The idea that “being too acid” contributes to disease susceptibility, especially cancer, has been around for a long time in the natural/integrative medicine world. This concept was easily discounted by conventional medicine as measuring blood pH on various types of diets showed no change.
Up until about 10 years ago, no research existed to counter this skepticism. However, since then, a growing body of research has documented not only that “acidosis” is a real phenomenon, but that it is now known to contribute to a wide range of diseases, such as metabolic syndrome, cancer, osteoporosis, kidney stones, and increased susceptibility to environmental toxins—and new research is adding to the list. In this editorial, I will review the biochemistry the various food components, soft drinks, prescription drugs, and metabolic dysfunctions affect the acid/base balance of cells. In addition, I will address how to determine body acid load and strategies using natural health products and diet to restore normal cellular function and reverse several diseases.
Acidosis Defined
We are talking here about acidosis as a process or a trend toward acidemia, not acidemia, which is an actual change in blood pH. Acidemia is defined as a blood pH of less than 7.35. This is very unlikely to occur, as the body has multiple mechanisms for ensuring a very stable blood pH. Acidosis only becomes acidemia when compensatory measures become overwhelmed. This typically only happens in “advanced disease” like kidney and lung failure. In many ways, we can consider acidosis as the constant pressure on the body’s physiology to compensate for all the acid-inducing challenges. Equally important, although the blood pH does not change, the pH in the cells and intracellular space becomes more acidic, causing disruption of enzyme function, loss of insulin sensitivity, and cellular metabolic adaptations.
There are 3 terms used in the research to measure acid load in the body. Although technically somewhat different from a research perspective, I will use them interchangeably here as clinically they are essentially the same1:
-
Net Endogenous Acid Production (NEAP)
NEAP represents the amount of net acid produced by the metabolic system every day; a combination of cellular metabolism and exogenous acid and base loads from the diet.
It can be calculated (and most often is) based on dietary constituents (estimated) or measured directly using diet/stool/urine samples.
-
Net Acid Excretion (NEA)
NEA, the net acid excretion by the kidneys, often very close in value to NEAP and usually considered equivalent.
Can be measured directly and includes urinary excretion of ammonium, titratable acids, and bicarbonate.
-
Potential Renal Acid Load (PRAL)
Calculated estimate of NEAP, based on protein and mineral intake of diet, but also dependent on body surface area.
Causes of Acidosis
The body is subjected to excess acid pressure primarily in 3 ways: diet/beverages, drugs, and disrupted metabolism.
Diet-induced Acidosis
When talking about diet-induced acidosis, please be clear we are not talking about the pH of the food or beverages consumed. Rather, this is about the acid/base changes induced by the food constituents. The preagricultural diets we evolved on are estimated to be base-producing, with a mean NEAP of negative 88 mEq/d. In contrast, according to NHANES III, the average diet in the United States is acid-producing, with an NEAP of positive 48 mEq/d.2 This is the equivalent of 4.9 g HCl being added to our metabolism every day. Although, as discussed below, the kidneys and lungs get rid of almost—but not all—of this excessive acidity, when these systems start to fail, calcium from bone is used instead as the buffer. The mineral content from 3 g of bone is needed to neutralize 1 g of acid. As I will show below, this excessive acidity turns out to be a seriously underrecognized cause of osteoporosis. The obvious question, then, is: What constituents of diet cause acidity, versus those that increase alkalinity? The answer is surprising.
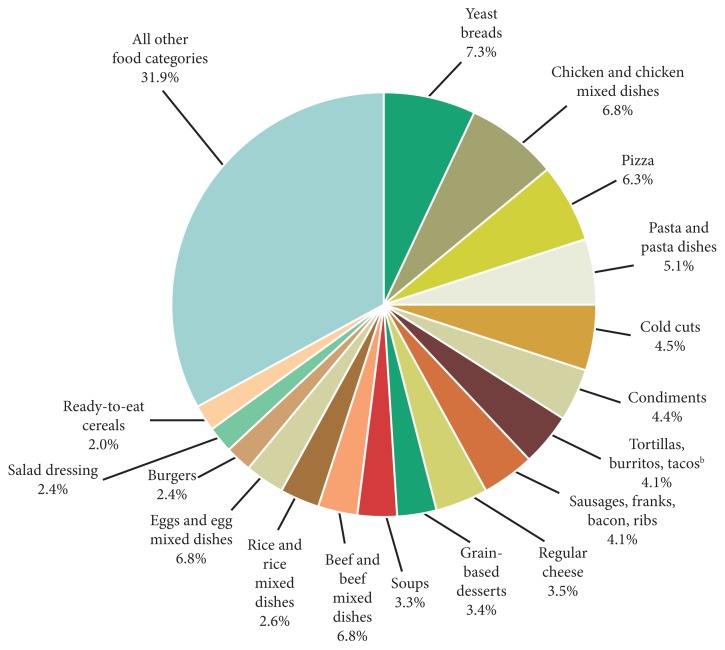
The primary sources of acidity in the diet are sulfur-containing amino acids, salt, and phosphoric acid in soft drinks (For a more complete discussion of the adverse effects of phosphates, see Lara Pizzorno’s article in IMCJ 13.6).3 You will likely immediately scoff that salt is neutral in pH and is not metabolized to anything that is acid—and you would be right. Nonetheless, research has clearly shown that—happily reversibly—NaCl accounts for 50% of the net acidity of the average American diet.4 The mechanism is not definitively known, it is currently thought to be impairment of the kidney’s ability to excrete acid compounds. Figure 1 shows the sources of salt in the typical Western diet. If you look closely, you will see that wheat products are the primary source of salt—which may account for the common belief that wheat products are acid forming (wheat itself is only slightly acid forming).
Figure 1.
Sources of NaCl in the American diet.a
aData are drawn from analyses of usual dietary intake conducted by the National Cancer Institute. Foods and beverages consumed were divided into 97 categories and ranked according to sodium contribution to the diet. “All other food categories” represent food categories that each contributes less than 2% total intake of sodium from foods.
bAlso includes nachos, quesadillas, and other Mexican mixed dishes.
Drug-induced Acidosis
Many commonly prescribed drugs induce acidity in the body through a variety of mechanisms.5 Most important are:
Impaired Renal H+ Excretion
NSAIDs; β-blockers; ACE inhibitors and angiotensin II type 1 receptor antagonists; K+-sparing diuretics, such as amiloride and triamterene; antibacterials, such as trimethoprim (commonly administered in combination with sulfamethoxazole as cotrimoxazole); and many more.
Lactic Acidosis
Biguanides, such as metformin; antiretrovirals; statins; and several others.
Metabolic-induced Acidosis
Several diseases disrupt metabolism in ways that cause excessive acidity (or is it the other way around or a vicious cycle). Most important are renal disease, diabetes, diarrhea, pancreatic drainage, biliary fistula, Sjogren’s syndrome, systemic lupus erythematosus, urinary tract obstruction, fever, aldosterone deficiency, and androgen deficiency.6 Interesting, acidosis causes insulin resistance and insulin resistance increases metabolic acidity—another vicious cycle.7
Metabolic Adaptation to Excessive Acid Load
The primary ways the body deals with excessive acidity are through renal adaptations, respiration, and buffering with calcium from bone.
Renal Adaptation
The renal adaptations are extensive:
Increased urinary excretion of sulfate, phosphate, urate, and chloride;
Increased urinary excretion of calcium;
Decreased urinary excretion of citrate;
Increased urinary excretion of ammonium ions; and
Kidney vasodilatation and increased glomerular filtration rate.
Be clear that the kidneys mitigate but do not eliminate all the excess acidity. After considering all the unnecessary work put on the kidneys to deal with this excess acidity, it occurred to me that perhaps this is a key cause for the loss of kidney function seen with aging. As the kidneys lose function with aging, their ability to excrete acid becomes impaired, which may be another explanation for the loss of bone with aging.8
Bone for Acid Buffering
The major reservoir of base is the skeleton (in the form of alkaline salts of calcium), which provides the buffer needed to maintain blood pH and plasma bicarbonate concentrations when renal and respiratory adaptations are inadequate. Acid-promoting diets are associated with increased urinary excretion of both calcium and bone matrix protein and decreased bone density.9 Neutralizing acid intake with diet or alkalinizing supplements decreases urine Ca and bone matrix protein excretion. Also, to a much smaller degree, skeletal muscle can act as a buffer.
Clinical Effects of Acidosis
A considerable amount of research is now showing a strong, causal relationship between acidosis and several common diseases. The most important are osteoporosis and kidney stones, but growing research is adding cancer, muscle wasting, metabolic syndrome, impaired glutathione synthesis, and others.
Osteoporosis
Dietary acidosis increases bone loss, osteoporosis, and fractures. Both pre- and postmenopausal women and older men eating an acid-forming diet have lower density in the spine and hip bones.10 Making the extracellular environment of the bones more acidic results in activation of osteoclasts and inhibition of osteoblasts causing a net loss of calcium from the bone.11 Surprisingly, a tiny extracellular pH reduction of 0.1 is sufficient to cause a doubling of calcium resorption from the bone.
Kidney Stones
Recent research has now shown that dietary acid load is the best predictor of both calcium oxalate and, surprisingly, urate stones.12 The key here are 3 factors in the renal adaptation to the excessive acidity: increased secretion of calcium (making the urine over saturated), decreased secretion of citrate (which solublizes calcium oxalate in the urine), and increased excretion of uric acid (this oversaturates the urine which has little capacity to solubilize uric acid)—all of these lead to precipitation and kidney stones.
Cancer
Although diet-induced acidosis may indeed increase risk of cancer, at this time there is no clinical research. Cell and animal studies have shown that lowering pH levels in the extracellular space promotes the invasive and metastatic potential of cancer cells.13 May be important, we just don’t know yet.
Assessment of Acid Load
Unfortunately, the traditional use of first morning urine for measuring body acidity is not valid. There are 2 reasons: (1) The pH paper typically used has very poor reproducibility, and (2) First morning urine does not correlate with standard measures such as NEAP.14,15 Fortunately, body acidity can be easily measured, with 24-hour urine, using a good-quality pH meter.
Intervention
A growing body of research has now clearly documented clinical benefit through diet and alkalinizing supplements. A diet rich in fruits and vegetables and low in animal protein and sodium chloride reduces acid load and is consistently associated with greater bone density.16 Alkalinization through supplementation with potassium and magnesium citrates decreases urinary excretion of calcium, increases bone density, and decreases fracture.17 This is especially interesting, as this reversal of osteoporosis is accomplished without increasing either vitamin D or calcium!18
Equally important is the research on prevention and treatment of kidney stones. Prospective studies have now shown that supplementation with potassium and magnesium citrates prevents recurrence of calcium oxalate stones by a remarkable 85%.19 More exciting, however, is research showing dissolution of kidney stones. One study found that 5 of 8 patients completely dissolved their stones after 6 months supplementation with potassium citrate/bicarbonate.20
Other areas are showing benefit from alkalinization such as strength training, aerobic exercise, and pain reduction.21,22,23
Contraindications to Alkalinizing Supplements
Although nutritional supplement intervention is typically safe, there are some contraindications that must be considered:
In congestive heart failure, sodium bicarbonate impairs arterial oxygenation and reduces systemic and myocardial oxygen consumption in these patients, which may lead to transient myocardial ischemia (although sodium bicarbonate is a very inexpensive alkalizing agent, I strongly recommend against as the sodium prevents most of the benefit).
In chronic obstructive pulmonary disease, bicarbonate loading may worsen exercise response (again, this is sodium bicarbonate, other alkalinizing agents may be fine).
Patients with kidney failure may develop elevated blood K levels and potentially fatal cardiac arrhythmias if given K alkali salts, or volume overload and breathing problems if given Na alkali salts.
Conclusion
Looking at the research on acidosis has been fascinating. I find it so encouraging when, yet again, an old-time concept is validated with modern research. No, they did not get it all right, as most have been using first morning urine to inaccurately assess body acid load and many use the much-less-effective sodium bicarbonate for alkalinization. Nonetheless, the concept of excessive acidity damaging health and increasing disease risk is sound, and I think we are seeing only the tip of the iceberg.
In this Issue
We continue featuring a keynote speaker of an upcoming conference of integrative medicine organizations we support. This interview of Jeffrey Bland, phd, and Patrick Hanaway, md, adds to my excitement for the next Institute for Functional Medicine symposium, “The Omics Revolution: Nature and Nurture.” The evolving research on how dramatically gene expression is modified by diet, environment, lifestyle, etc, is fundamentally revolutionizing how we think about health and disease.
I now serve on the science boards of 2 foundations that fund integrative medicine cancer research. In the 15 years I have been reviewing cancer research proposals, the most exciting has been the work of Gerald Krystal, phd. His innovative study of the effects of protein and carbohydrates on cancer is fascinating and the results with animal cancers phenomenal. I am very excited he agreed to be interviewed by IMCJ and hope his research soon advances to human clinical trials.
Whenever a submission comes to me and its title starts with “Integrative Treatment…” I immediately move it to the front of the line. Everyone who has practiced for several years realizes that few diseases have a single cause. Thus, curative medicine requires considering everything that went into the patient getting to their complex clinical situation. Childhood obesity is a very good example of this complexity. Jennifer A. Boisvert, phd, and W. Andrew Harrell, phd, provide us excellent guidance for helping children with this huge challenge for restoring their health. I especially appreciate how comprehensively they cover the social, environmental, psychological, and medical aspects that must be considered.
In final article of the 2-part series, Matthew J. Bull, bsc, phd; and Nigel T. Plummer, phd, continue to develop their important thesis of the huge impact of the human gut microbiome on health. I have a clinical anecdote that so validates this concept: I saw a young woman who had been suffering from inflammatory bowel disease and came to see me because her symptoms were not well controlled, and she was starting to experience significant adverse drug reactions. History revealed that her irritable bowel syndrome started about a year after taking daily broad spectrum antibiotics for her acne. Of course, her family doctor had not made the connection. In addition to eliminating allergic foods, we had her get a fecal transplant from her healthy father. Within a few weeks, her symptoms were substantially decreased, and within 3 months, she no longer needed any of the drugs. Her final comment to me, “My farts now smell like my dad’s!” says it all.
I was so sad to hear of Hal Huggins’s dds passing and pleased we are able to publish his obituary. He was truly a pioneer in integrative medicine, courageously raising the alarm about the dangers of mercury fillings. As a pioneer, he was aggressively persecuted by the vested interests he challenged so effectively. I still vividly remember our televised debate with Victor Herbert, md, jd, some 20 years ago. It was contentious; Herbert was constantly insulting, and Huggins kept his cool and effectively made his important points. (For those who are wondering, I ended up as the moderate between their two extremes.)
We are trying something new this issue: publication of poster abstracts from a conference, in this case the 15th Annual Science and Clinical Application of Integrative Holistic Medicine at Scripps. We prioritized those with clinical relevance. Would very much appreciate your letting us know whether these are useful to you, dear reader.
I fully realize that “Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health” by Katarzyna Maresz, phd, is the third time we have addressed the important topic. No apology, as this is an extremely important topic and the research continues to evolve. I still find surprising that diet rarely provides adequate vitamin K2. Makes me think this is likely due to the gut flora changes of Western diets that make endogenous conversion of K1 to K2 much less than the gut flora we evolved with.
As John Weeks so clearly presents, the last election resulted in a lot of turnover in senate committee chairs. Although most seem to think only liberals are interested in integrative medicine, many on the conservative side support us as well—though it seems unlikely we will ever again have such a strong and effective friend as Senator Tom Harkin (D-Iowa), who retired in 2014. Nonetheless, the message is clear—we need to commit to educating the new Senate leadership. Good medicine knows no political boundaries.
Bill Benda, md, in BackTalk continues his always insightful commentary/satire. His comments about the ridiculous fear mongering around Ebola reflect my concerns as well. I watched in amazement as the media on both sides of the political spectrum somehow found, in virtually every comment by politicians on Ebola, ammunition of some sort to show why they were—incompetent, out-of-touch, un-American, etc. Very unfortunate that fear appears to be such a strong attention grabber and motivator of behavior. Bill is right, we need leadership and vision, not fear mongering.
Joseph Pizzorno, nd, Editor in Chief
drpizzorno@innovisionhm.com
References
- 1.Pizzorno J, Frassetto LA, Katzinger J. Diet-induced acidosis: is it real and clinically relevant? Br J Nutr. 2010;103(8):1185–1194. doi: 10.1017/S0007114509993047. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC., Jr Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 2002;76(6):1308–1316. doi: 10.1093/ajcn/76.6.1308. [DOI] [PubMed] [Google Scholar]
- 3.Pizzorno L. Canaries in the Phosphate-Toxicity Coal Mines. Integr Med Clin J. 2014;13(6):24–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Frassetto LA, Morris RC, Jr, Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am J Physiol Renal Physiol. 2007;293(2):F521–F525. doi: 10.1152/ajprenal.00048.2007. [DOI] [PubMed] [Google Scholar]
- 5.Liamis G, Milionis HJ, Elisaf M. Pharmacologically-induced metabolic acidosis: a review. Drug Saf. 2010;33(5):371–391. doi: 10.2165/11533790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Gluck SL. Acid–base. Lancet. 1998;352(9126):474–479. doi: 10.1016/S0140-6736(98)03087-6. [DOI] [PubMed] [Google Scholar]
- 7.Souto G, Donapetry C, Calviño J, Adeva MM. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord. 2011;9(4):247–253. doi: 10.1089/met.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271(6 Pt 2):F1114–F1122. doi: 10.1152/ajprenal.1996.271.6.F1114. [DOI] [PubMed] [Google Scholar]
- 9.Buclin T, Cosma M, Appenzeller M, et al. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. 2001;12(6):493–499. doi: 10.1007/s001980170095. [DOI] [PubMed] [Google Scholar]
- 10.New SA, MacDonald HM, Campbell MK, et al. Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr. 2004;79(1):131–138. doi: 10.1093/ajcn/79.1.131. [DOI] [PubMed] [Google Scholar]
- 11.Arnett TR. Extracellular pH regulates bone cell function. J Nutr. 2008;138(2):415S–418S. doi: 10.1093/jn/138.2.415S. [DOI] [PubMed] [Google Scholar]
- 12.Trinchieri A, Lizzano R, Marchesotti F, Zanetti G. Effect of potential renal acid load of foods on urinary citrate excretion in calcium renal stone formers. Urol Res. 2006;34(1):1–7. doi: 10.1007/s00240-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 13.Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond) 2012;9(1):72. doi: 10.1186/1743-7075-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting SJ, Muirhead JA. Measurement of net acid excretion by use of paper strips. Nutrition. 2005;21(9):961–963. doi: 10.1016/j.nut.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 16.Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69(4):727–736. doi: 10.1093/ajcn/69.4.727. [DOI] [PubMed] [Google Scholar]
- 17.Jehle S, et al. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(1):207–217. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 18.Jehle S, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17(1):3213–3222. doi: 10.1681/ASN.2006030233. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A. Potassium magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158(6):2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri A, Esposito N, Castelnuovo C. Dissolution of radiolucent renal stones by oral alkalinization with potassium citrate/potassium bicarbonate. Arch Ital Urol Androl. 2009;81(3):188–191. [PubMed] [Google Scholar]
- 21.McNaughton, Backx K, Palmer G, Strange N. Effects of chronic bicarbonate ingestion on the performance of high intensity work. Eur J Appl Physiol Occup Physiol. 1999;80(4):333–336. doi: 10.1007/s004210050600. [DOI] [PubMed] [Google Scholar]
- 22.Dawson-Hughes B, Castaneda-Sceppa C, Harris SS, et al. Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporos Int. 2010;21(7):1171–1179. doi: 10.1007/s00198-009-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vormann J, Worlitschek M, Goedecke T, Silver B. Supplementation with alkaline minerals reduces symptoms in patients with chronic low back pain. J Trace Elem Med Biol. 2001;15(2–3):179–183. doi: 10.1016/S0946-672X(01)80064-X. [DOI] [PubMed] [Google Scholar]