Abstract
The human head louse is a cosmopolitan ectoparasite and frequently infests many people, particularly school-age children. Due to widespread pyrethroid resistance and the lack of efficient resistance management, there has been a considerable interest in the protection of uninfested people and prevention of reinfestation by disrupting lice transfer. In this study, two nonclinical model systems (in vitro and in vivo) were used to determine the efficacy of the infestation deterrents, Elimax lotion and Elimax shampoo, against human head lice or poultry chewing lice, respectively. With in vitro assessments, female head lice exhibited significantly higher avoidance responses to hair tufts treated with either of the test formulations, which led to significantly higher ovipositional avoidance when compared with female lice on control hair tufts. Additionally, both formulations were determined to be competent infestation deterrents in a competitive avoidance test in the presence of a known attractant (head louse feces extract). In in vivo assessments using a previously validated poultry model, Elimax shampoo was determined to be an efficacious deterrent against poultry chewing lice within Menopon spp. and Menacanthus spp.
Keywords: Pediculus humanus capitis, human head louse, Menopon spp., Menacanthus spp., poultry chewing lice
The human head louse, Pediculus humanus capitis (De Geer), is an obligatory hematophagous ectoparasite that frequently infests school-age children, their teachers and family members, and many others who have physically contacted infested people (Mumcuoglu 1996, Meinking 1999). Since the beginning of the modern era of synthetic insecticides, people have used a number of different pediculicides to control head lice (Durand et al. 2012). Most people find louse infestations intolerable and choose to use pediculicides, many of which pose a risk of adverse effects if not used properly. Misapplication and overuse may affect children in particular because of their small body size and higher sensitivity to chemicals (Goldman 1995).
Pediculicide sales in the United States were estimated to be >US$240 million per year in 1997 (Gratz 1997). This number increased to > US$350 million per year in 2003 (Jones and English 2003). The overall cost for dealing with head louse infestation (pediculosis), including cleaning household items, visiting doctor’s office or health clinic, purchasing prescription-based and over-the-counter medications, has been estimated at∼US$1 billion annually (Gratz 1997, Clark et al. 2009). Furthermore, almost 10% of school children in the United States have experienced the long-term consequences of school absences due to the “No-Nit” policy and the implementation of ineffective control measures (Gratz 1997, Williams et al. 2001, Frankowski and Weiner 2002, Lebwohl et al. 2007). A more current economic burden of losing workdays in the United States alone has been estimated ∼US$4–8 billion annually (Mumcuoglu et al. 2006).
The pyrethrins- and pyrethroids-containing formulations have been used extensively and intensely for >20 years (Durand et al. 2012), and clinical resistance has been widely reported as a consequence (Chosidow et al. 1994, Hipolito et al. 2001, Burgess et al. 2005, Hill et al. 2005). Louse resistance to pyrethroids under nonclinical settings has been documented in Czech Republic (Rupes et al. 1995), the United Kingdom (Downs et al. 1999), Denmark (Kristensen 2005), Israel (Mumcuoglu et al. 1995), the United States (Pollack et al. 1999, Lee et al. 2000, Lee et al. 2010), Argentina (Picollo et al. 1998), Japan (Tomita et al. 2003), and Australia (Hunter and Barker 2003).
Due to pediculicide resistance and the lack of efficient resistance management programs, there has been considerable interest in the protection of uninfested school children and prevention of reinfestation by interrupting lice transfer (Mumcuoglu et al. 1996, Semmler et al. 2012, Ketzis et al. 2014). Considering that head lice do not have wings or jumping legs, the mechanism used to transfer between hosts has been debated (Takano-Lee et al. 2005) and head-to-head transfer is generally accepted as a major mechanism of new infestation (Speare and Buettner 1999). Therefore, development of infestation deterrents that are safe to humans and can specifically discourage the transfer of lice from one host to another is a critical need in this niche market of affordable and effective louse control. Nevertheless, cost and time factors in clinical trials have been the major obstacles to researchers and sponsors who want to develop efficacious infestation deterrents. The current study provides data obtained from two nonclinical model systems (in vitro and in vivo) that can serve as efficient screening systems for developing deterrent formulations that are effective in the control of louse infestations.
Materials and Methods
In Vitro Avoidance Assays
Human Head Lice
Permethrin- and DDT-resistant human head lice (Pediculus humanus capitis, SF-HL strain), which were originally collected from infested children in Plantation and Homestead, FL, and maintained on an in vitro rearing system at the University of Massachusetts-Amherst, were used for three bioassays: oviposition avoidance, hatchability determination, and competitive avoidance.
Test Products
Elimax lotion and Elimax shampoo, which contain oligodecene oil, sesame oil, and acrylate, were supplied by Oystershell Laboratories NV, Drongen, Belgium. For positive controls, OFF! Deep Woods Insect Repellent VIII (OFF!, SC Johnson, Racine, WI), which contains 25% N,N-diethyl-meta-toluamide (DEET), was purchased locally and used in the avoidance bioassays.
Washing Solution
Sodium lauryl ether sulfate (SLES, ZETESOL NL-2U/ZETESOL 270, Zschimmer & Schwarz, Germany) was diluted to 12% (w/v) with distilled deionized water (ddH2O) and used to wash all hair tufts.
Hair Tuft Treatments
A semicircular shaped (15 mm radius), flattened (∼2 mm thick) hair tuft (Sally USA, Denton, TX) or a rectangular hair tuft (10.75 by 25 mm, ∼2 mm thick) was placed on a glass Petri dish and treated with Elimax lotion (1 g /g hair tuft) or Elimax shampoo (1 g /g hair tuft) using a pipette gun to dispense the test formulation to saturate the hair tuft. Using sterile forceps, the hair tuft was gently rubbed into either Elimax lotion or Elimax shampoo using a circular motion for 30 s until complete hair tuft coverage was achieved and ensured by visual inspection under a stereomicroscope. The treated hair tuft was transferred to a clean Petri dish and incubated for 15 min at room temperature. A positive control hair tuft was treated with sufficient amount of OFF! (1 g OFF!/g hair tuft) as above for 15 min at room temperature. A negative treatment control hair tuft was treated with sufficient amount of ddH2O (1 g ddH2O/g hair tuft) for 15 min at room temperature as described above for the Elimax lotion- or Elimax shampoo-treated tuft.
After the 15-min incubation, the treated hair tuft (either Elimax lotion, Elimax shampoo, OFF!, or ddH2O) was completely submerged in a 250-ml beaker containing 100 ml of 12% SLES by using a weighted paper binder clamp. The beaker was placed on a magnetic stir plate (Nuova II, Thernolyne), and a magnetic stir bar (7.9 by 25.4 mm, diameter × length) was added to the beaker. Individual hair tufts were washed by stirring the 12% SLES for 30 s. The controlling knob of the plate was set at 4–5 to generate a consistent mid-speed. The temperature of the 12% SLES solution was maintained between 25–30°C. This washing step was repeated two more times with fresh 12% SLES solution. Lastly, the treated and washed hair tufts were clamped, submerged, and rinsed in a beaker with 300 ml ddH2O for 90 s using the stirring method described above for the SLES washing step. The hair tufts were air-dried at room temperature on a stack of filter paper (Whatman No. 1) for 1 h on a laboratory bench.
Oviposition Avoidance Bioassay and Hatchability
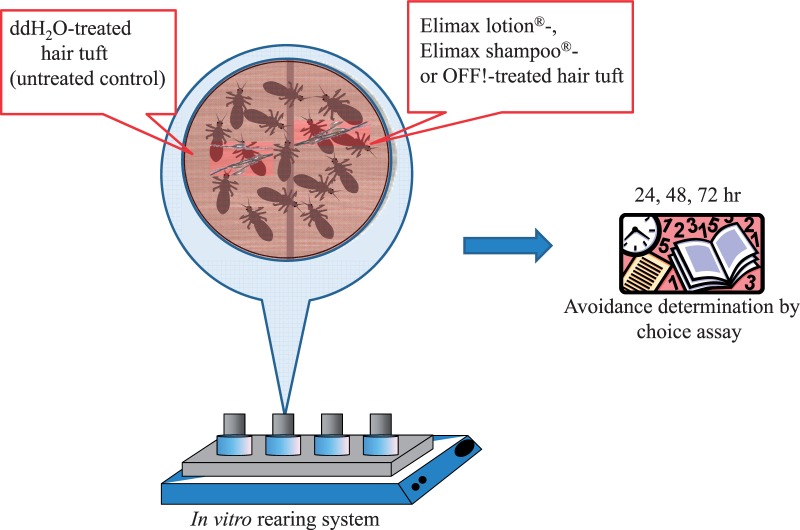
Freshly prepared hair tufts were used in each oviposition avoidance bioassay. A test arena was constructed on the blood-feeding membrane surface of an in vitro louse rearing unit (Yoon et al. 2006) by placing a test formulation (Elimax lotion- or Elimax shampoo-treated tuft) or a positive control (Off!-treated tuft) side by side next to a negative control (ddH2O-treated tuft) as shown in Fig. 1. Adult female lice (at least 3 d old, 10 lice per test) were then placed on the boundary between treated (either Elimax lotion, Elimax shampoo, or OFF!) and negative control (ddH2O-treated) hair tufts (Fig. 1). For control experiments, two semicircular hair tufts treated with only ddH2O were used. The number of female lice and eggs on each semicircular hair tuft were counted under a stereomicroscope and recorded after 24, 48, and 72 h. All experiments (treatment and control) were performed in triplicate. Ovipositional avoidance values were calculated using equation 1.
| (1) |
where nt = the total number of eggs on the treated tuft; Nt = the total number of eggs in the treated cup (treated plus control tufts); nc = the total number of eggs on the control tuft with the lower number of eggs; Nc = the total number of eggs in the control cup (both control tufts combined).
Fig. 1.
An assembled test arena for the determination of oviposional avoidance of female SF-HL in the presence of Elimax lotion, Elimax shampoo, or OFF! Insect Repellent VIII.
Immediately after the ovipositional avoidance bioassays, individual hair tufts with eggs were placed into new sterile Petri dishes, covered and moved to an incubator at 31°C and 70–80% relative humidity (RH). The number of first instars that hatched from the eggs were counted under a stereomicroscope and recorded over time until hatching ceased (∼7–10 d). Undeveloped eggs and stillborn lice were recorded as dead. The hatchability of the eggs on each hair tuft was calculated using equation 2.
| (2) |
where H = number of eggs hatched, N = total number of eggs oviposited.
Competitive Avoidance Bioassay
The competitive avoidance was determined based on the experimental design described previously (Mumcuoglu et al. 1996) with modifications. The rectangular test arena (75 by 25 by 25 mm) was constructed from standard glass microscope slides and held together with epoxy glue (Fig. 2). Individual cotton pads (10.2 by 10.2 cm, VWR International, Radnor, PA), soaked with ddH2O, ethanol, or hexane, respectively, were used to sequentially clean the test arena prior to each experiment. Four flattened hair tufts (18.75 by 25 mm) were prepared to fit the size of each of the four floor sections of the test arena (sections 1–4, Fig. 2). The rectangular hair tufts, which were fitted into the floor of the arena, allowed lice to move around freely without falling off or leaving the test arena.
Fig. 2.
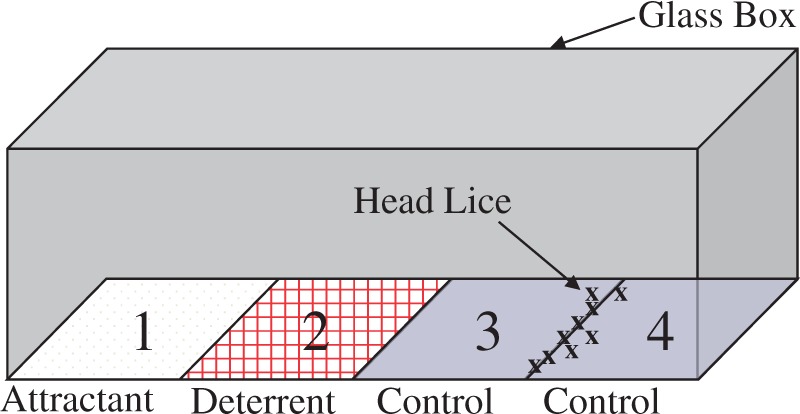
An assembled test arena for the determination of competitive avoidance using an open-top glass box (75 by 25 by 25 mm).
The test formulation (either Elimax lotion, Elimax shampoo, or OFF!)-treated tuft was placed on section 2, and the two ddH2O-treated control tufts were placed on sections 3 and 4 as shown in Fig. 2. An attractant-treated hair tuft was prepared as follows. Head louse feces collected from the in vitro rearing system was added to ddH2O and mixed well to prepare 1% (w/v) suspension. This suspension was centrifuged (13,000 × g) for 1 min and the resulting supernatant was transferred to an amber glass vial and used as a 1% head louse feces extract. A hair tuft was treated with 1% head louse feces extract (1 g head louse feces extract/g hair tuft) for 15 min. The attractant-treated hair tuft was then transferred to a clean Petri dish, completely dried for 2–3 h at room temperature and placed on section 1 (Fig. 2).
Adult female lice (10 lice per trial, SF-HL) were placed on the boundary of the two control hair tufts (interface of sections 3 and 4, Fig. 2) and the arena was transferred to an incubator (31 ± 1°C, 70–80% RH) and kept in the dark. The number of lice found on each hair tuft was recorded after 30 min. Lice counted on the attractant hair tuft were added to the lice counted on the deterrent hair tuft and this total number of females was used to determine competitive avoidance as determined using equation 3.
| (3) |
where N = total number of lice; n1 = total number of lice on the attractant- and deterrent-treated hair tufts; n2 = number of lice on the ddH2O-treated control hair tufts.
A test formulation was considered a competent infestation deterrent of females if the competitive avoidance was >50% and a semicompetent infestation deterrent if the competitive avoidance value was >25.0% but <50.0%.
In Vivo Avoidance Assays
This assay was based on the method described previously for Elimax lotion (Ketzis et al. 2014). An outline of the in vivo design is presented in Table 5.
Table 5.
In vivo study design
| Time point | Procedures |
|---|---|
| Day -3 | Chickens arrived; veterinary examination |
| Day -2 | Group 2a: E004490 used to remove lice |
| Group 2 housed separately until Day 0 | |
| Day 0 | Lice on all chickens counted |
| Group 1b: Elimax shampoo, applied | |
| Group 2: rinsed with water | |
| Groups 1, 2, and 3 housed together | |
| Day 0 + 8h | Lice counted |
| Days 1–7 (+24 to +172) | Lice counted |
| Treatment | |
| Group 1b | 100–144 ml Elimax shampoo, applied |
| 15 min wait | |
| Small amount of water used to create suds | |
| Rinsed with water | |
| Dried | |
| Group 2a | 100–110 ml E004490 applied |
| 15 min wait | |
| Soap used to wash off E004490 | |
| Rinsed with water | |
| Dried |
a Chickens treated with E004490 according to the instructions (Treatment).
b Chickens treated with Elimax shampoo according to the instructions (Treatment).
Animals and Housing
Thirty-three adult layer hens (31 white leg horn crosses and 2 Rhode Island red crosses) naturally infested with poultry chewing lice (Menopon spp. and Menacanthus spp., both type of hens had both species of lice) were acquired from a local supplier in St. Kitts, West Indies. None of the chickens had been treated with an insecticide previously. Individual chickens, identified by leg bands, were determined to be healthy by veterinary examinations. Chickens were group-housed in a 1.22- by 2.44-m pen with 4 perches. All husbandry procedures followed those described in the Ag Guide (Federation of Animal Science Societies [FAAS] Writing Committee 2010).
Test Products
Elimax shampoo and E004490 (the insecticidal oligodecene oil used in Elimax shampoo) were supplied by Oystershell NV. Dawn dish washing liquid (Proctor and Gamble, Cincinnati, OH) was purchased from a local supplier.
Treatments
The treatment procedure used was as per the proposed instructions for use on humans (Anonymous 2014). Six chickens, randomly selected from the 33 chickens, were treated with E004490 to kill all lice. E004490 was applied to individual chickens until fully covered (100–110 ml). The feathers were moved backwards and the skin and feathers were sprayed with E004490, using a hair dresser water spray bottle. After 15 min, the treated chickens were washed with Dawn dish washing liquid. Approximately 10 ml of dish washing liquid was mixed with water in a bucket, the chicken was placed into the bucket, and the feathers gently washed by hand. The washing liquid was rinsed off using a hose, the chickens were towel dried and gently air dried with a blow drier. Once chickens were completely dry, they were inspected to determine if all lice were killed. Lice-free chickens were assigned to the Group 2 (control group) and separately housed for 2 d. These chickens were examined for lice prior to the study start to ensure that no eggs had hatched and that they were still louse free.
After 2 d, the remaining 27 chickens were inspected to determine the number of lice on each chicken. Ten of the 27 chickens, randomly selected, were treated with Elimax shampoo. Treatment with Elimax shampoo consisted of spraying the formulation onto each chicken until the chicken was considered fully covered (100 to 144 ml of product per chicken was used depending on the size of the chicken). The feathers were moved backwards and the skin and feathers sprayed with the formulation until all feathers and skin were wet with the treatment. After 15 min, a small amount of water (<20 ml) was applied to the chicken to allow the soaping agent in the product to react. The chicken was gently washed by hand with the soap from the product and completely rinsed with water. The chickens were then towel dried and blow dried using cool air and assigned to the Group 1 (test group). The six chickens from Group 2 were rinsed with water and dried as above. The remaining 17 chickens without any treatments were assigned to the Group 3 (lice reservoir group). The timing of posttreatment intervals was begun at the time that rinsing commenced on Study Day 0.
Lice Assessment
All assessments were made by people blinded to the treatment for quantification of the deterrent properties over 7 d. The general design was based on the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines (Holdsworth et al. 2006) for assessing persistent activity of products for lice on cattle. To assess the number of lice on a chicken, five approximately 2- by 2-cm areas were inspected and the number of lice counted. The areas included the ventral and dorsal surface of each wing, the breast, the tail feathers, and the back. Initial lice assessments were performed on all chickens (Group 1, 2, and 3) 8 h ± 18 min posttreatment.
Study Days 1 to 7
Lice assessments were performed on all chickens (all groups) at + 24 h, +48 h, +72 h, + 96 h, + 120 h, + 148 h, and + 172 h ± 1 h posttreatment.
Data Analysis
The arithmetic and geometric mean number of lice for each group are presented in Table 6. To calculate geometric means with zero counts, 1 was added to all of the numbers and then subtracted from the calculated geometric mean. Groups 1 and 3 were compared pretreatment to determine that they were not statistically different using a Kruskal–Wallis test. A Fisher’s Exact test was used to compare the number of chickens with lice at each time point in Groups 1 and 2 using the total number of chickens infested at each time point as well as the accumulated number.
Table 6.
Average and mean lice counts for Group 1 (Elimax shampoo, infestation deterrent), Group 2 (re-infestation control group), and Group 3 (lice reservoir group)
| Group | Average lice counts (geometric meana)Hours posttreatment |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatmentb | +8 | +24 | +48 | +72 | +96 | +120 | +148 | +172 | |
| 1 | 33 (28) | 0.2 (0.2) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) | 0.8 (0.6) | 0.5 (0.4) | 0.7 (0.5) | 0.7 (0.5) |
| 2 | 0 (0) | 2 (2) | 2 (3) | 3 (3) | 2 (2) | 2 (2) | 2 (2) | 2 (1) | 2 (2) |
| 3 | 30 (26) | 35 (30) | 32 (27) | 33 (27) | 28 (23) | 32 (23) | 28 (19) | 26 (16) | 29 (17) |
a Geometric mean (GM) calculation: when a count contained zeros, 1 one was added to each number, the GM calculated and then 1 subtracted from the calculated GM.
b Groups 1 and 3 were not statistically significantly different (P = 0.688; 0.687 adjusted for ties; Kruskal–Wallis) pretreatment.
Results and Discussions
In Vitro Assessments
Ovipositional Avoidance
Female lice on the hair tufts treated with either Elimax lotion, Elimax shampoo, or OFF! exhibited significantly higher ovipositional avoidance responses compared with females on hair tufts treated with ddH2O only (Table 1, Cochran-Mantel-Haenszel test, P < 0.05). This finding indicates that Elimax lotion and Elimax shampoo are efficacious deterrents to ovipositioning over a 72-h period.
Table 1.
Comparative ovipositional avoidance of female SF-HL on either Elimax lotion-, Elimax shampoo-, or OFF!-treated hair tufts determined by choice assays performed on the in vitro rearing system
| Treatmenta | Washb | No. of ddH2O rinsesc | Time | Ncd or Nte | ncf or ntg | % OAh |
|---|---|---|---|---|---|---|
| Nc | nc | |||||
| ddH2O (Control group) | 12% SLES | 1 (90 s) | 24 h | 124 | 51 | – |
| 48 h | 318 | 135 | – | |||
| 72 h | 488 | 218 | – | |||
| Nt | nt | |||||
| OFF!* | 12% SLES | 1 (90 s) | 24 h | 172 | 7 | 90.1 |
| 48 h | 366 | 32 | 79.4 | |||
| 72 h | 576 | 58 | 77.5 | |||
| Nt | nt | |||||
| Elimax lotion*,+ | 12% SLES | 1 (90 s) | 24 h | 174 | 0 | 100 |
| 48 h | 358 | 0 | 100 | |||
| 72 h | 557 | 1 | 99.1 | |||
| Nt | nt | |||||
| Elimax shampoo*,+ | 12% SLES | 1 (90 s) | 24 h | 133 | 0 | 100 |
| 48 h | 353 | 0 | 100 | |||
| 72 h | 503 | 0 | 100 |
a Individual hair tufts were treated with ddH2O, OFF!, Elimax lotion, or Elimax shampoo (1 g treatment/g hair tuft) for 15 min.
b Individual hair tufts were washed using three separate 100 ml 12% SLES baths (stir bar method).
c Individual hair tufts were rinsed using a 300 ml ddH2O bath (stir bar method).
d Nc = the total number of eggs in the control cup (both control tufts combined).
e Nt = the total number of eggs in the treated cup (treated plus control tufts).
f nc = the total number of eggs on the control tuft with the lower number of eggs.
g nt = the total number of eggs on the treated tuft.
h OA (ovipositional avoidance, %) = 100 − [{(nt/Nt)/(nc/Nc)} × 100]
* % Avoidance is significantly different than control (Cochran-Mantel-Haenszel test, P < 0.05).
+ % Avoidance is significantly different than OFF! (Cochran-Mantel-Haenszel test, P < 0.05).
The female ovipositional avoidance of either Elimax lotion- or Elimax shampoo-treated hair tufts was also significantly higher than that of OFF!-treated hair tufts (Table 1, Cochran-Mantel-Haenszel test, P < 0.05), suggesting that Elimax lotion- and Elimax shampoo-treated hair tufts are more efficacious deterrent to oviposition than OFF! under the aforementioned experimental conditions.
Avoidance Response of Female Lice
Female lice exhibited significantly higher avoidance responses to the hair tufts treated with either Elimax lotion, Elimax shampoo, or OFF! compared with the female avoidance responses to the control ddH2O-treated hair tufts (Table 2, Cochran-Mantel-Haenszel test, P < 0.05). Although data collection was not based on the continuous monitoring of female movements on the hair tufts, the data from the three time points certainly provides a reasonable basis for the ovipositional avoidance reported and discussed above.
Table 2.
Comparative avoidance of female SF-HL on either Elimax lotion-, Elimax shampoo- or OFF!-treated hair tufts determined by choice assays performed on the in vitro rearing system
| Treatmenta | Washb | No. of ddH2O rinsesc | Time | Ncd or Nte | Ncf or ntg | % FAh |
|---|---|---|---|---|---|---|
| Nc | nc | |||||
| ddH2O (Control group) | 12% SLES | 1 (90 s) | 24 h | 30 | 8 | – |
| 48 h | 27 | 10 | – | |||
| 72 h | 29 | 12 | – | |||
| Nt | nt | |||||
| OFF! * | 12% SLES | 1 (90 s) | 24 h | 30 | 4 | 50.0 |
| 48 h | 30 | 3 | 73.0 | |||
| 72 h | 30 | 5 | 59.7 | |||
| Nt | nt | |||||
| Elimax lotion*,+ | 12% SLES | 1 (90 s) | 24 h | 30 | 0 | 100 |
| 48 h | 30 | 0 | 100 | |||
| 72 h | 30 | 0 | 100 | |||
| Nt | nt | |||||
| Elimax shampoo*,+ | 12% SLES | 1 (90 s) | 24 h | 30 | 0 | 100 |
| 48 h | 30 | 0 | 100 | |||
| 72 h | 30 | 0 | 100 |
a Individual hair tufts were treated with ddH2O, OFF!, Elimax lotion, or Elimax shampoo (1 g treatment/g hair tuft) for 15 min.
b Individual hair tufts were washed using three separate 100 ml 12% SLES baths (stir bar method).
c Individual hair tufts were rinsed using a 300 ml ddH2O bath (stir bar method).
d Nc = the total number of females in the control cup (both control tufts combined).
e Nt = the total number of females in the treated cup (treated plus control tufts).
f nc = the total number of females on the control tuft with the lower number of eggs.
g nt = the total number of females on the treated tuft.
h FA (female avoidance, %) = 100 − [{(nt/Nt)/(nc/Nc)} × 100]
* % Avoidance is significantly different than control (Cochran-Mantel-Haenszel test, P < 0.05).
+ % Avoidance is significantly different than OFF! (Cochran-Mantel-Haenszel test, P < 0.05).
Female avoidance responses to either Elimax lotion- or Elimax shampoo-treated hair tufts were also significantly higher than those determined using OFF!-treated hair tufts (Table 2, Cochran-Mantel-Haenszel test, P < 0.05), indicating that both Elimax lotion and Elimax shampoo are more effective female deterrents compared with OFF! under the experimental conditions used in current investigation.
Hatchability
Because the ovipositional avoidance values of female SF-HL in tests with either Elimax lotion- or Elimax shampoo-treated hair tufts were higher than 99.5% (Table 1), there were very few eggs, if any, laid on the test formulation-treated hair tufts. With such a small sample size, statistical analysis of hatchability was not feasible (Table 3). The hatchability values obtained from hair tufts treated with OFF! (trial 3 data excluded, as only one egg laid on the OFF!-treated hair tuft) were significantly lower than those obtained from ddH2O-treated hair tufts (Table 3, t-test, P < 0.05), suggesting that the OFF! product may contain an ovicidal agent. In fact, DEET often exhibits insecticidal activity and one of the nonvolatile ingredients, isopropyl myristate, has been reported as an effective agent removing hydrocarbons from head lice (Burgess et al. 2008, Barnett et al. 2012). It is possible that these two compounds in the OFF! product may be responsible for the ovicidal response, but further investigation is necessary to confirm this speculation.
Table 3.
Comparative percent hatchability of SF-HL eggs oviposited on differentially treated hair tufts
| Treatmenta | Washb | No. of ddH2O rinsesc | Trial | % Hatchability | |
|---|---|---|---|---|---|
| ddH2O | ddH2O | ||||
| ddH2O (Control group) | 12% SLES | 1 (90 s) | 1 | 96.1 (74/77) | 83.6 (56/67) |
| 2 | 78.9 (56/71) | 87.3 (62/71) | |||
| 3 | 88.5 (108/122) | 91.3 (73/80) | |||
| OFF! | ddH2O | ||||
| OFF! (25% DEET) | 12% SLES | 1 (90 s) | 1 | 86.5 (32/37)d | 92.6 (138/149) |
| 2 | 85.0 (17/20)d | 90.6 (145/160) | |||
| 3 | 100 (1/1) | 89.2 (140/157) | |||
| Elimax lotion | ddH2O | ||||
| Elimax lotion | 12% SLES | 1 (90 s) | 1 | NDe | 89.8 (177/197) |
| 2 | NDe | 96.4 (135/140) | |||
| 3 | 100 (1/1) | 95.5 (210/220) | |||
| Elimax shampoo | ddH2O | ||||
| Elimax shampoo | 12% SLES | 1 (90 s) | 1 | NDe | 95.0 (151/159) |
| 2 | NDe | 96.3 (130/135) | |||
| 3 | NDe | 97.6 (204/209) | |||
a Individual hair tufts were treated with ddH2O, OFF!, Elimax lotion, or Elimax shampoo (1 g treatment/g hair tuft) for 15 min.
b Individual hair tufts were washed using three separate 100 ml 12% SLES baths (stir bar method).
c Individual hair tufts were rinsed using a 300 ml ddH2O bath (stir bar method).
d Hatchability (trial 3 data excluded) on the OFF!-treated hair tuft was significantly lower that on treated hair tuft (t-test, P < 0.05).
e ND, not determined due to the lack of oviposition of eggs on the hair tuft.
Competitive Avoidance
All three formulations (Elimax lotion, Elimax shampoo, and OFF!) were determined to be competent deterrents at the end of the initial 30-min assay period (Table 4). Female avoidance (%) determined for OFF! treatment was significantly decreased from 75.0 to 22.2% at the end of the next 30-min period (t-test, P < 0.05). In a similar comparison, the avoidance values for either the Elimax lotion or Elimax shampoo formulations did not change at the end of the second 30-min period. Interestingly, the mean avoidance value (69.1 ± 10.3%) obtained from the assays with Elimax lotion-tufts was significantly higher than the avoidance value (22.2 ± 19.2%) obtained from assays with OFF!-tufts (ANOVA with Tukey’s test, P < 0.05). This result shows that Elimax lotion and Elimax shampoo elicited more consistent competitive avoidance over the 1-h duration of experiment, suggesting that both Elimax lotion and Elimax shampoo may be better deterrents against female head lice. Further research, including clinical trials, is necessary to confirm the above suggestion.
Table 4.
Competitive avoidance (%) of SF-HL females (10 lice per trial) on the glass arena with hair tufts treated with ddH2O, an attractant (1% feces), and a test formulation (OFF! Deep Woods Insect Repellent VIII, Elimax lotion, or Elimax shampoo)
| Trial 1 | Trial 2 | Trial 3 | Meana,b ± SD | |
|---|---|---|---|---|
| OFF! | ||||
| 30-min avoidance | 75.0 | 75.0 | 75.0 | 75.0 ± 0a |
| 60-min avoidance | 33.3 | 0 | 33.3 | 22.2 ± 19.2A |
| Elimax lotion | ||||
| 30-min avoidance | 33.3 | 100 | 57.1 | 63.5 ± 33.8a |
| 60-min avoidance | 75.0 | 75.0 | 57.1 | 69.1 ± 10.3B |
| Elimax shampoo | ||||
| 30-min avoidance | 57.1 | 75.0 | 75.0 | 69.1 ± 10.3a |
| 60-min avoidance | 75.0 | 57.1 | 33.3 | 55.2 ± 20.9A,B |
a A formulation is considered to be a competent deterrent to infestation when % avoidance is >50.0 %.
b 30-min avoidance means followed by the same lower-case letter are not statistically different by ANOVA (P > 0.05); 60-min avoidance means followed by the same upper-case letter are not statistically different by ANOVA (P > 0.05).
Comparative avoidance (%) = 100 – [{(n1/N)/(n2/N)} × 100], where N = total number of lice; n1 = total number of lice on the attractant- and deterrent-treated hair tufts; n2 = number of lice on the ddH2O-treated control hair tufts (Fig. 2).
In vivo Assessments
All of the chickens initially had heavy louse infestations associated with the wings, feathers, and body. For those in Group 2, no live lice were found after the E004490 treatment, but their infestation levels prior to lice removal indicated that they were capable of having heavy louse infestations. Groups 1 (treated with deterrent) and 3 (reservoir for lice) were not statistically different in regards to their louse counts pretreatment (Kruskal–Wallis test; P = 0.688; 0.687 adjusted for ties), indicating that all of the chickens were able to maintain equivalent levels of louse infestations (Table 6).
During the treatment applications, the lice tended to move away from the area of application (Group 1) and stopped moving quickly (Groups 1 and 2). Elimax shampoo (Group 1) washed off of the chickens relatively easily resulting in very clean chickens; E004490 (Group 2) left a slight oily film on the chickens for ∼24 h. This difference posed a challenge in regards to blinding. Therefore, all chickens were thoroughly examined for lice (e.g., additional locations examined) whenever a count of zero was determined. Following treatments, all of the chickens (Groups 1, 2, and 3) were housed together and shared the perches in a common pen. After treatments, none of the chickens were avoided by the other chickens, indicating that there was sufficient and equal opportunity for lice to transfer from chicken to chicken and no pecking order appeared to influence contact between the chickens.
Average louse counts per group over time post treatment and the number of infested chickens in each group are presented in Tables 6 and 7, respectively. All chickens in Group 3 (reservoir group) were infested with lice at all of the time points post treatment (3 to 93 lice per chicken). All six Group 2 chickens, used to demonstrate natural reinfestation without protection, had lice within 8 h of being exposed to the Group 3 chickens and continued to have lice during the 7-d experimental time period, except for one chicken at +24 h. While the infestation levels were low (1–7 lice per chicken), the Group 2 chickens consistently had lice.
Table 7.
Number of chickens infested in Group 1 (Elimax shampoo, infestation deterrent) and Group 2 (re-infestation control group) posttreatment
| Group | Number in group | Number of positive chickens (accumulated number of positive chickens) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| +8 | +24 | +48 | +72 | +96 | +120 | +148 | +172 | ||
| 1 | 10 | 2 | 1 (3) | 1 (3) | 1 (3) | 5 (6) | 5 (8) | 5 (9) | 5 (9) |
| 2 | 6 | 6* | 5* | 6* | 6* | 6 | 6 | 6 | 6 |
An asterisk indicates a significant difference in infested:noninfested chickens between Groups 1 and 2 (P < 0.01; Fisher’s Exact test).
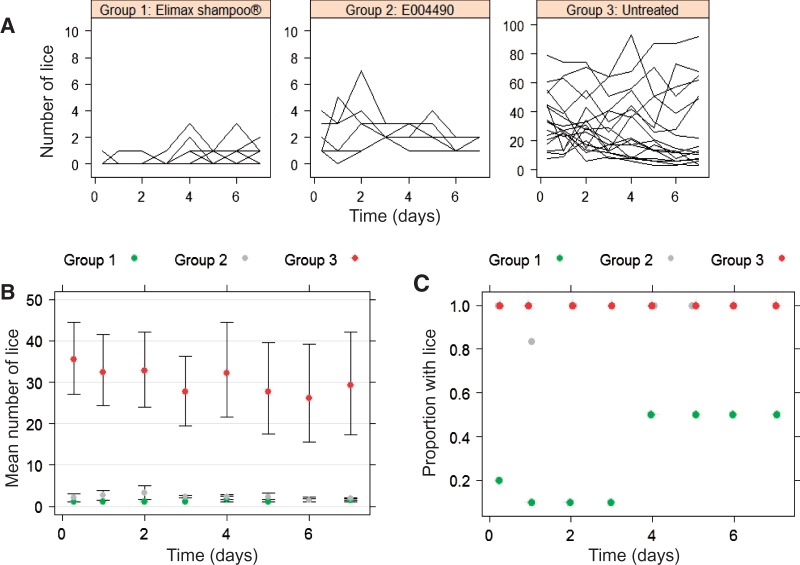
Group 1 chickens, treated with Elimax shampoo, had low louse counts (0 to 3) during the 7-d experimental period (Fig. 3A). At 8 h after being exposed to the lice-infested Group 3 chickens (reservoir group), two of the 10 Group 1 chickens had one louse each. From 24 to 72 h after being exposed to the Group 3 chickens, only 1 of the 10 Group 1 chickens had lice (1 louse). At 4 d (96 h) after treatment and exposure to the Group 3 chickens, the Group 1 chickens began to become as infested as the Group 2 chickens (Fig. 3B and C).
Fig. 3.
Analysis of data obtained from in vivo avoidance assays. (A) Changes in number of lice determined on individual chickens superimposed by treatment group. (B) Average (±SD) number of lice for lousy chickens. (C) Proportion of lousy chickens. ( ) group 1 treated with Elimax shampoo, (
) group 1 treated with Elimax shampoo, ( ) group 2 treated with E004490, and (
) group 2 treated with E004490, and ( ) group of lousy chickens.
) group of lousy chickens.
The proportion of infested chickens in Groups 2 was significantly higher than that of infested chickens in Group 1 during a period from 8 to 72 h (Fig. 3C). In other words, the Group 2 chickens were infested faster than the Group 1 chickens during the 8–72 h period. By 96 h posttreatment and onward, the chickens in Group 1 appeared to be able to maintain an increased number of lice, indicating that the deterrent properties of Elimax shampoo may no longer be effective.
In Vitro and In Vivo Assessments to Evaluate Deterrent Formulations Against Head Lice
Based on results from in vitro and in vivo assessment, Elimax lotion and Elimax shampoo can be considered as effective infestation deterrents against head lice. In the in vivo study, Elimax shampoo effectively killed the existing chewing lice on chickens and provided significant protection from reinfestation for 72 h.
Acknowledgments
This research was supported by Oystershell Laboratories (Belgium).
References Cited
- Barnett E., Palma K. G., Clayton B., Ballard T. 2012. Effectiveness of isopropyl myristate/cyclomethicone D5 solution of removing cuticular hydrocarbons from human head lice (Pediculus humanus capitis). BMC Dermatol. 12: 15–5945-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess I. F., Brown C. M., Lee P. N. 2005. Treatment of head louse infestation with 4% dimeticone lotion: Randomised controlled equivalence trial. BMJ 330: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess I. F., Lee P. N., Brown C. M. 2008. Randomised, controlled, parallel group clinical trials to evaluate the efficacy of isopropyl myristate/cyclomethicone solution against head lice. Pharm. J. 371–375. [Google Scholar]
- Chosidow O., Chastang C., Brue C., Bouvet E., Izri M., Monteny N., Bastuji-Garin S., Rousset J., Revuz J. 1994. Controlled study of malathion and d-phenothrin lotions for Pediculus humanus var capitis-infested schoolchildren. Lancet 344: 1724–1727. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lee S. H., Yoon K. S., Strycharz J. P., Kwon D. H. 2009. Human head lice: Status, control and resistance, pp. 73–88. In Clark J. M., Bloomquest J. R., Kawada H. (eds.), Advances in human vector control. American Chemical Society, Washington, DC. [Google Scholar]
- Downs A.M.R., Stafford K. A., Coles G. C. 1999. Head lice: Prevalence in schoolchildren and insecticide resistance. Parasitol. Today 15: 1–4. [DOI] [PubMed] [Google Scholar]
- Durand R., Bouvresse S., Berdjane Z., Izri A., Chosidow O., Clark J. M. 2012. Insecticide resistance in head lice: Clinical, parasitological and genetic aspects. Clin. Microbiol. Infect. 18: 338–344. [DOI] [PubMed] [Google Scholar]
- Federation of Animal Science Societies (FAAS) Writing Committee. 2010. Guide for the care and use of agricultural animals in research and teaching, vol. 3, 3rd ed. Federation of Animal Science Societies, Champaign, IL. [Google Scholar]
- Frankowski B. L., Weiner L. B. 2002. Head lice. Pediatrics 110: 638–43. [PubMed] [Google Scholar]
- Goldman L. R. 1995. Children–unique and vulnerable. Environmental risks facing children and recommendations for response. Environ. Health Perspect. 103: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz N. G. 1997. Human lice: Their prevalence, control and resistance to insecticides, vol. WHO/CTD/WHOPES/97.8. World Health Organization, Switzerland. [Google Scholar]
- Hill N., Moor G., Cameron M. M., Butlin A., Preston S., Williamson M. S., Bass C. 2005. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ 331: 384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipolito R. B., Mallorca F. G., Zuniga-Macaraig Z. O., Apolinario P. C., Wheeler-Sherman J. 2001. Head lice infestation: Single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics 107: E30. [DOI] [PubMed] [Google Scholar]
- Holdsworth P. A., Vercruysse J., Rehbein S., Peter R. J., Letonja T., Green P., and World Association for the Advancement of Veterinary Parasitology. 2006. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of ectoparasiticides against biting lice, sucking lice and sheep keds on ruminants. Vet. Parasitol. 136: 45–54. [DOI] [PubMed] [Google Scholar]
- Hunter J. A., Barker S. C. 2003. Susceptibility of head lice (Pediculus humanus capitis) to pediculicides in Australia. Parasitol. Res. 90: 476–478. [DOI] [PubMed] [Google Scholar]
- Jones K. N., English 3rd J. C. 2003. Review of common therapeutic options in the United States for the treatment of pediculosis capitis. Clin Infect Dis 36: 1355–1361. [DOI] [PubMed] [Google Scholar]
- Ketzis J. K., Clements K., Honraet K. 2014. Use of a poultry model to assess the transfer inhibition effect of head lice (Pediculus humanus capitis) products. Parasitol. Res. 113: 1943–1948. [DOI] [PubMed] [Google Scholar]
- Kristensen M. 2005. Identification of sodium channel mutations in human head louse (Anoplura: Pediculidae) from Denmark. J. Med. Entomol. 42: 826–829. [DOI] [PubMed] [Google Scholar]
- Lebwohl M., Clark L., Levitt J. 2007. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics 119: 965–974. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Clark J. M., Ahn Y. J., Lee W. J., Yoon K. S., Kwon D. H., Seong K. M. 2010. Molecular mechanisms and monitoring of permethrin resistance in human head lice. Pectic. Biochem. Physiol. 97: 109–114. [Google Scholar]
- Lee S. H., Yoon K. S., Williamson M. S., Goodson S. J., Takano-Lee M., Edman J. D., Devonshire A. L., Clark J. M. 2000. Molecular analysis of kdr-like resistance in permethrin-resistant strains of head lice, Pediculus capitis. Pestic. Biochem. Physiol. 66: 130–143. [Google Scholar]
- Meinking T. L. 1999. Head lice infestations: biology, diagnosis, and management. Curr. Prob. Dermatol. 75–118. [Google Scholar]
- Mumcuoglu K. Y. 1996. Control of human lice (Anoplura: Pediculidae) infestations: Past and present. Am. Entomol. 42: 175–178. [Google Scholar]
- Mumcuoglu K. Y., Hemingway J., Miller J., Ioffe-Uspensky I., Klaus S., Ben-Ishai F., Galun R. 1995. Permethrin resistance in the head louse Pediculus capitis from Israel. Med.Vet. Entomol. 9: 427–432. [DOI] [PubMed] [Google Scholar]
- Mumcuoglu K. Y., Galun R., Bach U., Milller J., Magdassi S. 1996. Repellency of essential oils and their components to the human body louse, Pediculus humanus humanus. Entomol. Exp. Appl. 78: 309–314. [Google Scholar]
- Mumcuoglu K. Y., Meinking T. A., Burkhart C. N., Burkhart C. G. 2006. Head louse infestations: The “no nit” policy and its consequences. Int. J. Dermatol. 45: 891–896. [DOI] [PubMed] [Google Scholar]
- Picollo M. I., Vassena C. V., Casadio A. A., Massimo J., Zerba E. N. 1998. Laboratory studies of susceptibility and resistance to insecticides in Pediculus capitis (Anoplura; Pediculidae). J. Med. Entomol. 35: 814–817. [DOI] [PubMed] [Google Scholar]
- Pollack R. J., Kiszewski A., Armstrong P., Hahn C., Wolfe N., Rahman H. A., Laserson K., Telford S.R., III, Spielman A. 1999. Differential permethrin susceptibility of head lice sampled in the United States and Borneo. Arch. Pediatr. Adolesc. Med. 153: 969–973. [DOI] [PubMed] [Google Scholar]
- Rupes V., Moravec J., Chmela J., Ledvinka J., Zelenkova J. 1995. A resistance of head lice (Pediculus capitis) to permethrin in Czech Republic. Cent. Eur. J. Public Health 3: 30–32. [PubMed] [Google Scholar]
- Semmler M., Abdel-Ghaffar F., Al-Quraishy S., Al-Rasheid K. A., Mehlhorn H. 2012. Why is it crucial to test anti-lice repellents? Parasitol. Res. 110: 273–276. [DOI] [PubMed] [Google Scholar]
- Speare R., Buettner P. G. 1999. Head lice in pupils of a primary school in Australia and implications for control. Int. J. Dermatol. 38: 285–290. [DOI] [PubMed] [Google Scholar]
- Takano-Lee M., Edman J. D., Mullens B. A., Clark J. M. 2005. Transmission potential of the human head louse, Pediculus capitis (Anoplura: Pediculidae). Int. J. Dermatol. 44: 811–816. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yaguchi N., Mihara M., Takahashi M., Agui N., Kasai S. 2003. Molecular analysis of a para sodium channel gene from pyrethroid-resistant head lice, Pediculus humanus capitis (Anoplura: Pediculidae). J. Med. Entomol. 40: 468–474. [DOI] [PubMed] [Google Scholar]
- Williams L. K., Reichert A., MacKenzie W. R., Hightower A. W., Blake P. A. 2001. Lice, nits, and school policy. Pediatrics 107: 1011–1015. [DOI] [PubMed] [Google Scholar]
- Yoon K. S., Strycharz J. P., Takano-Lee M., Edman J. D., Clark J. M. 2006. An improved in vitro rearing system for the human head louse allows the determination of resistance to formulated pediculicdes. Pestic. Biochem. Physiol. 86: 195–202. [Google Scholar]