Abstract
Context
Osteoarthritis, sometimes called degenerative joint disease, is the most common form of arthritis. It affects more than 20 million people in the United States, who mostly are older than age 45 y. No specific treatment exists to halt the progressive cartilage degeneration of osteoarthritis or to repair the damaged cartilage. Alternatives to pharmaceuticals include natural therapies and nutritional supplements.
Objective
The present study examined the clinical response to daily supplementation with bioactive protein complex containing a collagen type 2 network, with associated growth factors, and osteoinductive proteins, known as bone morphogenetic proteins (BMPs).
Design
The research team designed an open-label preliminary study.
Setting
Joint evaluations were self-administered by participants at their residences.
Participants
Participants were 44 individuals with self-reported osteoarthritis in the hip, knee, or ankle (ie, in weight-bearing joints).
Intervention
Participants self-administered orally a low-dose form of the ingredient (ie, 150 mg of Cyplexinol® Standard, once daily for 4 wk).
Outcome Measures
Four parameters—pain intensity, frequency of pain, activity level, and strength—were evaluated using a visual analog scale (VAS), both at baseline and at the end of the 4-wk period.
Results
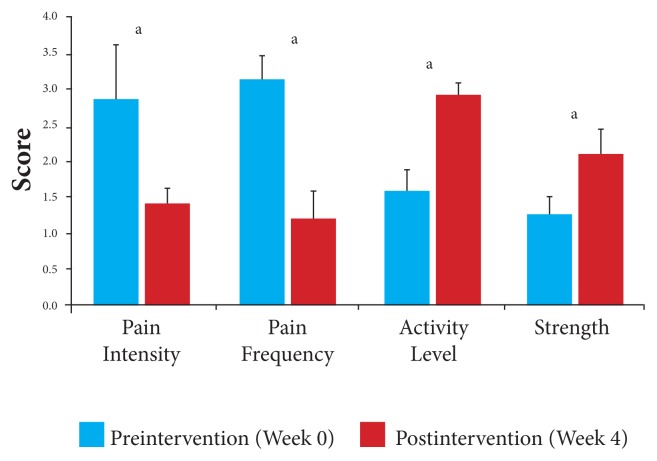
Pain intensity decreased 45% on a 10-cm VAS from 2.85 cm (P = .0001; 95% CI, 2.54, 3.16) at baseline to 1.41 cm (P = .0001; 95% CI, 1.18, 1.64) at wk 4. Pain frequency decreased 55% from 3.16 cm (P = .0001; 95% CI, 2.88, 3.44) at baseline to 1.22 cm (P = .0001; 95% CI, 0.85, 1.59) at wk 4. Activity level increased from 1.58 cm (P = .0001; 95% CI, 1.34, 1.82) at baseline to 2.91 cm (P = .0001; 95% CI, 2.71, 3.11) at wk 4. Strength increased 80% from 1.24 cm (P = .0001; 95% CI, 0.91, 1.57) at baseline to 2.10 cm (P = .0001; 95% CI, 1.73, 2.47) at wk 4.
Conclusions
The 44 participants reported subjective improvements in pain frequency and intensity as well as in activity level following administration of Cyplexinol®. The results from this study identify the possible pathways associated with cartilage degradation, pain, and inflammation, which should be the focus of future research, and they suggest that the ingredient can be effective in helping to maintain joint homeostasis.
Arthritis defines more than 100 conditions that affect joints and other parts of the body. Osteoarthritis, sometimes called degenerative joint disease, is the most common form of arthritis, affecting more than 20 million people in the United States, who mostly are older than age 45 years.1 Osteoarthritis is caused by the progressive breakdown and eventual loss in the joints of cartilage that acts as a cushion between the bones. Most individuals show the effects in the hands, feet, spine, and large weight-bearing joints, such as the hips and knees.2
No specific treatment exists to halt the progressive degeneration of cartilage that osteoarthritis causes or to repair the damaged cartilage.3 Current treatments aim to reduce joint pain and inflammation, while improving and maintaining joint function.4 Acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) are common treatments for patients with osteoarthritis.5 Although those therapies have known therapeutic value, they have also shown significant negative effect on human health.6 The issues include kidney damage and gastrointestinal side effects ranging from upset stomach, cramps, and diarrhea to ulcers and internal bleeding.6–8 The side effects have proven fatal in some cases.9
Alternatives to pharmaceuticals include natural therapies and nutritional supplements, such as glucosamine and chondroitin.10 Clegg et al11 found that both of those supplements were safe, with positive effects in patients with joint problems associated with osteoarthritis. However, that study’s findings were preliminary and not substantiated by results for its full cohort, providing mixed results at best.
For many years, clinicians and researchers alike have sought to uncover or develop either natural or synthetic therapies that can achieve cartilage tissue repair, while they bypass the negative effects of cyclooxygenase 2 (COX-2) inhibitors and other NSAIDs. Bone morphogenetic proteins (BMPs) have been extensively investigated for the last 4 decades. They are now being targeted by leading biomedical and biotechnology companies as therapeutic agents that can reduce the cartilage damage that is associated with arthritis as well as contribute to regenerative medicine.12,13
In addition, recent studies have shown that BMPs were more effective when used in combination with growth factors, such as insulin-like growth factor (IGF).14 IGF is one of the key growth factors present in the currently investigated ingredient, Cyplexinol®, which is a protein complex that contains a combination of growth factors and BMPs that have been implicated in enhanced bone and joint health. Accordingly, the aim of the current study is to evaluate the clinical response to the ingredient to provide indications as to which pathways should be the focus of future research.
Methods
Participants
The current study was an open-label, prospective trial with 44 participants who had self-reported osteoarthritis or degenerative joint disease. All participants were 55 years of age or older and were experiencing joint pain and stiffness.
Individuals were included in the study if they (1) had a self-reported, osteoarthritic condition of the hip, knee, or ankle (ie, of a weight-bearing joint); (2) had been suffering from regular, spontaneous pain and stiffness 5 times per week for at least 1 year prior to the study; (3) had pain-and-stiffness scores between 2 and 6 on a scale of 0 to 10; (4) had a body mass index (BMI) between 18.5 and 40.0; and (5) were able to provide consent and to understand and follow the protocol. Self-reported osteoarthritis was confirmed by an initial baseline evaluation that was administered over the phone at enrollment.
Although a clinical diagnosis was not a prerequisite for participation in the study, 68.2% of enrolled participants reported a diagnosis of osteoarthritis by their primary care physician. Osteoarthritis was not confirmed by a physician for the remaining 31.8%. However, during the screening process, the participants were asked questions designed to determine whether the reported pain could be attributed to conditions other than osteoarthritis, such as a traumatic or neuromuscular injury. Participants whose answers indicated that the reported symptoms might not be due to osteoarthritis were excluded from the study.
Individuals were excluded from the study if they (1) were aged 54 years or younger at the start of the study; (2) had rheumatoid or other forms of arthritis; (3) had joint pain as a result of nerve or muscle damage, accidents, falls, trauma, etc; (4) had comorbidities often associated with osteoarthritis, such as diabetes, cardiovascular disease, elevated cholesterol requiring medical intervention, renal insufficiencies, asthma, or hypertension requiring medical intervention; (5) had cancer within the prior 5 years; (6) were taking any other herbal product or other supplement for pain or inflammation of joints health, such as glucosamine/chondroitin/methylsulfonylmethane, S-adenosylmethionine, or omega-3; (7) were taking any NSAID, COX-2 inhibitor, disease-modifying antirheumatic drug, steroid or corticosteroid, tumor necrosis factor blockers, narcotics, or a controlled substance for pain as prescribed by a medical physician for any condition; (8) were smokers; (9) were heavy alcohol consumers; (10) were pregnant or nursing women; (11) had shellfish allergies; and (12) had a BMI lower than 18.5 (underweight) or greater than 40.0 (morbidly obese) or a body weight exceeding 225 lbs (102 kg). Individuals who were unwilling or unable to participate in a 4-week washout period prior to beginning the trial were also excluded from the study. Participants’ incomes were not used as selection criteria and, therefore, were not reported.
Participants were recruited from within the continental United States. A rolling-admission model was used, beginning in August 2011. By September 2011, 59 individuals had been screened, and 44 were enrolled in the trial, which began on October 2011 and was concluded in November 2011. They all provided written informed consent to participate in the study and agreed to comply with all guidelines for its duration. The demographics of the patient population are listed in Table 1.
Table 1.
Patients’ Demographics (N = 44)
| Demographic | Results |
|---|---|
| Age, mean ± SD (y) | 64.6 ± 12.3 |
| Average BMI, mean ± SD | 26.9 ± 3.1 |
| Weight, lbs, mean ± SD | 160.3 ± 15.1 |
| Geographic distribution | Continental United States |
| Gender | |
| Men | 55% |
| Women | 45% |
| Ethnicity | |
| Caucasian | 87.7% |
| African American | 9.3% |
| Other (Asian, Latino, Native American, etc) | 3.0% |
| Pain descriptors | |
| Mean pain levels at screening (0–10), ± SD | 2.85 ± 0.7 |
| Duration | ≥1 y |
| Occurrences of pain | ≥5/wk |
Abbreviations: SD, standard deviation; BMI, body mass index.
Intervention
ZyCal Bioceuticals Healthcare Company (Toms River, NJ, USA) has developed an all-natural, bioactive protein complex called Cyplexinol® Standard, with the goal of providing a more effective ingredient for patients with the reduced joint function, impaired mobility, pain, and inflammation that is associated with degenerative joint disease. The product is a ingredient and, therefore, this trial was not registered with the Food and Drug Administration.
The complex is an isolated form of certified organic bovine femurs. The hydroxyapatite component of the bone is removed, leaving behind the native, partially hydrolyzed collagenous network of type 1 collagen and associated growth factors. Chief among these growth factors are (1) transforming growth factor beta (TGF-β), (2) IGF, (3) basic fibroblastic growth factor (bFGF), (4) vascular endothelial growth factor (VEGF), and (5) isoforms of BMPs that have been investigated and reported in previous studies12,15,16 but are included at a lower concentration.
Through a proprietary process, ZyCal is able to maintain the natural physiological balance of the proteins in the product, ensuring that each lot is osteoinductive. Osteoinductivity refers to the ability of BMPs to activate, stimulate, and differentiate mesenchymal stem cells (MSCs) into osteoblasts and chondrocytes, for de novo growth of bone and cartilage.15,17,18 The proteins exhibit capabilities for repair of both joint and bone tissue as well as intrinsic anti-inflammatory properties, both essential for repairing tissue damage in arthritic patients.19–21
Participants were asked to undergo a 4-week washout period during which time they ceased all dietary supplements for bones, joints, and-inflammation as well as nonprescription medications, including over-the-counter (OTC) NSAIDs. Prescription medication that was not related to joint health was permitted, as was OTC rescue medication that was taken only on an as-needed basis. Participating individuals were also asked not to change any aspects of their lives during the 4-week trial. Lifestyle variables were unaltered, including but not limited to diet, fitness regimens, and work-related tasks. In addition, pain management therapies that did not include the application or ingestion of therapeutic agents were permitted, including but not limited to thermo- or cryotherapies, physical therapy, joint manipulation, massages, and stretching exercises of all types. Participants were asked to continue the use of these lifestyle approaches for pain management on an as-needed basis throughout the duration of the study.
Participants who passed the initial screening over the phone were provided with 1 bottle containing 30 capsules of Cyplexinol® Standard and 2 paper copies of a visual analog scale (VAS) questionnaire. Each participant was asked to fill out a baseline VAS questionnaire and return it to the investigator before beginning to take the capsules. Participants were given the study’s ingredient in the form of single O-size gelatin capsules. Each capsule comprised 150 mg of Cyplexinol® and approximately 300 mg of rice-flour filler. Thirty pills per bottle were prepared by an outside vendor (Atlantic Essential Products, Hauppauge, NY, USA), and all participants were given the ingredient from the same manufacturing lot and pill-press run. The raw materials were prepared by ZyCal in a good manufacturing process (GMP)-certified plant using approved standard operating procedures, which are also used to make the commercially available product. The material was triple tested for basic biochemistry, heavy metals—in accordance with Californian Proposition 65 Chemical list and bioactivity using an indirect enzyme-linked immunosorbent assay.
The participants were contacted 30 days later to confirm whether they had taken all 30 pills, 1 per day, and to remind them to fill out the remaining VAS questionnaire. All participants reported that they adhered to the regimen. On completion of the trial, each participant had returned the second VAS questionnaire.
Outcome Measures
A VAS scale of 0 to 10 for pain, frequency of pain, activity levels, and strength was employed to evaluate the 4 measures before and after 4 weeks of supplementation. VAS scores were reported by asking the participants to mark responses on a 10-cm line. The line contained both end anchor points and 3 additional descriptors that were evenly spaced along the VAS scale at 2.5-cm intervals to help orient the participant. Definitions for the descriptors for each scale are listed in Table 2.
Table 2.
Internal Descriptors for the VAS Scale
| VAS Value (cm) | Pain | Frequency of Pain | Activity Level | Strength |
|---|---|---|---|---|
| 0 | No pain | No pain | Can do all activities | No pain with heavy lifting |
| 2.5 | Mild pain | Occasional pain—25% of the day | Can do most activities | Increased pain with heavy lifting |
| 5 | Moderate pain | Intermediate pain—50% of the day | Can do some activities | Increased pain with moderate lifting |
| 7.5 | Severe pain | Frequent pain—75% of the day | Can do few activities | Increased pain with lightlifting |
| 10 | Worst possible pain | Constant pain—100% of the day | Cannot do any activities | Increased pain with any lifting |
Abbreviation: VAS, visual analog scale.
At the start of the evaluation, participants were told to notify the research team immediately if any mild, moderate, or severe adverse events occurred during the period of supplementation. Criteria for those adverse events were described to participants. Participants were also instructed to notify the team should they require any rescue treatment, such as OTC NSAIDs to allow them to document the need. During the 30-day follow-up phone conversation, participants were once again asked whether they had experienced any adverse events or required any form of rescue treatment during the supplementation period, and none were reported.
The criteria for mild, moderate, and severe adverse events were as follows: (1) mild—an adverse event that does not interfere with usual day-to-day activities and requires no special intervention or treatment; (2) moderate—an adverse event that can affect usual daily activities and that can be addressed with simple therapeutic treatments; (3) severe—an adverse event that requires therapeutic intervention (hospitalization automatically implies a severe event).
Statistical Analysis
Data gathered from the VAS questionnaires underwent statistical analysis using a 2-tailed, paired t test with the Graphpad PRISM statistical analysis software, version 5.0 (La Jolla, CA, USA). The alpha that was used for statistical significance was .05.
Results
Of the 44 participants enrolled in the study, none withdrew or were removed due to medical complications or lack of compliance with the study’s protocol. The number of participants and their self-reported osteoarthritic joint(s) are listed in Table 3.
Table 3.
Participants and Self-reported Osteoarthritic Joints (N = 44)
| Affected Joint | No. of Participants | Incidence |
|---|---|---|
| Ankles | 2 | 4.5% |
| Back (spine) | 8 | 18.2% |
| Feet (metatarsals) | 1 | 2.3% |
| Hands | 6 | 13.6% |
| Hips | 5 | 11.4% |
| Knees | 14 | 31.8% |
| Shoulders | 4 | 9.1% |
| Fibromyalgia | 4 | 9.1% |
All Participants
In comparison with baseline, the results for all participants at the end of the 4 weeks for all measures were statistically significant. Overall, participants showed a 49% decrease in joint pain, from a mean VAS score of 2.85 (P = .0001; 95% CI, 2.54, 3.16) at baseline to 1.41 (P = .0001; 95% CI, 1.18, 1.64) postintervention, and a 54% decrease in the frequency of pain, from a mean VAS score of 3.16 (P = .0001; 95% CI, 2.88, 3.44) at baseline to 1.22 postintervention (P = .0001; 95% CI, 0.85, 1.59), after the 4 weeks of the study (Figure 1, Table 4). Further, a 57% improvement in activity levels, from a mean VAS score of 1.58 (P = .0001; 95% CI, 1.34,1.82) at baseline to 2.91 postintervention (P = .0001; 95% CI, 2.71, 3.11), and an 80% improvement in affected joint strength, from a mean VAS score of 1.24 (P = .0001; 95% CI, 0.91, 1.57) at baseline to 2.10 postintervention (P = .0001; 95% CI, 1.73, 2.47), was observed after the 4 weeks.
Figure 1.
All participants: Changes in mean VAS scores in response to 4 weeks of daily supplementation.
Abbreviation: VAS, visual analog scale.
aP < .05.
Table 4.
Primary Outcomes for All Participants After 4 Weeks of Daily Supplementation
| Primary Outcome | Number | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|---|
| Mean VAS Scores | SD | 95% CI | Mean VAS Scores | SD | 95% CI | ||
| Pain Intensity | 44 | 2.85 | 0.7 | 2.54, 3.16 | 1.41 | 0.5 | 1.18, 1.64 |
| Pain Frequency | 44 | 3.16 | 0.7 | 2.88, 3.44 | 1.22 | 0.9 | 0.85, 1.59 |
| Activity Level | 44 | 1.58 | 0.6 | 1.34, 1.82 | 2.91 | 0.5 | 2.71, 3.11 |
| Strength | 44 | 1.24 | 0.8 | 0.91, 1.57 | 2.10 | 0.9 | 1.73, 2.47 |
Abbreviations: VAS, visual analog scale; SD, standard deviation; CI, confidence interval.
Measurements by Joint
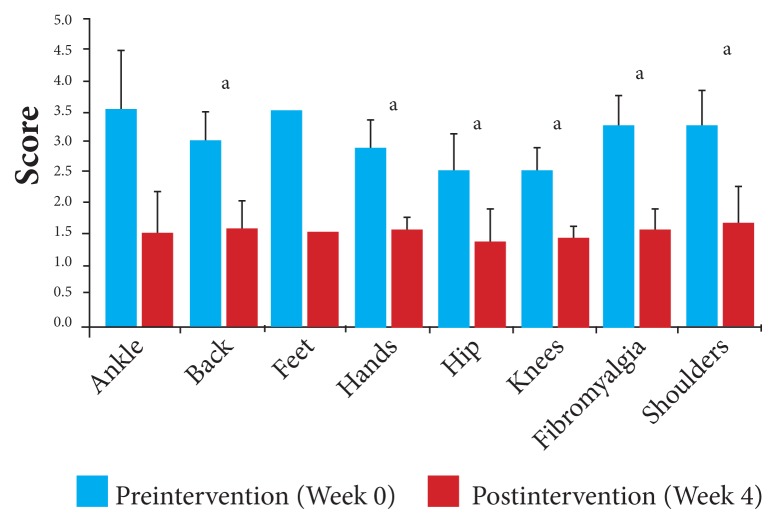
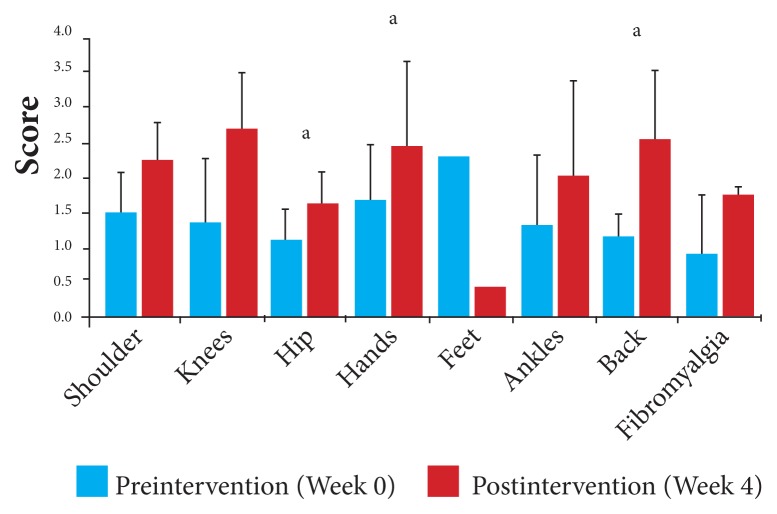
Using the data for each of the 4 measures for each joint, changes between baseline and the end of the study were calculated. With a mean decrease overall of 49% in pain intensity from baseline, the scores by joint for pain intensity ranged from a 46% reduction for those participants with affected hip and knee joints to a 57% reduction for those with affected ankle and feet joints. Pain intensity, pre- and postintervention, for individual joints is displayed in Figure 2 and Table 5.
Figure 2.
Pain intensity by affected joint: Changes in mean VAS in response to 4 weeks of daily supplementation.
Abbreviation: VAS, visual analog scale.
aP < .05.
Table 5.
Changes in Pain Intensity by Affected Joint After 4 Weeks of Daily Supplementation
| Affected Joints | Number | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|---|
| Mean VAS Scores | SD | 95% CI | Mean VAS Scores | SD | 95% CI | ||
| Ankle | 2 | 3.50 | 0.71 | 2.52, 4.48 | 1.50 | 0.71 | 0.79, 2.48 |
| Back | 8 | 3.00 | 0.46 | 2.54, 3.32 | 1.56 | 0.68 | 1.09, 2.03 |
| Feet | 1 | 3.50 | --- | --- | 1.50 | --- | --- |
| Hands | 6 | 2.88 | 0.59 | 2.41, 3.35 | 1.50 | 0.32 | 1.25, 1.75 |
| Hip | 5 | 2.50 | 0.71 | 1.88, 3.12 | 1.35 | 0.49 | 0.92, 1.78 |
| Knees | 14 | 2.54 | 0.77 | 2.14, 2.94 | 1.38 | 0.42 | 1.16, 1.60 |
| Fibromyalgia | 4 | 3.25 | 0.50 | 2.76, 3.74 | 1.50 | 0.41 | 1.10, 1.90 |
| Shoulders | 4 | 3.25 | 0.65 | 2.62, 3.88 | 1.63 | 0.75 | 0.88, 2.36 |
Abbreviations: VAS, visual analog scale; SD, standard deviation; CI, confidence interval.
With a mean reduction overall of 54% in frequency of pain from baseline, the scores by joint for frequency of pain ranged from 45% for those participants with affected knee joints, with a mean VAS score of 2.86 (95% CI, 2.28, 3.44) at baseline decreasing to 1.59 (95% CI, 0.99, 2.19) postintervention, to 86% for those with affected ankle joints, with a mean VAS score of 3.30 (95%, CI, 2.71, 3.89) at baseline decreasing to 0.45 (95% CI, 0.35, 0.55) postintervention (Figure 3, Table 6).
Figure 3.
Pain frequency by affected joint: Changes in mean VAS scores in response to 4 weeks of daily supplementation.
Abbreviation: VAS, visual analog scale.
aP < .05.
Table 6.
Changes in Pain Frequency by Affected Joint After 4 Weeks of Daily Supplementation
| Preintervention | Postintervention | ||||||
|---|---|---|---|---|---|---|---|
| Affected Joints | Number | Mean VAS Scores | SD | 95% CI | Mean VAS Scores | SD | 95% CI |
| Shoulder | 4 | 3.25 | 0.29 | 2.97, 3.53 | 1.38 | 0.75 | 0.63, 2.11 |
| Knees | 14 | 2.86 | 1.10 | 2.28, 3.44 | 1.59 | 1.15 | 0.99, 2.19 |
| Hip | 5 | 3.32 | 0.46 | 2.92, 3.72 | 1.62 | 1.39 | 0.40, 2.84 |
| Hands | 6 | 3.00 | 1.10 | 2.12, 3.88 | 1.50 | 1.26 | 0.49, 2.51 |
| Feet | 1 | 3.00 | --- | --- | 0.50 | --- | --- |
| Ankles | 2 | 3.30 | 0.42 | 2.71, 3.89 | 0.45 | 0.07 | 0.35, 0.55 |
| Back | 8 | 3.14 | 0.59 | 2.73, 3.55 | 1.38 | 0.74 | 0.86, 1.90 |
| Fibromyalgia | 4 | 3.18 | 0.62 | 2.57, 3.79 | 1.13 | 0.25 | 0.89, 1.37 |
Abbreviations: VAS, visual analog scale; SD, standard deviation; CI, confidence interval.
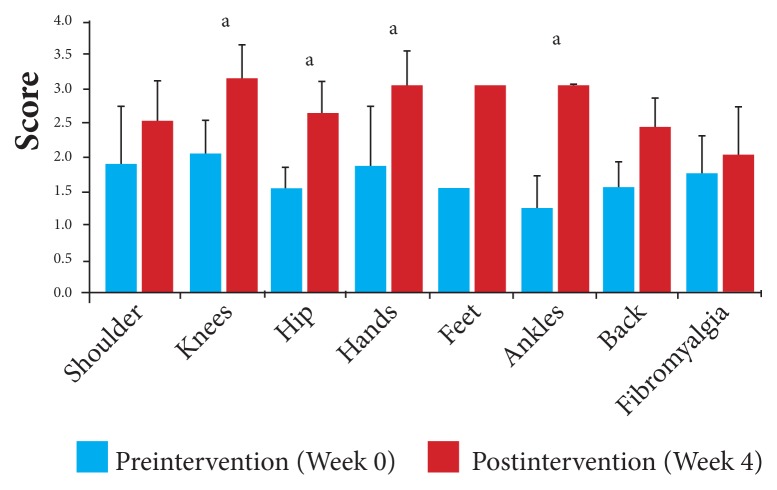
With a mean improvement overall of 57% in activity levels from baseline, the scores by joint for activity levels ranged from 14% for those participants with self-reported fibromyalgia, with a mean VAS score of 1.75 (95% CI, 1.2, 2.24) at baseline to 2.00 (95% CI, 1.31, 2.69) postintervention, to 140% for those affected with ankle-joint problems, with a mean VAS score of 1.25 (95% CI, 0.76, 1.74) at baseline to 3.00 (95% CI 3.00, 3.00) postintervention (Figure 4, Table 7).
Figure 4.
Activity level by affected joint: Changes in mean VAS in response to 4 weeks of daily supplementation.
Abbreviation: VAS, visual analog scale.
aP < .05.
Table 7.
Changes in Activity Level by Affected Joint After 4 Weeks of Daily Supplementation
| Preintervention | Postintervention | ||||||
|---|---|---|---|---|---|---|---|
| Affected Joints | Number | Mean VAS Scores | SD | 95% CI | Mean VAS Scores | SD | 95% CI |
| Shoulder | 4 | 1.88 | 0.85 | 1.04, 2.72 | 2.50 | 0.58 | 1.93, 3.07 |
| Knees | 14 | 2.00 | 0.98 | 1.49, 2.51 | 3.14 | 0.86 | 2.69, 3.59 |
| Hip | 5 | 1.50 | 0.35 | 1.19, 1.81 | 2.60 | 0.55 | 2.12, 3.08 |
| Hands | 6 | 1.83 | 1.13 | 0.93, 2.73 | 3.00 | 0.71 | 2.43, 3.57 |
| Feet | 1 | 1.50 | --- | --- | 3.00 | --- | --- |
| Ankles | 2 | 1.25 | 0.35 | 0.76, 1.74 | 3.00 | 0.00 | 3.00, 3.00 |
| Back | 8 | 1.56 | 0.50 | 1.22, 1.90 | 2.44 | 0.56 | 2.05, 2.83 |
| Fibromyalgia | 4 | 1.75 | 0.50 | 1.26, 2.24 | 2.00 | 0.71 | 1.31, 2.69 |
Abbreviations: VAS, visual analog scale; SD, standard deviation; CI, confidence interval.
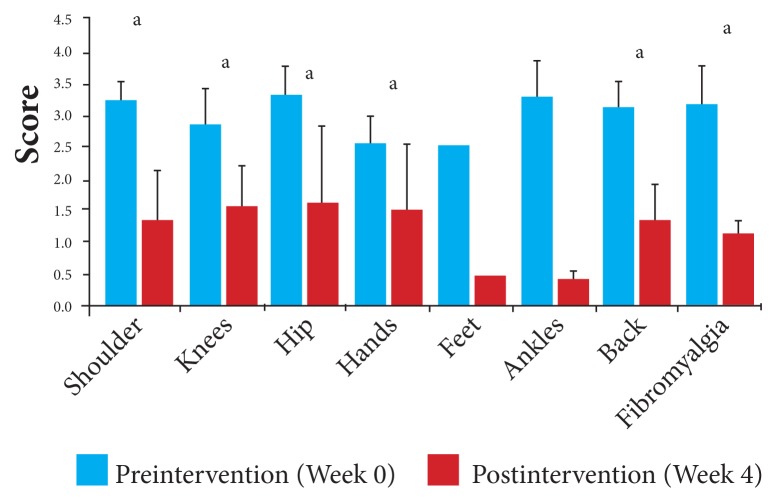
With a mean increase overall of 80% in joint strength from baseline, the scores by joint for joint strength ranged from 45% for affected hand and knee joints, to 128% for affected back (spine) joints, with a mean VAS score of 1.13 (95% CI, 0.89, 1.37) at baseline to 2.56 postintervention (95% CI, 1.88, 3.24). Joint strength, pre- and postintervention, for individual joints is displayed in Figure 5 and Table 8.
Figure 5.
Joint strength by affected joint: Changes in mean VAS scores in response to 4 weeks of daily supplementation.
Abbreviation: VAS, visual analog scale.
aP < .05.
Table 8.
Changes in Level of Strength by Affected Joint
| Preintervention | Postintervention | ||||||
|---|---|---|---|---|---|---|---|
| Affected Joints | Number | Mean VAS Scores | SD | 95% CI | Mean VAS Scores | SD | 95% CI |
| Shoulder | 4 | 1.50 | 0.58 | 0.93, 2.07 | 2.25 | 0.50 | 1.76, 2.74 |
| Knees | 14 | 1.36 | 0.86 | 1.11, 1.81 | 2.71 | 0.75 | 2.32, 3.10 |
| Hip | 5 | 1.10 | 0.42 | 1.10, 1.47 | 1.60 | 0.42 | 1.23, 1.97 |
| Hands | 6 | 1.67 | 0.82 | 1.02, 2.32 | 2.42 | 1.20 | 1.46, 3.38 |
| Feet | 1 | 1.50 | --- | --- | 3.00 | --- | --- |
| Ankles | 2 | 1.25 | 1.06 | −0.22, 2.72 | 2.00 | 1.41 | 0.04, 3.96 |
| Back | 8 | 1.13 | 0.35 | 0.89, 1.37 | 2.56 | 0.98 | 1.88, 3.24 |
| Fibromyalgia | 4 | 0.88 | 0.85 | 0.04, 1.72 | 1.38 | 0.48 | 0.97, 1.85 |
Abbreviations: VAS, visual analog scale; SD, standard deviation; CI, confidence interval.
Weight-bearing Joints
The data were pooled and analyzed from the cohort of those participants affected in a weight-bearing joint— hips, knees, and ankles. This cohort accounted for 48% of participants and showed improvements similar to the entire group. The weight-bearing joint subgroup reported a 47% decrease in joint pain, from a mean VAS score of 2.62 (95% CI, 2.28, 2.96) at baseline to 1.38 (95% CI, 1.21, 1.55) postintervention, P = .001, and a 51% reduction in the frequency of pain, from a mean VAS score of 3.01 (95% CI, 2.61, 3.41) at baseline to 1.49 (95% CI, 0.99, 1.99) postintervention, P = .001, after 4 weeks.
In addition, this cohort reported a 66% increase in activity levels, from a mean VAS score of 1.81 (95% CI, 1.44, 2.18) at baseline to 3.00 (95% CI, 2.67, 3.33; P = .001) and an 85% increase in joint strength levels, from a mean VAS score of 1.28 (95% CI 0.96, 1.62) at baseline to 2.38 (95% CI, 2.01, 2.75) postintervention, P = .001, after the 4 weeks of the supplement.
Responders Groups
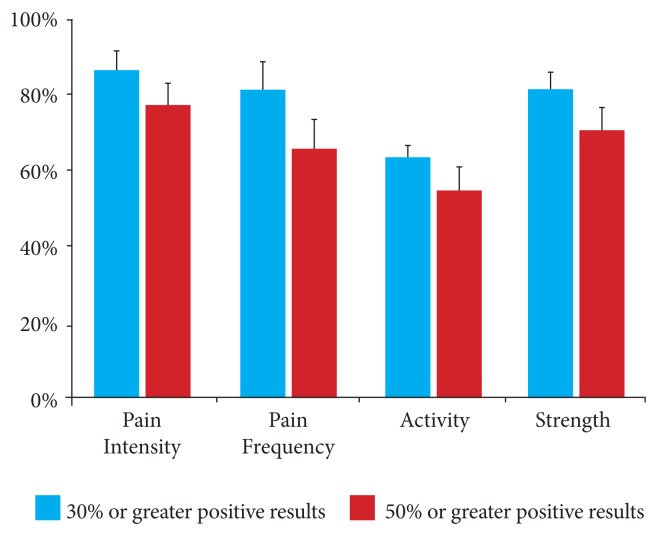
The results of this study were also stratified according to the number of people who reported a greater than 30% improvement and a greater than 50% improvement from baseline. The results showed that 86% of all participants reported a greater than 30% reduction in pain intensity from baseline in each of the 4 measures, with 77% reporting a greater than 50% reduction (Figure 6, Table 9). Similarly, 82% reported at least a 30% reduction in the frequency of pain from baseline, with 66% reporting at least a 50% reduction.
Figure 6.
Percentage of participants who reported at least a 30% improvement and at least at a 50% improvement from baseline.
Table 9.
Percentage of Participants Who Reported at Least a 30% Improvement and at Least at a 50% Improvement From Baseline
| % of People Who Reported Improvement | Pain Intensity | Pain Frequency | Activity | Strength | ||||
|---|---|---|---|---|---|---|---|---|
| % | Error | % | Error | % | Error | % | Error | |
| 30% or greater positive results | 86% | 5% | 82% | 7% | 64% | 3% | 82% | 4% |
| 50% or greater positive results | 77% | 6% | 66% | 8% | 55% | 6% | 71% | 6% |
In addition, 64% of all participants reported at least a 30% improvement in their activity levels in comparison to baseline, with 55% reporting at least a 50% improvement. These results were supported by the finding that 82% of the participants reported at least a 30% improvement in strength in the affected joint in comparison with baseline, and 71% reported at least a 50% improvement in the affected joint.
Summary of Adverse Effects
No adverse effects were reported during the study, meaning that participants reported no negative changes in health that would have impaired or affected their daily activities when compared with baseline. No participant required any rescue treatment for pain during the month of supplementation, and no adverse health outcomes were reported by participants during the study.
Discussion
Osteoarthritis is the most common form of arthritis in the United States.1 Glucosamine and chondroitin— natural substances found in and around the cells in cartilage—are the most widely used natural supplements for osteoarthritis and joint pain.
Glucosamine is an amino sugar that the body produces and distributes in cartilage and other connective tissue, whereas chondroitin is a complex carbohydrate that helps cartilage retain water.22 Some studies have found them to be safe and effective for addressing the symptoms of arthritis compared with some NSAIDs.23,24
The Glucosamine/Chondroitin Arthritis Intervention Trial in 2006, sponsored by the National Institutes of Health (NIH), found that glucosamine combined with chondroitin sulfate provided statistically significant pain relief for a subset of participants in comparison with a placebo.11 The study was funded by the National Center for Complementary and Alternative Medicine and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which both are components of the NIH. That 4-year, multicenter study concluded that the supplements were not effective overall; however, a small subgroup of patients experiencing moderate-to-severe pain11 did show positive improvement.
The aim of the current study was to determine the clinical response to the use of 150 mg of Cyplexinol® Standard in people with pain, stiffness, and loss or reduction in activity and strength due to self-reported osteoarthritis or degenerative joint disease. The BMPs in that ingredient are believed to target MSCs, which differentiate into chondrocytes, thus initiating chondrogenesis.15,17,19,25 Further, the BMPs in the product may elicit a natural immunoprotective effect by exhibiting antagonistic activity toward key proinflammatory cytokines, specifically interleukin 1 (IL-1) and interleukin 6 (IL-6), and transcription factors associated with inflammation.12,14,16,20,21 More important, the simultaneous mechanisms of those activities might make the ingredient a unique, natural regimen that can address the process that causes inflammation, with its ensuing pain, as well as cartilage tissue degradation from arthritis and other degenerative joint conditions.
The results of the current study demonstrated that statistically significant changes (P < .05) occurred for each parameter that was measured on the VAS. In particular, for all participants, pain intensity decreased from 2.85 cm at baseline to 1.41 cm at week 4 on a 10-cm VAS, which reflects a mean reduction in joint pain of 49% for the 44 participants who took the ingredient for 4 weeks. Similarly, pain frequency decreased from 3.16 cm at baseline to 1.22 cm at week 4. This change reflects a mean reduction in pain frequency of 39%.
In addition, an increase in activity level and strength was observed for participants. Activity level at baseline was 1.58 cm and 2.91 cm at week 4, reflecting a mean increase of 57%. Level of strength at baseline was 1.24 cm and 2.10 cm at week 4, reflecting a mean increase of 80%. These findings support the use of the current, novel protein complex that has been investigated previously both in clinical practice and clinical studies.
Moreover, at week 4 participants taking the low dose (150 mg) of Cyplexinol® Standard in the current study reported results similar to participants who participated in the previously reported CHAMPIONS Trial. That trial was a multicentered, prospective study of patients with moderate-to-severe osteoarthritis, who took 150 mg per day of Cyplexinol® Pro for 4 weeks. Those participants completed a VAS questionnaire at baseline and weekly thereafter, and the results demonstrated a reduction in joint pain and stiffness due to the supplementation.26
Similarly, participants in the current study reported subjective improvements in all parameters measured, regardless of the joint that was affected. The results in those affected in weight-bearing joints, which accounted for nearly one-half the participants, mirrored the group that participated in the CHAMPIONS Trial. This finding indicates that Cyplexinol® is not selective in joint uptake and can be used for people with a wide variety of joint dysfunction, degeneration, pain, and stiffness.
However, the rapid subjective improvement that was reported with the ingredient was not due to a chondrogenic response, as such physiological changes would not be observed within 4 weeks. Both medical and experimental studies have demonstrated that the BMPs within the ingredient have the ability to stimulate regenerative activity in the cartilage (chondrogenesis) and that they have anti-inflammatory activity as well16 from a clinical assessment of low-dose osteoinductive protein.
BMPs have also been shown through research to be the key osteoinductive proteins that are responsible for activating MSCs.27 In addition, BMPS are supported by key growth factors, such as TGF-β, IGF, and bFGF, for cellular maturation, and those osteoinductive proteins exhibit capabilities for joint-tissue repair as well as intrinsic anti-inflammatory properties, both of which are essential for repairing tissue damage in osteoarthritic patients.15,17,25
Further, excessive levels of IL-1 in the synovial tissue are associated with greater attrition of bone and cartilage, but BMPs have an immunosuppressive effect on the IL-1 activation pathway and its downstream components.16,21 In addition, interstitial collagenases such as matrix metalloproteinase MMP 1 (MMP-1), MMP-8, and MMP-13 are up regulated by IL-1 and degrade type 2 collagen in cartilage tissue. In particular, elevated levels of MMP-1 and MMP-13 are observed in arthritic tissues.
Accordingly, current research is focused on blocking the target sites of these catabolic cytokines and the downstream pathways that lead to arthritic conditions, because blocking or inhibiting these targets may allow the repair of joint function and damage.
To investigate alternative therapies, ZyCal developed the Cyplexinol® complex, which is a natural-protein complex containing BMPs belonging to the TGF-β superfamily of proteins. This includes BMP-2 and TGF-β, both of which have been shown through previous research to inhibit the expression of MMP-1 and MMP-13, resulting in a protective effect against collagen degradation.16,27,28 Further, in vivo and in vitro studies have previously shown that many of the proteins in Cyplexinol®, including BMP-4, BMP-5, and IGF, play an important role in joint homeostasis by activating cartilage tissue repair.14,15,25 In addition, preclinical, in vivo studies have demonstrated that oral administration of TGF-β and various BMPs results in cartilage remodeling and the reversal of osteoarthritis symptoms.
Therefore, the proteins and growth factors within Cyplexinol®, specifically the BMPs, show promise as natural alternatives to those new pharmacotherapies, and future studies will focus on confirming the mechanisms involved in the biochemical and physiological responses.
The study had some limitations, including the lack of an evaluation of joint homeostasis in healthy participants and the absence of prespecified safety end points in the study’s design (eg, liver enzymes and blood pressure). Both limited the research team’s objective assessment of the outcomes and the safety of Cyplexinol®. Further, the study’s design did not include a measurement of biomarkers, which may have further substantiated previous studies that addressed the mechanisms for the BMPs and growth factors contained in the supplement. The results did, however, indicate that the participants who took it reported experiencing fewer symptoms after 4 weeks.
Conclusion
The current study has demonstrated that Cyplexinol® Standard 150 mg was effective in reducing pain intensity and frequency as well as improving activity level and strength in affected joints. The results also provide possible indications as to pathways associated with cartilage degradation, pain, and inflammation that should be the focus of alternative therapies for osteoarthritis. Therefore, the ingredient in the current study offers a new therapeutic option for those experiencing joint pain and stiffness associated with osteoarthritis or other degenerative bone and- joint conditions.
Footnotes
Author Disclosure Statement
All research was funded and conducted by ZyCal Bioceuticals.
References
- 1.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(1):4–7. [PubMed] [Google Scholar]
- 3.Flannery CR. Novel therapies in OA. Curr Drug Targets. 2010;11(5):614–619. doi: 10.2174/138945010791011884. [DOI] [PubMed] [Google Scholar]
- 4.Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanos SP, Galluzzi KE. Topical therapies in the management of chronic pain. Postgrad Med. 2013;125(4) suppl 1:25–33. doi: 10.1080/00325481.2013.1110567111. [DOI] [PubMed] [Google Scholar]
- 6.Smalley WE, Ray WA, Daugherty JR, Griffin MR. Nonsteroidal anti-inflammatory drugs and the incidence of hospitalizations for peptic ulcer disease in elderly persons. Am J Epidemiol. 1995;141(6):539–545. doi: 10.1093/oxfordjournals.aje.a117469. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N Engl J Med. 1999;340(24):1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 8.Tamblyn R, Berkson L, Dauphinee WD, et al. Unnecessary prescribing of NSAIDs and the management of NSAID-related gastropathy in medical practice. Ann Intern Med. 1997;127(6):429–438. doi: 10.7326/0003-4819-127-6-199709150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Fries J. Toward an understanding of NSAID-related adverse events: the contribution of longitudinal data. Scand J Rheumatol Suppl. 1996;102:3–8. doi: 10.3109/03009749609097225. [DOI] [PubMed] [Google Scholar]
- 10.Fransen M, Agaliotis M, Nairn L, et al. LEGS study collaborative group. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens [published online ahead of print January 6, 2014] Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-203954. [DOI] [PubMed] [Google Scholar]
- 11.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 12.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31(6):773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavenis K, Heussen N, Schmidt-Rohlfing B. Effects of low concentration BMP-7on human osteoarthritic chondrocytes: comparison of different applications. J Biomater Appl. 2012;26(7):845–859. doi: 10.1177/0885328210388439. [DOI] [PubMed] [Google Scholar]
- 14.Chubinskaya S, Hakimiyan A, Pacione C, et al. Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2007;15(4):421–430. doi: 10.1016/j.joca.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Stimulation of articular cartilage repair in established arthritis by local administration of transforming growth factor-beta into murine knee joints. Lab Invest. 1998;78(2):133–142. [PubMed] [Google Scholar]
- 16.Aigner T, Soeder S, Haag J. IL-1beta and BMPs—interactive players of cartilage matrix degradation and regeneration. Eur Cell Mater. 2006 Oct;12:49–56. doi: 10.22203/ecm.v012a06. [DOI] [PubMed] [Google Scholar]
- 17.Pfeilschifter J, Oechsner M, Naumann A, Gronwald RG, Minne HW, Ziegler R. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990;127(1):69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- 18.Campbell WI, Lewis S. Visual analogue measurement of pain. Ulster Med J. 1990;59(2):149–154. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14(11):1126–1135. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Ronzière MC, Aubert-Foucher E, Gouttenoire J, Bernaud J, Herbage D, Mallein-Gerin F. Integrin alpha1beta1 mediates collagen induction of MMP-13 expression in MC615 chondrocytes. Biochim Biophys Acta. 2005;1746(1):55–64. doi: 10.1016/j.bbamcr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Yang CW, Jin DC, Chang YS, Bang BK, Kim YS. Bone morphogenetic protein-7 inhibits constitutive and interleukin-1 beta-induced monocyte chemoattractant protein-1 expression in human mesangial cells: role for JNK/AP-1 pathway. J Immunol. 2003;170(5):2557–2563. doi: 10.4049/jimmunol.170.5.2557. [DOI] [PubMed] [Google Scholar]
- 22.Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids. Int J Rheumatol. 2011;2011:969012. doi: 10.1155/2011/969012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matheson AJ, Perry CM. Glucosamine: a review of its use in the management of osteoarthritis. Drugs Aging. 2003;20(14):1041–1060. doi: 10.2165/00002512-200320140-00004. [DOI] [PubMed] [Google Scholar]
- 24.Uebelhart D, Thonar EJ, Delmas PD, Chantraine A, Vignon E. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarthritis Cartilage. 1998;6(suppl A):39–46. doi: 10.1016/s1063-4584(98)80011-3. [DOI] [PubMed] [Google Scholar]
- 25.Bramlage CP, Häupl T, Kaps C, et al. Decrease in expression of bone morphogenetic proteins 4 and 5 in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther. 2006;8(3):R58. doi: 10.1186/ar1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garian R, Donar A, DeFabio D, Gahles N, Scaffidi JJ. An osteoinductive protein complex that stimulates regeneration of bone and cartilage for treatment of moderate to severe osteoarthritis. Integr Med Clin J. 2012;11(5):16–21. [Google Scholar]
- 27.Takiguchi T, Kobayashi M, Suzuki R, et al. Recombinant human bone morphogenetic protein-2 stimulates osteoblast differentiation and suppresses matrix metalloproteinase-1 production in human bone cells isolated from mandibulae. J Periodontal Res. 1998;33(8):476–485. doi: 10.1111/j.1600-0765.1998.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Li D, Saldeen T, Mehta JL. TGF:beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2003;284(5):H1612–H1617. doi: 10.1152/ajpheart.00992.2002. [DOI] [PubMed] [Google Scholar]