variety of opportunistic bacterial pathogens occurs.
Overgrowth of Clostridium difficile is commonly associated with AAD and is also implicated in the most serious adverse events relate to it.25 Probiotics have been found to play an important role in the prevention of AAD and C difficile diarrhea. Probiotics assist in re-establishing the disrupted intestinal microbiota, enhancing the host’s immune response and clearing pathogens and their toxins from the host’s intestines.26 Meta-analyses have shown that administration of probiotic strains, either alone or in combination, is effective for the prevention of AAD in the general population.26–28 Generally, the best results have been seen when the probiotic was coadministered with the antibiotic.24 In the case of C difficile diarrhea, a placebo-controlled pilot study that monitored 150 hospital inpatients showed that administration of a probiotic containing both Lactobacillus acidophilus and Bifidobacterium bifidum reduced the incidence of C difficile diarrhea during hospitalization.29
Probiotics and IBS
IBS is a functional gastrointestinal disorder characterized by abdominal pain or discomfort associated with abnormal bowel habits. During recent years, evidence for the involvement of the gut microbiota in the pathogenesis and pathophysiology of IBS has increased, and it appears that IBS patients may have an altered microbiota when compared with healthy individuals.30
A large number of articles cover studies and trials that have investigated the efficacy of probiotic use in patients with IBS. Moayyedi et al31 conducted a large-scale analysis of clinical trials of probiotics in the treatment of IBS, which included 18 randomized, controlled clinical trials using a variety of probiotic organisms and examined results for more than 1600 patients with IBS.31 When measured as a dichotomous outcome (eg, improvement or no improvement), a reduction in IBS symptoms was observed in 918 individuals.31 Of the 18 total trials, 15 found that probiotics caused a statistically significant improvement in IBS symptoms when compared with a placebo.31 Little difference in the magnitude of the effect was found between Lactobacillus, Bifidobacterium, and Streptococcus, or combination probiotics, and all showed a trend toward symptomatic improvement.31 Measureable improvements in pain scores, flatulence, and bloating were observed. No significant incidence of adverse effects was associated with probiotic supplementation in patients with IBS.12,31 However, as different probiotics have different microbiological characteristics that will inevitably impact their efficacy, results from meta-analyses, which group the outcomes of different studies together, should be interpreted with caution.32 Consequently, despite the requirement for these large-scale analyses, adapting the findings from meta-analyses into direct clinical guidance (eg, probiotics improve global symptoms of IBS) implies that administration of all probiotics will result in a similar outcome, which may not be the case.32
Probiotics and IBD
IBDs, including both Crohn’s disease (CD) and ulcerative colitis (UC), are complex immune disorders that exhibit a genetic basis. Genome-wide association studies (GWASs) have shown that many of a host’s genes correlate with the development of CD and UC, although not every individual presenting with genetic abnormalities will develop disease. In effect, the host’s genetic background may set the parameters for the response of the gut microbiota to environmental factors, in turn shifting the composition of the gut microbiota and leading to IBD development.33
Sheil et al34 reviewed evidence on the efficacy of probiotics in patients with IBD. Probiotics, especially Bifidobacteria, have been shown to influence a host’s immune-system function and are linked to suppression of mucosal inflammation in animal models of IBD.35 However, the anti-inflammatory effects of probiotics that are often observed in vitro and in animal studies do not always translate to clinically beneficial effects.12 This result may be due to the complexity of the immunemodulatory effects of probiotics, with the overall effect on patients’ symptoms difficult to predict and potentially specific to the probiotic strain administered.34
Studies undertaken in genetically modified mice using several strains of Lactobacillus and Bifidobacterium have shown that some IBD characteristics are relieved by probiotics, improving gut function,36 reducing inflammation,37 and preventing disease progression to UC.38,39 In humans, however, the evidence for probiotic efficacy is less positive, with a recent meta-analysis of 8 clinical trials indicating that probiotics do not seem to be a therapeutic option for maintenance of remission of CD.40 Rahimi et al41 found that the inclusion of probiotics with conventional treatment for UC did not to improve the overall remission rates in mild-to-moderate UC, but that it was possible to generate a slight decrease in disease activity. At the current stage, the conclusion has been reached that use of probiotics in IBD cannot be recommended.42 Studies have been conducted using a diversity of bacterial strains in different clinical situations, but critically, only a few patients have been enrolled in these studies and, hence, more randomized trials with strict enrollment criteria are needed to investigate further the effects of probiotics on IBD.43
Probiotics and Atopic Eczema
In the last several decades, an increasing number of children, up to 20% of the general population, have developed allergies in a clinical progression called the atopic march.44 Allergic disorders have been associated with aberrant gut microbiota, and factors associated with allergy such as (1) birth delivery mode (ie, cesarean section vs vaginal delivery); (2) antibiotic use in the newborn and infant; and (3) non-breast-milk diets are also associated with shifts in the gut microbiota.45,46 The hygiene hypothesis, formulated as an explanation for the observed rise in the prevalence of allergic diseases, suggests that increased cleanliness, reduced family size, and decreased incidence of childhood infections have lowered exposure to microbes, which play a vital role in the maturation of children’s immune systems during the first years of their lives.47
With this increase in mind, a substantial effort has occurred to assess the potential role of probiotics in the prevention and/or treatment of allergic diseases, particularly by feeding of probiotics to infants. Exposure to probiotic bacteria may stimulate the immune system and train it to produce an appropriate response to allergens. When Lactobacillus rhamnosus GG was administered to high-risk infants (ie, those with at least 1 relative with atopic eczema or asthma), a 50% reduction in the incidence of atopic eczema was observed.48 Also, the skin condition of children with atopic eczema improved when they were given whey formula supplemented with the L rhamnosus or Bifidobacterium animalis subsp lactis for 2 months.49 A systematic review of the effect of nutritional supplementation on atopic dermatitis in children found that the best effects were observed when both mothers and infants were supplemented with probiotics.50
Probiotics and Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is an IBD that is one of the most common gastrointestinal emergencies in newborn infants and that causes portions of the bowel to undergo necrosis (ie, tissue death).51 It often occurs within the first 3 months of life in infants with very low birth weights (VLBWs) (ie, less than 1 kg). Premature neonates (ie, younger than 28 wk of gestation) are the most susceptible, accounting for 90% of cases.52 NEC has a mortality rate of up to 50%, representing a significant clinical problem.53 Initial symptoms include feeding intolerance, bloody diarrhea, temperature instability, lethargy, apnea, delayed gastric emptying, abdominal distension and tenderness, and respiratory stress.53 The large intestine is the most commonly affected region in NEC, although any segment of the GIT can be involved, including the stomach.53
In the advanced stages of NEC, pathological findings include gastrointestinal bleeding, inflammation, bacterial overgrowth, intestinal perforation, coagulative necrosis, hypotension, septic shock, and, eventually, death in approximately 30% of cases.53,54 Probiotics may prevent NEC in VLBW infants by promoting colonization of the gut with beneficial organisms, preventing colonization by pathogens, improving the maturity and function of the mucosal barrier of the gut, and enhancing the host’s immune response.55 A systematic review and meta-analysis of 7 clinical trials that administered probiotics to 1393 preterm, VLBW infants found that mortality from of all causes was reduced by 53% and NEC by 64% when compared with a control group of neonates. In addition, the time taken for infants to be able to consume full feeds of milk was also significantly reduced, by an average of approximately 3 days, in those who received probiotic supplementation.56 The consumption of milk drives the maturation of the gut microbiota in neonates.57 Based on current data, experts have subsequently stated that those who wish to offer probiotic supplementation as a routine therapy for preterm neonates cannot be faulted.55
Probiotics and Systemic Metabolic Diseases: Obesity and Type 2 Diabetes
Modulation of the gut microbiota has been considered as a potential target in cases of obesity and diabetes. Requena et al58 have reviewed extensively the interaction between the obese human host, food, and the gut microbiota. Recent studies in both humans and animals have suggested that particular strains of Lactobacillus and Bifidobacterium show beneficial effects on molecular markers of obesity59,60 and type 2 diabetes.61 The consumption of specific Lactobacillus and Bifidobacterium strains has been shown to ameliorate the progression of obesity and diabetes in mice and rats, suggesting that modulation of the gut microbiota is a potential therapy for obesity and diabetes.62,63 In a 12-week, randomized, placebo-controlled intervention, participants who had a body mass index (BMI) of greater than 24 received either Lactobacillus gasseri in fermented milk or a control milk fermented without L gasseri; the study showed significant reductions in body weight and abdominal fat tissue in the probiotic group.64 In contrast, the administration of Lactobacillus salivarius to 25 obese adolescents over a 12-week period did not reduce weight or indicators of metabolic syndrome relative to a control group.65 Comparison of bacterial-cell numbers did not reveal any significant shifts inside the groups that could be attributed to probiotic consumption, but the ratios of specific bacterial populations in the probiotic group after the intervention significantly differed from the ratios before intervention, specifically the ratio of the Bacteroides-Prevotella-Porphyromonas group bacteria to those belonging to the Firmicutes was significantly increased after administration of L salivarius.66 It has been suggested that the probiotic effect may be dependent on the bacterial strain administered, but further large-scale, randomized, placebo-controlled, double-blind human studies are needed to examine the efficacy of probiotics in treating symptoms of obesity and other metabolic diseases.
Potential Areas of Application for Probiotics via the Gut−Brain Axis: Cold and Flu symptoms, Liver Disease, and Stress
Respiratory tract infections (RTIs) in children represent a major health care burden, and hospital admissions related to them in children younger than 15 years of age have increased by 22% since 1999.67 The immunomodulatory effects of probiotics offer a potential opportunity to prevent or reduce the symptoms of RTIs. A double-blind, placebo-controlled trial of 428 children showed that prophylactically supplementing dietary intake with a probiotic for 6 months reduced the incidence and duration of fever, rhinorrhoea, and cough, as well as the incidence of antibiotic prescriptions, when compared with a placebo group.68 A difference in effect was observed for single- and multiple-strain probiotics. The group treated with a single-strain probiotic exhibited reduced incidence of fever and cough, and the group treated with the multiple-strain probiotic exhibited reduced incidence of fever, cough, and rhinorrhoea.68 Both the single- and multiple-strain treatment groups exhibited significantly reduced symptom duration and antibiotic use.68 Impacting the need for antibiotic use early in life may have important longer-term benefits, such as decreasing overall adverse reactions to antibiotics, lowering costs, and lessening the risk of antimicrobial resistance.
Minimal hepatic encephalopathy (MHE) is a preclinical condition of chronic liver disease that has the potential to progress to hepatic encephalopathy, which manifests clinically as brain dysfunction, confusion, and altered consciousness, and in the worst cases, may result in death.69 The exact pathogenesis of hepatic encephalopathy is not completely understood; however, ammonia and other nitrogen-containing compounds that are produced widely by enteric bacteria have been implicated.70 The potential for the use of probiotics to treat MHE is related to the ability of probiotics to exclude pathogenic organisms competitively through bacterial urease activity and to reduce intestinal inflammation.69 In a small study, 25 individuals with early-stage liver disease received a probiotic yoghurt daily for 6 months, and MHE pathology was reversed in 71% of individuals with early-stage liver disease.69
The hepatic manifestation of the metabolic syndrome nonalcoholic fatty liver disease (NAFLD) has a prevalence of 20% to 30% in Western countries.71 Among morbidly obese patients undergoing bariatric surgery, approximately 90% have NAFLD. Amelioration of NAFLD by weight loss and/or lifestyle changes is often unsuccessful at all ages, frequently requiring implementation of pharmacological or surgical treatments.72 The use of probiotics appears to be an interesting and reasonable option acting on the malfunction of the gut–liver axis through the modulation of diet-driven, obesogenic, and inflammatory intestinal microbiota. Although strictly a synbiotic intervention, rather than just probiotic, an open-label, randomized controlled trial of 20 obese adult patients with biopsy-proven NAFLD, received a 6 month synbiotic formula containing 5 strains of probiotic bacteria—4 lactobacilli and B bifidum—and a prebiotic, fructooligosaccharide (FOS). Treatment resulted in a significant reduction of liver fat.73 Prebiotic and synbiotic supplements are discussed in greater detail in subsequent sections.
The enteric nervous system (ENS) and central nervous systems (CNS) are linked bidirectionally by the sympathetic and parasympathetic pathways that form the brain–gut axis. Acute stress induces responses in the upper and lower GIT, increasing colonic motility and stress-hormone levels.74 Bested et al75–77 comprehensively reviewed the gut–brain axis and the history of probiotic use in the treatment of stress and other mental health disorders. Lactobacillus helveticus and Bifidobacterium longum, in combination, were fed to volunteers for 1 month in a placebo-controlled study, and improvements in scores on anxiety, stress, and depression scales were observed, together with improvements in day-to-day depression, anger, and anxiety.78 In addition, lower levels of the stress hormone cortisol were noted among otherwise healthy adults taking a daily probiotic supplement, compared with the placebo control group.78
Prebiotics
In 2007, Roberfroid79 defined a prebiotic as “a selectively fermented ingredient that allows specific changes, both in the composition of and/or activity in the gastrointestinal microflora that confers benefits upon a host’s well-being and health.” This definition means that the ingredients of prebiotics should not be metabolized by a human host’s cells and may only be metabolized by members of the gut microbiota considered to be important to gut health, such as the lactobacilli and bifidobacteria. The finer implications of this definition mean that the prebiotic ingredient should be able to travel through the majority of the GIT to move to a position in close physical proximity to its targeted bacterial cells. Prebiotics must be sufficiently resistant to gastric acidity, hydrolysis by mammalian enzymes, and gastrointestinal absorption such that a quantity great enough to stimulate growth of targeted bacteria reaches the large intestine.79 Roberfroid79 suggested that only 2 food ingredients, inulin and galactooligosaccharides (GOSs), fulfill these criteria.
Chemically, inulin is a heterogeneous collection of fructose polymers, composed of between 2 and 60 fructose residues.80 Some researchers refer to materials composed primarily of shorter-chain molecules (ie, between 2 and 10 residues) as FOS and of longer-chain molecules as inulin, but the authors of the current article make no distinction. Inulin and FOSs are present in more than 36 000 plant species and are used as storage carbohydrates in a number of vegetables and plants, including wheat, onion, bananas, garlic, and chicory.81 Inulin has been shown to be resistant to low pH, mammalian hydrolysis, and absorption in many studies and has also been shown, in vitro and in vivo, to have a growth-promoting effect on both bifidobacteria and lactobacilli.82–84
From an intervention perspective, the evidence for prebiotic efficacy in treatment of obesity and metabolic disorders is mixed.85,86 The proposed role for prebiotics is based on the findings that the population of bifidobacteria and other bacteria belonging to the Firmicutes phylum is proportionally smaller in individuals with obesity than in lean people87 and also in individuals with type 2 diabetes when compared with individuals without type 2 diabetes.88 This suggests that the lower numbers of bifidobacteria may be implicated in obesity and type 2 diabetes and their associated gastrointestinal and systemic diseases. When the prebiotic inulin was fed to both obese and diabetic mice, the proportion of bifidobacteria increased significantly compared with mice on a standard diet that did not contain a prebiotic.89,90 In addition, inulin administration was linked with a reduction in the expression of several genes in the host that were related to adiposity and inflammation.70 The amount of evidence, however, is limited, and a definitive beneficial effect on metabolic disturbances remains to be shown in large, prospective, randomized controlled trials.86
GOSs are more diverse than inulin in their chemistry but are generally composed of between 2 and 8 linked galactose residues, with an attached terminal glucose residue.91 They are produced commercially from lactose, a sugar found in cow’s milk, using an enzymatic transglycosylation reaction.92 In vitro studies have shown that GOS is able to support the growth of Bacteroides, most lactobacilli, enterobacteria, streptococci, and bifidobacteria, which achieved the most vigorous growth.79 In vivo human studies with volunteers showed that supplementation with GOS resulted in significant increases in fecal bifidobacteria93 and lactobacilli94 and in significant decreases in potentially harmful Bacteroides and Candida.94 Feeding infants with formula milk that was supplemented with a mixture of oligosaccharides—90% GOSs and 10% inulin—has been shown to result in an increase in fecal bifidobacteria.95
Synbiotics
When probiotics and prebiotics are administered simultaneously, the combination is termed a synbiotic. Synbiotics have been proposed as increasing the levels of beneficial bacteria such as subspecies of Bifidobacterium and Lactobacillus, with the result being that the increased level of total anaerobes induces an increased production of SCFAs in the gut. The prebiotic in the synbiotic mixture improves the survival of the probiotic bacteria and stimulates the activity of the host’s endogenous bacteria, and administration of synbiotics can result in elevated bifidobacterial numbers due to the prebiotic component.96
Among patients receiving liver transplantation, hepatectomy for biliary cancer, and pancreatoduodenectomy, those who received a synbiotic combination of Lactobacillus plus fiber developed significantly fewer bacterial infections. 96 In addition, although the measures did not reach statistical significance, the mean duration of antibiotic therapy, the mean total hospital stay, and the stay on the intensive care unit were all shorter in the group receiving the synbiotic.96 In 65 multiple-trauma patients, the decrease in the incidence of infectious complications in the group receiving synbiotics were significantly greater than those in the group without synbiotics (49% vs 77%); inflammatory markers such as TNF-α and IL-6 also decreased in the synbiotics group.97
Antibiotics
Considering the growing evidence of the central role of the gut microbiota in intestinal dysbiosis, a paucity of data exists regarding treatment with antibiotics. Despite this weak evidence, their use has a long tradition. One of the more recently developed antibiotics to treat gastrointestinal diseases is rifaximin, a broad-spectrum, low-absorption antibiotic that reaches high fecal concentrations, making it an excellent therapeutic agent that is currently approved for treating traveller’s diarrhea.98,99 The effectiveness of rifamixin for treating a variety of chronic gastrointestinal diseases has also been demonstrated in several small-scale studies. Two randomized, double-blind, placebo-controlled trials indicated that rifamixin may improve IBS symptoms,100,101 and further trials show efficacy in patients with UC102 and CD.103
Fecal Microbial Transplantation
FMT is a novel approach to modulation of the gastrointestinal microbiota, particularly in situations where microbial dysbiosis has occurred.104 Van Nood et al105 recently reviewed the efficacy of FMT in treating a variety of dysbiotic states. The objective of FMT is to reintroduce and re-establish a stable community of gastrointestinal bacteria from a healthy donor, supplanting a dysbiotic community in a diseased individual, via a transcolonoscopic infusion in most cases. The treatment has shown a success rate of up to 90% in treating severe gastrointestinal dysbiosis caused by C difficile infections.106 Unlike probiotics, which aim to alter the metabolic or immunological activity of the native gut microbiota, the objective of FMT is to introduce an entirely novel community of gut microorganisms, ultimately to repair or replace the disrupted indigenous microbiota.105 A study showed at both 2 weeks and 1 month post-FMT treatment that the composition of the fecal microbiota of a patient with persistent C difficile, associated with diarrhea, consisted predominantly of the bacteria derived from a healthy donor. The treatment was also associated with normalization of the patient’s bowel function.107 The ability of FMT to stabilize the gastrointestinal microbiota in radically dysbiotic states makes it an interesting basis for novel treatments of gut-associated diseases. Evidence for its efficacy is currently on a case-by-case basis, however, and further systematic trials will be required before the process can be declared both safe and effective.
Conclusions and Perspective
Although long recognized for their importance, the bacteria constituting the gut microbiota in humans have been largely ignored by science because of both the complexity of the populations and the technical limitations of classic microbiological techniques. Microbial ecologists, clinicians, immunologists, physiologists, nutritionists, and computer scientists are now beginning to work together, to build a new science of personalized medicine, and maybe even future biotherapeutics. Current evidence supports the role of probiotics, prebiotics, and synbiotics in a broad range of gastrointestinal conditions. Part of the etiology of IBS may also involve the use of antibiotics, and the overwhelming amount of data again indicates that probiotics are effective in ameliorating symptoms, although the consistency of benefits across clinical studies is difficult to discern due to variation in strains, product dosages, and duration of trials. The fact that much of the evidence is positive despite the inconsistencies, however, potentially bears witness to the possible benefits of probiotic administration.
With regard to immune-function regulation, evidence exists to indicate that probiotics may be effective in the prevention of atopic eczema and may also be of benefit with established disease in reducing sensitization, and hence, symptom load. Atopic eczema is a very powerful predictor of future development of asthma and rhinitis, with a 50% to 80% risk factor; the expectation is that the frequency of the expressions of the allergic state will decline with the use of probiotics. This area holds great promise for probiotics because the medical sector currently has no therapeutic option available for reducing incidence of type 1 hypersensitivity. Indications also exist that improving the colonization and succession of the microbiota in the first months and years of life may have benefits beyond the reduction in allergy. These benefits may include the potential to reduce susceptibility to enteric and general, RTIs; propensity to obesity; and possibly, even the risk of developing certain autoimmune diseases.
Unfortunately, IBD continues to be a difficult-to-treat disease, where probiotics, prebiotics, and synbiotics have shown only limited efficacy in fully resolving symptoms. However, these diseases are difficult to manage and intractable under any circumstances, and as such, the limited benefits shown to date are still useful adjuncts in therapy.
Finally, the potential for probiotics to offer benefits in health improvement and disease-risk reduction in areas such as obesity, metabolic disease, and brain and neural function merely attests to the understanding that the gut has a role in human physiology. This role is much more profound than simply the digestion and assimilation of food, and the gut microbiome, as an integral, inseparable, and highly active component of the human GIT, must also play a significant role. Probiotics and prebiotics may offer tools to manipulate the gut microbiome, potentially opening a new channel for health care.
References
- 1.Metchnikoff E. In: The Prolongation of Life: Optimistic Studies. Mitchell PC, translator. New York, NY: GP Putnam’s Sons; 1908. [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations; World Health Organization. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Córdoba, Argentina: Food and Agriculture Organization of the United Nations, World Health Organization; 2001. [Google Scholar]
- 3.Food and Agriculture Organization of the United Nations; World Health Organization. Guidelines for the Evaluation of Probiotics in Food. London, ON: Food and Agriculture Organization of the United Nations, World Health Organization; 2002. [Google Scholar]
- 4.Sanders ME, Marco ML. Food formats for effective delivery of probiotics. Annu Rev Food Sci Technol. 2010;1:65–85. doi: 10.1146/annurev.food.080708.100743. [DOI] [PubMed] [Google Scholar]
- 5.Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46(suppl 2):S58–S61. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 6.Metchnikoff E. Lactic acid as inhibiting intestinal putrefaction. In: Mitchell PC, translator. The Prolongation of Life: Optimistic Studies. New York, NY: GP Putnam’s Sons; 1907. pp. 161–183. [Google Scholar]
- 7.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 8.Parker RB. Probiotics, the other half of the antibiotic story. Anim Nutr Health. 1974;29:4–8. [Google Scholar]
- 9.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365–378. [PubMed] [Google Scholar]
- 10.Havenaar R, Huis in’t Veld JH. Probiotics: a general view. In: Wood BJ, editor. The Lactic Acid Bacteria. Vol. 1. New York, NY: Springer US; 1992. pp. 151–170. [Google Scholar]
- 11.Schaafsma G. State of the art concerning probiotic strains in milk products. Int Dairy Food Nutr News Lett. 1996;5:23–24. [Google Scholar]
- 12.Ringel Y, Quigley EM, Lin HC. Using probiotics in gastrointestinal disorders. Am J Gastroenterol Suppl. 2012;1(1):34–40. [Google Scholar]
- 13.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care. 2006;9(6):717–721. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- 14.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson-Henry KC, Hagen KE, Gordonpour M, Tompkins TA, Sherman PM. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9(2):356–367. doi: 10.1111/j.1462-5822.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 16.Tejero-Sariñena S, Barlow J, Costabile A, Gibson GR, Rowland I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe. 2012;18(5):530–538. doi: 10.1016/j.anaerobe.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol. 2005;5(6):596–603. doi: 10.1016/j.coph.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 19.Medellin-Peña MJ, Wang H, Johnson R, Anand S, Griffiths MW. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl Environ Microbiol. 2007;73(13):4259–4267. doi: 10.1128/AEM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendall MM, Sperandio V. Quorum sensing by enteric pathogens. Curr Opin Gastroenterol. 2007;23(1):10–15. doi: 10.1097/MOG.0b013e3280118289. [DOI] [PubMed] [Google Scholar]
- 21.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72(6):3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SO, Sheikh HI, Ha SD, Martins A, Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8(12):1958–1971. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 23.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(supplement 1):S19–S31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 24.Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;(11):CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 25.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008;3(5):563–578. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 26.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Sazawal S, Hiremath G, Dhingra U, Malik P, Deb S, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6(6):374–382. doi: 10.1016/S1473-3099(06)70495-9. [DOI] [PubMed] [Google Scholar]
- 28.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 29.Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7(1):59–62. [PubMed] [Google Scholar]
- 30.Ohman L, Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS) Curr Gastroenterol Rep. 2013;15(5):323. doi: 10.1007/s11894-013-0323-7. [DOI] [PubMed] [Google Scholar]
- 31.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 32.Whelan K, Quigley EM. Probiotics in the management of irritable bowel syndrome and inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29(2):184–189. doi: 10.1097/MOG.0b013e32835d7bba. [DOI] [PubMed] [Google Scholar]
- 33.Leone V, Chang EB, Devkota S. Diet, microbes, and host genetics: the perfect storm in inflammatory bowel diseases. J Gastroenterol. 2013;48(3):315–321. doi: 10.1007/s00535-013-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheil B, Shanahan F, O’Mahony L. Probiotic effects on inflammatory bowel disease. J Nutr. 2007;137(3) suppl 2:819S–824S. doi: 10.1093/jn/137.3.819S. [DOI] [PubMed] [Google Scholar]
- 35.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 36.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 37.O’Mahony L, Feeney M, O’Halloran S, et al. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15(8):1219–1225. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 38.Schultz M, Munro K, Tannock GW, et al. Effects of feeding a probiotic preparation (SIM) containing inulin on the severity of colitis and on the composition of the intestinal microflora in HLA-B27 transgenic rats. Clin Diagn Lab Immunol. 2004;11(3):581–587. doi: 10.1128/CDLI.11.3.581-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz M, Veltkamp C, Dieleman LA, et al. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8(2):71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi R, Nikfar S, Rahimi F, et al. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease. Dig Dis Sci. 2008;53(9):2524–2531. doi: 10.1007/s10620-007-0171-0. [DOI] [PubMed] [Google Scholar]
- 41.Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573. doi: 10.1002/14651858.CD005573.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Prantera C, Scribano ML. Antibiotics and probiotics in inflammatory bowel disease: why, when, and how. Curr Opin Gastroenterol. 2009;25(4):329–333. doi: 10.1097/MOG.0b013e32832b20bf. [DOI] [PubMed] [Google Scholar]
- 43.Prantera C. Probiotics for Crohn’s disease: what have we learned? Gut. 2006;55(6):757–759. doi: 10.1136/gut.2005.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160(1):1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–440. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Williams HC, Grindlay DJ. What’s new in atopic eczema? An analysis of systematic reviews published in 2007 and 2008, I: definitions, causes and consequences of eczema. Clin Exp Dermatol. 2010;35(1):12–15. doi: 10.1111/j.1365-2230.2009.03733.x. [DOI] [PubMed] [Google Scholar]
- 47.Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132(3):e666–e676. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 48.Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 49.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol. 1997;99(2):179–185. doi: 10.1016/s0091-6749(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 50.Foolad N, Brezinski EA, Chase EP, Armstrong AW. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149(3):350–355. doi: 10.1001/jamadermatol.2013.1495. [DOI] [PubMed] [Google Scholar]
- 51.Illi S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 52.Updegrove K. Necrotizing enterocolitis: the evidence for use of human milk in prevention and treatment. J Hum Lact. 2004;20(3):335–339. doi: 10.1177/0890334404266972. [DOI] [PubMed] [Google Scholar]
- 53.Schnabl KL, Van Aerde JE, Thomson AB, Clandinin MT. Necrotizing enterocolitis: a multifactorial disease with no cure. World J Gastroenterol. 2008;14(14):2142–2161. doi: 10.3748/wjg.14.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Embleton N, Berrington JE. Probiotics reduce the risk of necrotising enterocolitis (NEC) in preterm infants. Evid Based Med. 2013;18(6):219–220. doi: 10.1136/eb-2013-101260. [DOI] [PubMed] [Google Scholar]
- 55.Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. 2010;125(5):921–930. doi: 10.1542/peds.2009-1301. [DOI] [PubMed] [Google Scholar]
- 56.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369(9573):1614–1620. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 57.Wagner CL, Taylor SN, Johnson D. Host factors in amniotic fluid and breast milk that contribute to gut maturation. Clin Rev Allergy Immunol. 2008;34(2):191–204. doi: 10.1007/s12016-007-8032-3. [DOI] [PubMed] [Google Scholar]
- 58.Requena T, Cotter P, Shahar DR, et al. Interactions between gut microbiota, food and the obese host. Trends Food Sci Technol. 2013;34(1):44–53. [Google Scholar]
- 59.Aronsson L, Huang Y, Parini P, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4) PLoS One. 2010;5(9):e13087. doi: 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HY, Park JH, Seok SH, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761(7):736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, et al. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104(12):1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]
- 62.Naito E, Yoshida Y, Makino K, et al. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110(3):650–657. doi: 10.1111/j.1365-2672.2010.04922.x. [DOI] [PubMed] [Google Scholar]
- 63.Chen JJ, Wang R, Li XF, Wang RL. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal Reg I gene expression. Exp Biol Med (Maywood) 2011;236(7):823–831. doi: 10.1258/ebm.2011.010399. [DOI] [PubMed] [Google Scholar]
- 64.Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 65.Gøbel RJ, Larsen N, Jakobsen M, Mølgaard C, Michaelsen KF. Probiotics to adolescents with obesity: effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;55(6):673–678. doi: 10.1097/MPG.0b013e318263066c. [DOI] [PubMed] [Google Scholar]
- 66.Larsen N, Vogensen FK, Gøbel RJ, et al. Effects of Lactobacillus salivarius Ls-33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32(6):935–940. doi: 10.1016/j.clnu.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Gill PJ, Goldacre MJ, Mant D, et al. Increase in emergency admissions to hospital for children aged under 15 in England, 1999–2010: national database analysis. Arch Dis Child. 2013;98(5):328–334. doi: 10.1136/archdischild-2012-302383. [DOI] [PubMed] [Google Scholar]
- 68.Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124(2):e172–e179. doi: 10.1542/peds.2008-2666. [DOI] [PubMed] [Google Scholar]
- 69.Bajaj JS, Saeian K, Christensen KM, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterol. 2008;103(7):1707–1715. doi: 10.1111/j.1572-0241.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- 70.Vyas U, Ranganathan N. Probiotics, prebiotics, and synbiotics: gut and beyond. Gastroenterol Res Pract. 2012;2012:872716. doi: 10.1155/2012/872716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56(12):1760–1769. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59(4):859–871. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 73.Wong VW, Won GL, Chim AM, et al. Treatment of nonalcoholic steatohepatitis with probiotics: a proof-of-concept study. Ann Hepatol. 2013;12(2):256–262. [PubMed] [Google Scholar]
- 74.Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 2007;56(11):1495–1497. doi: 10.1136/gut.2007.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances, I: autointoxication revisited. Gut Pathog. 2013;5(1):5. doi: 10.1186/1757-4749-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances, II: contemporary contextual research. Gut Pathog. 2013;5(1):3. doi: 10.1186/1757-4749-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances, III: convergence toward clinical trials. Gut Pathog. 2013;5(1):4. doi: 10.1186/1757-4749-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755–764. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 79.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137(3) suppl 2:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 80.Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24(5):701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 81.Niness KR. Inulin and oligofructose: what are they? J Nutr. 1999;129(7) suppl:1402S–1406S. doi: 10.1093/jn/129.7.1402S. [DOI] [PubMed] [Google Scholar]
- 82.Tako E, Glahn RP, Welch RM, Lei X, Yasuda K, Miller DD. Dietary inulin affects the expression of intestinal enterocyte iron transporters, receptors and storage protein and alters the microbiota in the pig intestine. Br J Nutr. 2008;99(3):472–480. doi: 10.1017/S0007114507825128. [DOI] [PubMed] [Google Scholar]
- 83.Roberfroid MB, Van Loo JA, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128(1):11–19. doi: 10.1093/jn/128.1.11. [DOI] [PubMed] [Google Scholar]
- 84.Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 2002;87(suppl 2):S159–S162. doi: 10.1079/BJNBJN2002532. [DOI] [PubMed] [Google Scholar]
- 85.Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(suppl 2):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 86.Kootte RS, Vrieze A, Holleman F, et al. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(2):112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 87.Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 88.Wu X, Ma C, Nawaz M, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol. 2010;61(1):69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 89.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 90.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crittenden RG, Playne MJ. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol. 1996;7(11):353–361. [Google Scholar]
- 92.Mussatto SI, Mancilha IM. Non-digestible oligosaccharides: a review. Carbohydr Polym. 2007;68(3):587–597. [Google Scholar]
- 93.Bouhnik Y, Flourié B, D’Agay-Abensour L, et al. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr. 1997;127(3):444–448. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 94.Ito M, Kimura M, Deguchi Y, Miyamori-Watabe A, Yajima T, Kan T. Effects of transgalactosylated disaccharides on the human intestinal microflora and their metabolism. J Nutr Sci Vitaminol (Tokyo) 1993;39(3):279–288. doi: 10.3177/jnsv.39.279. [DOI] [PubMed] [Google Scholar]
- 95.Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Probiotics, prebiotics infant formula use in preterm or low birth weight infants: a systematic review. Nutr J. 2012 Aug;11:58. doi: 10.1186/1475-2891-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rastall RA, Maitin V. Prebiotics and synbiotics: towards the next generation. Curr Opin Biotechnol. 2002;13(5):490–496. doi: 10.1016/s0958-1669(02)00365-8. [DOI] [PubMed] [Google Scholar]
- 97.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30(10):1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 98.Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26(1):17–25. doi: 10.1097/MOG.0b013e328333dc8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang ZD, Ke S, Palazzini E, Riopel L, Dupont H. In vitro activity and fecal concentration of rifaximin after oral administration. Antimicrob Agents Chemother. 2000;44(8):2205–2206. doi: 10.1128/aac.44.8.2205-2206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145(8):557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 101.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101(2):326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 102.Guslandi M, Petrone MC, Testoni PA. Rifaximin for active ulcerative colitis. Inflamm Bowel Dis. 2006;12(4):335. doi: 10.1097/01.MIB.0000215092.85116.6c. [DOI] [PubMed] [Google Scholar]
- 103.Shafran I, Johnson LK. An open-label evaluation of rifaximin in the treatment of active Crohn’s disease. Curr Med Res Opin. 2005;21(8):1165–1169. doi: 10.1185/030079905x53252. [DOI] [PubMed] [Google Scholar]
- 104.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2011;9(2):88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 105.van Nood E, Speelman P, Nieuwdorp M, Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. 2014;30(1):34–39. doi: 10.1097/MOG.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 106.Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe. 2009;15(6):285–289. doi: 10.1016/j.anaerobe.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 107.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44(5):354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]