Abstract
Context
Little is known about the effect of nutritional supplementation on metabolic optimization for enhancing adaptation and recovery of the connective tissue elements that support musculoskeletal function.
Objective
The study aimed to determine the potential effect of supplementation with a novel, hydrolyzed chicken sternal cartilage extract—called BioCell Collagen—on biomarkers and functional indices of recovery from intense exercise.
Design
The research team designed a randomized, double-blind, placebo-controlled pilot study.
Setting
The study was conducted at the Center for Applied Health Sciences in Stow, OH, USA.
Participants
Participants were 8 healthy, recreationally active individuals, with a mean age of 29.3 y.
Intervention
Participants ingested either 3 g of a novel, hydrolyzed chicken sternal cartilage extract called BioCell Collagen (“supplement”) or 3 g of a placebo daily for 6 wk prior to challenge with an upper-body, muscle-damaging resistance exercise (UBC) on day 43 and a rechallenge on day 46 to assess functional recovery.
Outcome Measures
Primary endpoints were levels of 3 blood biomarkers—creatine kinase (CK), lactate dehydrogenase (LDH), and C-reactive protein (CRP)— and scores on a clinical pain scale and a perceived recovery scale (PRS).
Results
The extract attenuated the post-UBC increase in serum markers for muscle tissue damage: CK, LDH, and CRP. For the intervention group vs the placebo group, the mean changes were as follows: (1) an increase in CK of 20 U/L vs 4726 U/L, respectively; (2) a decrease in LDH of 3.5 U/L vs an increase of 82.9 U/L, respectively; and (3) an increase in CRP of 0.07 mg/L vs an increase of 0.7 mg/L, respectively. The performance decrement in bench press repetitions to failure was 57.9% on day 43 and 57.8% on day 46 for the intervention group vs 72.2% on day 43 and 65% on day 46 for the placebo group. The overall trend for the performance decrement, together with the results for the PRS, suggested that a more robust muscular recovery and adaptive response occurred with use of the extract. The supplement was well tolerated.
Conclusions
The study’s preliminary data are promising with regard to the beneficial effects of the extract on connective tissue protection and recovery in those engaged in routine resistance training and cardiovascular exercise. A larger study is warranted to confirm and refine these findings.
The use of mechanical stressors that overload skeletal muscle and associated connective tissue often result in some degree of exercise-induced muscular damage (EIMD). EIMD results in delayed-onset muscle soreness (DOMS) in association with an increased presence of muscle-specific proteins in the blood, muscle edema, and fiber swelling.1 Traditionally, DOMS has largely been explained by myogenic factors associated with structural alteration of the sarcolemmal membrane, damage to Z disks of sarcomeres and disruption of cytoskeletal proteins (ie, dystrophin, desmin, titin, integrins, and fibronectin).2,3 However, it is clear that shear stress and injury to other extramuscular elements of connective tissue (extracellular matrix [(ECM)] proteins; basal lamina; types 1, 3, 4, and 6 collagen; proteoglycan/glycosoaminoglycans [GAGs]; epimysium/perimysium/endomysium; and tendon)—are also involved.4,5 This structural alteration of the skeletal muscle tissue is known to limit performance in repetitive activities due to a decreased range of motion, decreased force production, and pain.
It is well established that ECMs of both the muscles and tendons are sensitive to the mechanical stimuli and tension generated by muscular contractions, particularly by a lengthening or eccentric muscle action against resistance from either isotonic, isometric, or isokinetic modalities. A substantial body of evidence has demonstrated that exercise leads to local enhancement of metabolic activity, circulatory responses, and collagen turnover, together with an elevated expression of growth factors, such as insulin growth factor 1 (IGF-1) and transforming growth factor β (TGF-β).4
The resulting biochemical and molecular responses within both skeletal muscles and tendons promote adaptation to mechanical loading and physical activity. Hence, although high-force eccentric contractions are associated with EIMD and microtrauma or injury, they are also known to provide, paradoxically, significant protection against future injury through what is known as the repetitive bout effect (RBE).4,6,7 For example, compared with a novel bout of eccentric exercise that causes muscle damage and loss of strength, a subsequent bout of eccentric exercise, when performed days or even weeks later, is associated with less loss of contractile force, a decreased soreness, and a reduction in the amount of muscle proteins in the blood, the biomarkers of tissue disruption, when compared with the first bout of exercise.
In a rat model, RBE was shown to occur as early as 2 days after the first bout of contractions of the dorsiflexor muscles in the left hind limbs, and it persisted for several weeks. This protective effect occurred against different types of the second EIMD.6 More studies need to be conducted, particularly in humans, to further the understanding of specific agents or conditions that enhance the role of the musculoskeletal ECM in mechanotransduction and force transmission and in the RBE (ie, the protective effect that a novel exercise bout has on subsequent exercise sessions).
Nutritional agents that are capable of metabolic optimization of the ECM and the connective tissue components of muscle and its myotendinous junctions have the potential to provide safe and cost-effective tools that can aid in the recovery from intense or unaccustomed exercise bouts. Because the supplement used in the current study contains collagen peptides and GAGs of low molecular weight that are derived from cartilage ECM, its intake possibly could increase the pool of building blocks for ECM molecules of the musculoskeletal connective tissue, providing the nutritional replenishment or balance necessary for its dynamic homeostasis.
Recent reports from human studies on the supplement used in the current study suggest that daily supplementation with it can improve the health of joint and dermal connective tissue. First, daily intake of the supplement has been shown to reduce symptoms associated with moderate and advanced-stage osteoarthritis, such as pain and physical difficulties.8 Schwartz and Park9 found that the supplement also attenuated various signs of facial aging, such as wrinkles, while enhancing blood microcirculation and potentially affecting dermal collagen content. In addition, intake of hydrolyzed chicken cartilage or collagen hydrolysate has been shown to increase the concentrations of collagen-derived peptides, such as prolyl-hydroxyproline, in human blood; in vitro and animal studies have suggested that collagen-derived peptides are biologically active in stimulating or protecting cells residing in various connective tissues.10,11
Thus, the current research team has hypothesized that the extract might positively affect the metabolism of musculoskeletal connective tissue in response to intense strength training. In the current study, the team has studied the ability of the extract to attenuate several biochemical markers of damage to connective and skeletal muscle tissues and improved functional resilience against intense exercise in recreationally active individuals.
Methods
Experimental Approach
A contract research organization, the Center for Applied Health Sciences in (Stow, OH, USA) designed the current single-center, randomized, double-blind, placebo-controlled, parallel-groups study. Subjects were recruited from northeastern Ohio, a typical suburban region, by word of mouth and posted announcements. Each potential candidate received a physical examination and completed a questionnaire on their health history prior to acceptance into the study. Subjects were required to have normal values for lipids, liver, and kidney function tests and be willing to maintain their usual dietary and physical activity habits. Subjects were excluded from the study if they had a body mass index (BMI) greater than 40 kg/m2; thyroid disease; hypogonadism; a history of musculoskeletal, autoimmune, or neurologic disease; or if they were currently taking thyroid, hyperlipidemic, hypoglycemic, antihypertensive, or anticoagulant medications. A randomization schedule for allocation was generated to assign consecutive individuals to 1 of 2 study groups in a 1:1 ratio.
The study was conducted in compliance with an independent institutional review board (IRB) and according to the International Conference on Harmonization Good Clinical Practice Guidelines. The protocol was reviewed, and all procedures were approved by an independent IRB (IntegReview, Austin, TX, USA; protocol No. BCT-001-2013, approved 28 October 2013). All participants provided written informed consent for participation prior to commencement of any study-related activities.
Intervention
The dietary supplement tested in the study was BioCell Technology (Newport Beach, CA, USA) BioCell Collagen, a hydrolyzed chicken sternal cartilage extract composed of a naturally occurring matrix of hydrolyzed collagen type 2 (approximately 1.5 to 2.5 kDa) and of chondroitin sulfate and hyaluronic acid of low molecular weights. Each capsule contained 500 mg of the supplement: 300 mg of hydrolyzed collagen type 2, 100 mg of chondroitin sulfate, and 50 mg of hyaluronic acid. Uncharacterized components of sternal cartilage account for the remaining 50 mg. The placebo capsule contained 500 mg of cellulose and was indistinguishable from the intervention group’s capsule on examination by blinded investigators. Based on their groups, participants were instructed to take either 3 capsules (1.5 g) of the supplement or the placebo in the morning and 3 capsules (1.5 g) in the evening.
Each participant ingested the 3 g daily of either the supplement or the placebo for 6 weeks prior to the posttest protocol.
Testing Protocol
Table 1 shows the testing protocol for the study.
Table 1.
Testing Protocol
| Test | Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | ||
| Informed Consent | x | |||||||||
| Physical Activity (Yale) Questionnaire | x | x | x | |||||||
| Health History Questionnaire | x | |||||||||
| Physical Exam | x | x | ||||||||
| Comprehensive Blood Chemistry | x | x (pre) | ||||||||
| Serum CK, LDH, CRP | x | x (pre) | x | x | x | x | x | x | ||
| Vitals | x | x | x | x | x | |||||
| Height | x | |||||||||
| Body Weight | x | x | x | x | x | |||||
| PRS | x | x | x | x | ||||||
| Anchored 150 mm VAS (pain, soreness) | x | x | x | x | x | x | ||||
| Upper-body Exercise Challenge/Performance | x | x | ||||||||
| Diet Record Analysis | x | x | ||||||||
| Side Effect Questionnaire | x | x | x | x | ||||||
Abbreviations: CK, creatine kinase; LDH, lactate dehydrogenase; CRP, C-reactive protein; PRS, perceived recovery status; VAS, visual analogue scale.
Note: “Pre” indicates the outcome measures that were done before the upper-body stress challenge.
Upper-body Resistance Exercise Stress Challenge (UBC)
The upper-body resistance exercise stress challenge (UBC) consisted of 8 sets of barbell bench presses at 75% of body weight. Participants completed as many repetitions as possible using a 4-second eccentric phase, with no pause at the bottom of the movement and an explosive concentric-phase tempo of execution. Rest periods between sets were 90 seconds. The number of repetitions performed and completed within each set was recorded for comparison of set-to-set performance and to identify the total repetitions completed during the entire 8-set challenge.
The repetition decrement was calculated simply from the difference between the number of repetitions completed on the initial set (ie, the first set used as a reference set) and the number of repetitions completed in the subsequent 7 sets. The test was performed after 6 weeks of supplementation, on day 43, and then again 3 days later on day 46 to assess recovery of muscle strength and endurance and return of functional capacity.
Control of Diet, Exercise Training, and Physical Activity
A research dietitian met with each participant to explain the proper procedures for recording dietary intake (serving sizes, times of day, methods of preparation, etc).
Each participant’s baseline diet, which included 3 days—2 weekdays and 1 weekend day—was analyzed via NutriBase IV Clinical Edition (Cybersoft Inc, Phoenix, AZ, USA) to determine its energy and macronutrient content. Additional 3-day diet records were analyzed during the last day of testing on day 49 to verify that eating habits remained consistent throughout the study. In addition, 24 hours prior to each bench press test, participants duplicated the diets that they had eaten before the prior test using their records.
Participants were asked to maintain their usual physical activity patterns during the study. Each participant’s activity was assessed via the Yale Physical Activity Questionnaire at baseline (ie, prior to enrollment) and on the last day of testing (day 49).
Compliance with the supplementation protocol was monitored by having participants complete a supplementation log. In addition, participants were required to return their empty supplement containers, and they were reminded of details associated with the study’s protocols through weekly text messages and/or e-mails. Participants were instructed to refrain from using other dietary supplements in the course of the study.
Outcome Measures
As shown in Table 1, the primary endpoints were levels of 3 blood biomarkers—creatine kinase (CK), lactate dehydrogenase (LDH), and C-reactive protein (CRP)—and scores on a clinical pain scale and a perceived recovery scale (PRS). The clinical pain scale was a 150-mm visual analog scale (VAS) that measured soreness of different affected muscle groups, discomfort, and pain with activities or movement of the upper body. The PRS measurement was based on a self-reported description of readiness to exercise on a graded scale (0–10), which is known to be negatively associated with high-volume resistance exercise (lowered PRS) together with a rise in serum indices of muscle damage such as CK.13
The primary endpoints were measured (1) at baseline (ie, presupplementation); (2) at 42 days postintervention, before the UBC; and (3) on days 43, 44, 45, 46, 47, 48, and 49. Secondary endpoints, measured after 6 weeks of supplementation (ie, on day 43, and again on day 46) measured functional performance (ie, the difference between groups in total repetitions performed on the repeated stress test/challenge) to assess decrements and return of function (repeat UBC). Tertiary endpoints regarding safety were measured (1) at baseline (ie, presupplementation); (2) postsupplementation on day 43; and (3) on day 49, the final day of the study. They included vital signs—heart rate and blood pressure—and comprehensive, clinical chemistry panels of plasma and whole-blood-cell counts.
Statistical Analyses
Data from the current study were independently entered into separate Microsoft Excel (Microsoft, Redmond, WA, USA) spreadsheets. The 2 spreadsheets were compared for discrepancies, which were resolved by a third party, and then the final data was imported into the SPSS statistical software, version 21.0 (IBM, Armonk, NY, USA) for purposes of analysis.
Because the current pilot study had only 4 participants in each arm, formal statistical analyses (ie, determination of P values) were de-emphasized. Instead, data were analyzed descriptively (means, standard deviation [SD], range, etc), and the effect sizes were calculated with the mean and SD for 2 independent samples of equal size using the following formula:
where x1 and x2 were the means of the intervention and the placebo groups, and σ1 and σ2 were the SDs of the 2 groups. Effect sizes on various outcome measures were used to measure the magnitude of the treatment effects from use of the supplement and to determine the sample size necessary to achieve statistical significance in a larger follow-up study. The current research team decided not to include P values in the current article because the team believed the values would not appropriately represent the potential effect of the current preliminary data, as sample size and variability would prevent effective statistical significance.
Safety
Treatment-dependent adverse events (AEs) and side effects were coded using MedDRA (Medical Dictionary for Regulatory Activities). Typically, AEs occurring in more than 2% of subjects are presented. However, because this pilot study was small scale and exploratory, any and all side effects and AEs were reported. Participants were contacted on a weekly basis via e-mail or telephone and queried for AEs.
Results
A total of 8 individuals, 6 males and 2 females, were enrolled in the study. Participants were 18 to 55 years old, healthy, and recreationally active but not highly trained. Table 2 shows that the baseline characteristics of participants were comparable between the intervention and placebo groups. It should be noted that participants in the 2 groups were of similar fitness status and levels of physical activity per the Yale Physical Activity Score (data not shown). All the participants completed the study per protocol.
Table 2.
Baseline Characteristics of Participants (N = 8)
| Intervention Group | Placebo Group | ||
|---|---|---|---|
| Gender, n (%) | Male | 3 (75%) | 3 (75%) |
| Female | 1 (25%) | 1 (25%) | |
| Age, y | Mean ± SD | 32.5 ± 12.8 | 25.8 ± 2.2 |
| Min, Max | 21, 49 | 23, 28 | |
| Weight, lbs | Mean ± SD | 166.4 ± 41.31 | 173.8 ± 18.14 |
| Min, Max | 121, 204.6 | 153, 197 | |
| Height, inches | Mean ± SD | 67.0 ± 3.16 | 69.3 ± 3.30 |
| Min, Max | 63, 70 | 65, 73 | |
| BMI, kg/m2 | Mean ± SD | 25.7 ± 4.16 | 25.5 ± 2.39 |
| Min, Max | 21.4, 29.4 | 22.4, 28.3 | |
| Systolic BP, mm Hg | Mean ± SD | 124.3 ± 13.05 | 124.5 ± 3.70 |
| Min, Max | 107, 138 | 120, 129 | |
| Diastolic BP, mm Hg | Mean ± SD | 71.0 ± 15.45 | 67.3 ± 11.18 |
| Min, Max | 48, 80 | 53, 80 |
Abbreviations: BMI, body mass index; BP, blood pressure; SD, standard deviation; Min, minimum; Max, maximum.
Plasma Biomarkers
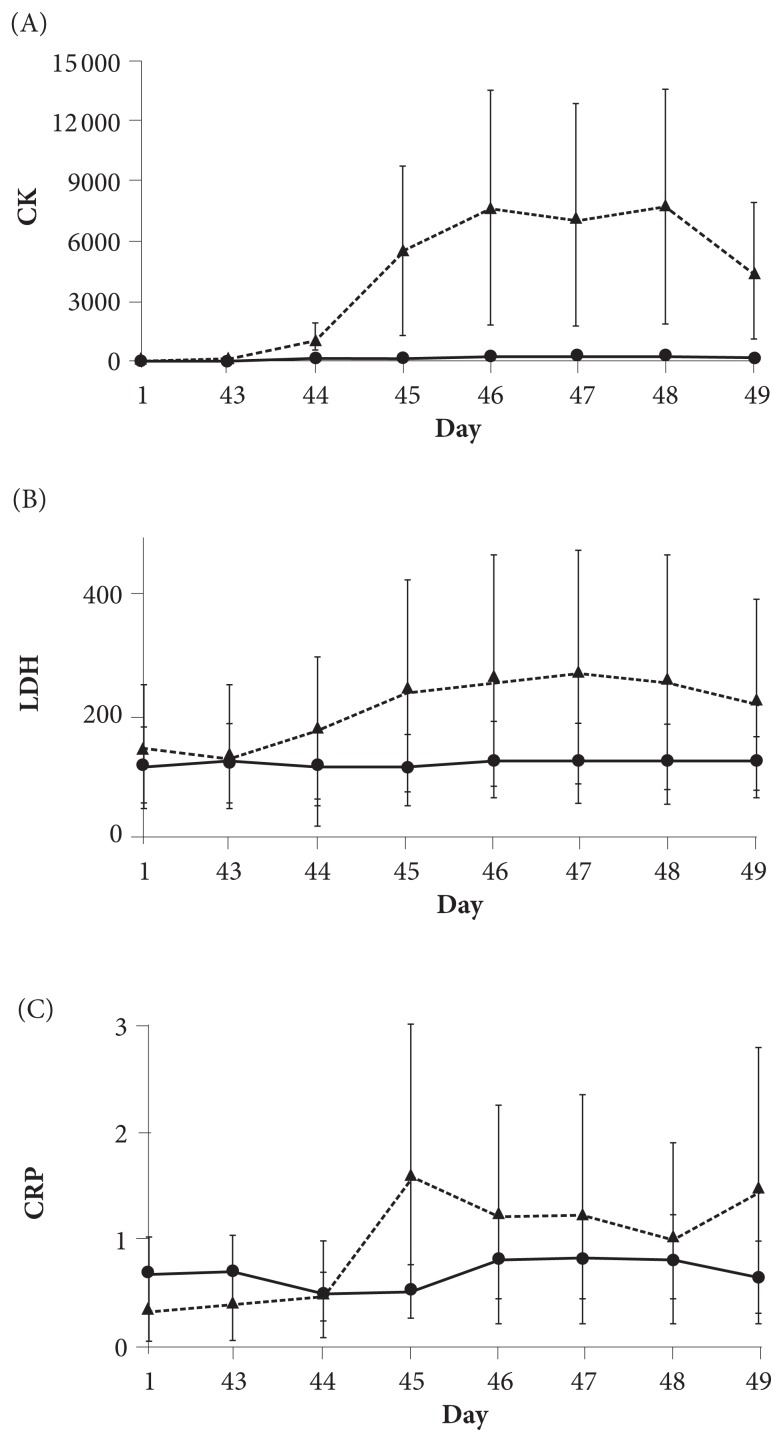
Figure 1 shows changes in the concentrations of biomarkers for muscle tissue damage post-UBC. In response to the first UBC, levels increased in both groups, but the participants in the intervention group showed a much smaller increase in the concentrations of CK and LDH from day 44 to day 49 than those supplemented with the placebo. Specifically, post-UBC, the mean levels of CK and LDH for the intervention group decreased by 9.3 U/L, moving from 81.6 to 72.3 U/L, and by 7.5 U/L, moving from 9.8 to 2.3 U/L, respectively. For the same measures, the placebo group’s levels increased by 935.0 U/L, moving from 6678.5 to 7613.5 U/L, and by 26.0 U/L, from 103 to 129 U/L, respectively. Further, post-UBC levels of the plasma biomarker for inflammation, CRP, also appeared to be lower in the intervention group, ranging from a decrease of 0.23 mg/L to an increase of 0.13 mg/L, than it was in the placebo group, ranging from an increase of 0.15 mg/L to an increase of 1.23 mg/L. The range of effect sizes in each biomarker was 0.98 to 1.0 for CK, 0.96 to 1.09 for LDH, and 0.61 to 1.9 for CRP.
Figure 1.
Concentrations of biomarkers for muscle tissue damage post-UBC. Figure 1A shows CK; Figure 1B shows LDH; and Figure 1C shows CRP. A solid line represents a change in the plasma concentration of each biomarker in the intervention group, whereas a dotted line represents a change for the placebo group. All values are mean ± SD.
Abbreviations: UBC, upper-body, muscle-damaging, resistance exercise; CK, creatine kinase; LDH, lactate dehydrogenase; CRP, C-reactive protein; SD, standard deviation.
Performance Decrement and RBE
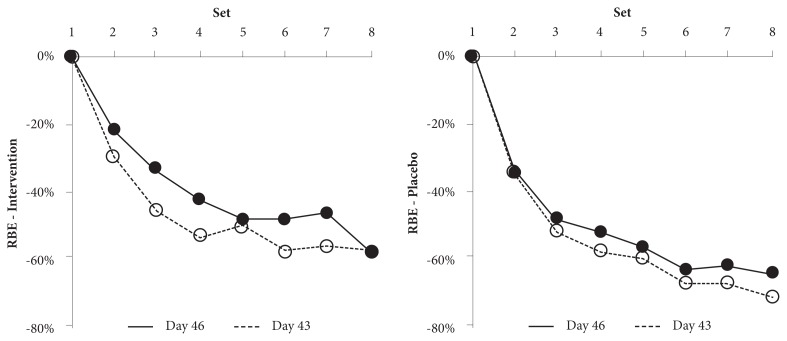
For postbaseline bench press performance, Table 3 shows the change in the repetition number from the first set. The intervention group appeared to show a consistently smaller decrease in performance than the placebo group on both day 43 and day 46. During the final set, the decrement in bench press repetitions to failure was 57.9% for day 43 and 57.8% for day 46 for the intervention group versus 72.2% for day 43 and 65.0% for day 46 for the placebo group, suggesting that the supplement was more effective than the placebo in enhancing the resilience of the musculoskeletal tissue to intense upper-body exercise. In addition, both groups displayed a smaller performance decrement on day 46 as compared with that on day 43, suggesting that the RBE was present in both the intervention and the placebo groups. Figure 2 shows, however, that the RBE might have been augmented in the intervention group.
Table 3.
Change in Number of Completed Bench-press Repetitions From First Set for Postbaseline Bench Press
| (A) Day 43 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Set | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Intervention | 9.5 ± 1.0 | 6.7 ± 1.3 | 5.2 ± 1.0 | 4.5 ± 0.8 | 4.7 ± 1.3 | 4.0 ± 1.0 | 4.2 ± 1.0 | 4.0 ± 0.6 |
| Change | −29.5% | −45.3% | −52.6% | −50.5% | −57.9% | −55.8% | −57.9% | |
| Placebo | 11.5 ± 11.2 | 7.5 ± 6.1 | 5.5 ± 9.4 | 4.7 ± 9.6 | 4.5 ± 10.1 | 3.7 ± 10.3 | 3.7 ± 10.3 | 3.2 ± 10.1 |
| Change | −34.8% | −52.2% | −59.1% | −60.9% | −67.8% | −67.8% | −72.2% | |
| (B) Day 46 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Set | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Intervention | 8.3 ± 2.5 | 6.5 ± 2.2 | 5.5 ± 2.2 | 4.8 ± 1.9 | 4.3 ± 2.5 | 4.3 ± 2.5 | 4.5 ± 2.6 | 3.5 ± 1.5 |
| Change | 0.0% | −21.7% | −33.7% | −42.2% | −48.2% | −48.2% | −45.8% | −57.8% |
| Placebo | 10.0 ± 6.8 | 6.5 ± 3.1 | 5.2 ± 5.0 | 4.7 ± 5.9 | 4.2 ± 6.3 | 3.5 ± 5.8 | 3.7 ± 5.9 | 3.5 ± 5.8 |
| Change | 0.0% | −35.0% | −48.0% | −53.0% | −58.0% | −65.0% | −63.0% | −65.0% |
Abbreviation: SD, standard deviation.
Note: All values are mean ± SD.
Figure 2.
Intergroup comparison of potential RBE. Figure 2A shows the RBE in the intervention group, and 2B shows it for the placebo group. The solid line represents the performance decrement on day 46, whereas the dotted line represents the performance decrement on day 43. All values are mean ± SD for sets 1 through 8 of the bench press exercise. The error bars were omitted for clarity.
Abbreviations: RBE, repetitive bout effect; SD, standard deviation.
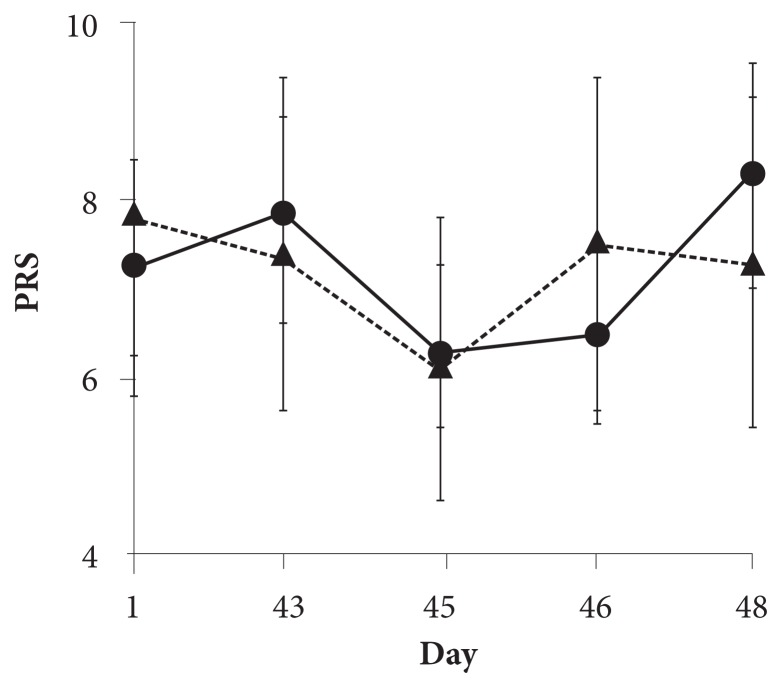
Perceived Recovery Scale
Figure 3 shows the changes in the PRS during the study. Six weeks of supplementation did not seem to affect PRS on day 43 for either group, as pre-UBC PRS was 7.8 in the intervention group and 7.5 in the placebo group. The initial bout of muscle-damaging UBC resulted in a decrease in the PRS of 1.5 units on day 45 for the intervention group and 1.3 units for the placebo group. PRS increased on the following day to the pre-UBC level in the placebo group, whereas it increased by 0.2 in the intervention group. After the second UBC on day 46, however, PRS increased by 1.8 within 2 days for the intervention group, whereas it decreased by 0.2 in the placebo group, resulting in the PRS on day 48 being 8.3 in the intervention group and 7.3 in the placebo group (effect size = 0.66).
Figure 3.
PRS scores during the study. The solid line represents the change in the PRS in the intervention group, whereas the dotted line represents the change in the placebo group. All values are mean ± SD.
Abbreviations: PRS, perceived recovery scale; SD, standard deviation.
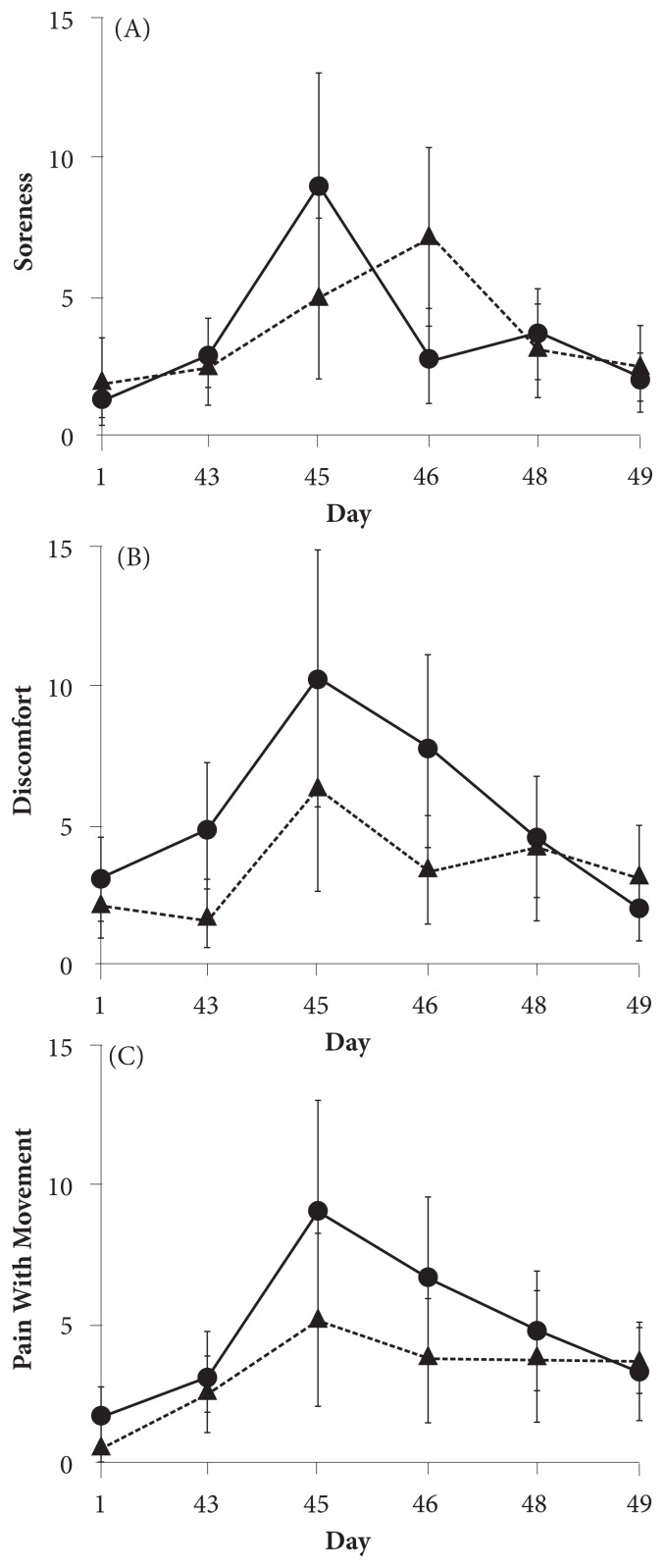
VAS Measures of DOMS
Figure 4 displays the changes in the upper-body DOMS during the study. After the initial bout of muscle-damaging UBC, on day 45, the DOMS appeared to peak, as expected, at 48 hours post-UBC for both groups, with a pattern of greater magnitude in the intervention group. However, similar to the pattern of PRS after rechallenge on day 46, the intervention group appeared to show an accelerated improvement in DOMS by day 48 as compared with the placebo group. For the intervention group versus the placebo group, the VAS measurements on day 48 were as follows: (1) soreness—2.3 versus 2.8, respectively; (2) discomfort—2.1 versus 3.1, respectively; and (3) pain with movement—3.3 versus 3.6, respectively, showing that the score on the overall clinical pain scale for the intervention group was lower than that for the placebo group on day 48. The effect sizes on day 48 were 0.11 for soreness, 0.64 for discomfort, and 1.12 for pain with movement.
Figure 4.
VAS scores for DOMS. Figure 4A shows soreness, 4B shows discomfort, and 4C shows pain with movement. The solid line represents the change in DOMS in the intervention group, whereas the dotted line represents the change in the placebo group. All values are mean ± SD.
Abbreviations: VAS, visual analog scale; DOMS, delayed-onset muscle soreness; SD, standard deviation.
Diet, Training, and Activity
No changes in diet, training, or habitual physical activity were noted during the study (data not shown).
Safety
During the study, 2 participants in the placebo group experienced nonserious AEs, whereas those in the intervention group did not experience any AEs. Each AE that occurred during the study was mild in intensity, transient in duration, and deemed unlikely to be related to the study’s supplement, placebo, or treatment. None were documented as serious (SAE).
Discussion
The daily intake of 3 g of the supplement was well tolerated by participants during the study, which was consistent with its safety profile as observed in earlier human studies, where daily doses of 1 g and 2 g were ingested by healthy females for 12 weeks and by osteoarthritic patients for 10 weeks, respectively.8,9 In an animal study, 33 times the human daily dose was also shown to be well tolerated by rats.13
The present, proof-of-concept pilot study demonstrated that 6 weeks of supplementation with BioCell Collagen, as compared with the placebo, attenuated biochemical markers of skeletal muscle tissue damage in plasma following 2 bouts of muscle-damaging exercise. The magnitude of the changes in these measures and the substantial effect sizes, ranging from 0.98 to 1.9 at peak times, support the research team’s hypothesis that the supplement effectively mitigated the muscle-damaging effects of intense, eccentric loading and resistance exercise stress.
In addition, for both day 43 and day 46, the decrease in the total number of repetitions performed, 57.9% and 57.8%, respectively, was lower for the intervention group in the course of the 8 sets of upper-body stress tests than for the placebo group, which had a 72.2% decrement on day 43 and a 65.0% decrement on day 46. This finding suggests that the supplement containing hydrolyzed, low-molecular-weight ECM molecules, such as collagen peptides and GAGs derived from the chicken cartilage, might support the connective tissue/ECM integrity of skeletal muscle, such that an enhanced stress resilience to exhaustive, high-intensity resistance exercise occurred. Further, although a typical RBE (ie, the protective effect of an initial bout of exercise relative to muscle damage, soreness, and markers of damage in subsequent bouts of exercise) was observed in both groups, the intervention group appeared to demonstrate a more robust RBE. Because RBE is considered to occur through the interaction of multiple factors, including nerves, sarcomeres, and connective tissue,4,6,7 the amplified RBE as well as the lower performance decrement in the intervention group appear to give a tentative support to the current research team’s hypothesis that the supplement might enhance not only protective adaptation but also recovery of the musculoskeletal tissue to the stress of exhaustive resistance exercise. Supplement-mediated protective adaptation could also explain the attenuated concentrations of CK, LDH, and CRP of the intervention group as compared with the placebo group.
It is intriguing that the reduced levels of biomarkers and the lower performance decrement in the intervention group after the first UBC did not appear to be in accordance with the increased VAS measurements of soreness and discomfort. However, this discordance was apparent only after the initial bout of stressful bench presses on day 43. By the final test on day 48, 2 days after rechallenge, the intervention group seemed to have experienced an augmented or accelerated recovery, such that the group demonstrated less soreness and generally improved PRS. The research team considers this phenomenon to be consistent with an enhanced remodeling response related to skeletal muscle connective tissue/ECM in the intervention group that was not as apparent in the placebo group.
Given the different physiologic mechanisms of pain perception and nociception, the current research team believes it is reasonable to conjecture that the supplement, although it provided nutritional and metabolic support to the ECM and connective tissue elements of skeletal muscle, did not interfere with the inflammatory response that sensitized delta sensory nerves and small C-fiber nociceptive afferent neurons.14 Hence, this result might explain the soreness present in the intervention group after the initial stress bout, followed by an accelerated recovery and adaptation after the repeat bout.
Taken together, the plasma/performance/PRS data were consistent with the study’s hypothesis that the supplement might enhance the musculoskeletal tissue and/or ECM remodeling in response to repeated, intense resistance exercise. Based on the observed effect sizes in the current pilot study, it was calculated that approximately 30 finishers per group would be required for 80% power and statistical significance at P < .05. Future larger studies would also need to include additional, more specific markers of connective tissue recovery/turnover (eg, matrix metalloproteinase [MMPs], carboxyterminal propeptide of type 1 procollagen/carboxy-terminal telopeptide of type 1 collagen [PICP/ICTP], and type 3 procollagen amino terminal propeptide [P3NP]) and measure changes in body composition and adaptations to training with time.
Conclusions
The study’s preliminary data are promising with respect to the beneficial effects of the extract on connective tissue protection and recovery in those engaged in routine resistance training and cardiovascular exercise. A larger study is warranted to confirm and refine these findings.
Acknowledgements
The authors would like to thank the participants in the study and the sponsor (BioCell Technology). The presentation of results of this study does not constitute endorsement by the any of the investigators, the Center for Applied Health Sciences, or the journal in which it is published.
Footnotes
Author Disclosure Statement
The study was sponsored by BioCell Technology, LLC. Hector L. Lopez and Tim N. Ziegenfuss have no conflict of interest related to the study. Joosang Park was a salaried employee of BioCell Technology at the time the study was completed. This work has been presented previously as poster presentation in the Annual Meeting of International Society of Sports and Nutrition (2014).
References
- 1.Brentano MA, Martins Kruel LF. A review on strength exercise-induced muscle damage: applications, adaptation mechanisms and limitations. J Sports Med Phys Fitness. 2011;51(1):1–10. [PubMed] [Google Scholar]
- 2.Kanda K, Sugama K, Hayashida H, et al. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. 2013;19:72–85. [PubMed] [Google Scholar]
- 3.Clarkson PM, Tremblay I. Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol (1985) 1988;65(1):1–6. doi: 10.1152/jappl.1988.65.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Kjaer M, Magnusson P, Krogsgaard M, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208(4):445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21(6):749–757. doi: 10.1111/j.1600-0838.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Dipasquale DM, Bloch RJ, Lovering RM. Determinants of the repeated-bout effect after lengthening contractions. Am J Phys Med Rehabil. 2011;90(10):816–824. doi: 10.1097/PHM.0b013e3182240b30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McHugh MP, Connolly DA, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med. 1999;27(3):157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Schauss AG, Stenehjem J, Park J, Endres JR, Clewell A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: a randomized, double-blind, placebo-controlled trial. J Agric Food Chem. 2012;60(16):4096–4101. doi: 10.1021/jf205295u. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz SR, Park J. Ingestion of BioCell Collagen(®), a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin Interv Aging. 2012;7:267–273. doi: 10.2147/CIA.S32836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oesser S, Seifert J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003;311(3):393–399. doi: 10.1007/s00441-003-0702-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohara H, Iida H, Ito K, Takeuchi Y, Nomura Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci Biotechnol Biochem. 2010;74(10):2096–2099. doi: 10.1271/bbb.100193. [DOI] [PubMed] [Google Scholar]
- 12.Sikorski EM, Wilson JM, Lowery RP, et al. Changes in perceived recovery status scale following high-volume muscle damaging resistance exercise. J Strength Cond Res. 2013;27(8):2079–2085. doi: 10.1519/JSC.0b013e31827e8e78. [DOI] [PubMed] [Google Scholar]
- 13.Schauss AG, Merkel DJ, Glaza SM, Sorenson SR. Acute and subchronic oral toxicity studies in rats of a hydrolyzed chicken sternal cartilage preparation. Food Chem Toxicol. 2007;45(2):315–321. doi: 10.1016/j.fct.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Michaelis M, Vogel C, Blenk KH, Amarson A, Jänig W. Inflammatory mediators sensitize acutely axotomized nerve fibers to mechanical stimulation in the rat. J Neurosci. 1998;18(18):7581–7587. doi: 10.1523/JNEUROSCI.18-18-07581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]