Abstract
Objectives. We explored the contribution of missed primary HIV care visits (“no-show”) to observed disparities in virological failure (VF) among Black persons and persons with injection drug use (IDU) history.
Methods. We used patient-level data from 6 academic clinics, before the Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care intervention. We employed staged multivariable logistic regression and multivariable models stratified by no-show visit frequency to evaluate the association of sociodemographic factors with VF. We used multiple imputations to assign missing viral load values.
Results. Among 10 053 patients (mean age = 46 years; 35% female; 64% Black; 15% with IDU history), 31% experienced VF. Although Black patients and patients with IDU history were significantly more likely to experience VF in initial analyses, race and IDU parameter estimates were attenuated after sequential addition of no-show frequency. In stratified models, race and IDU were not statistically significantly associated with VF at any no-show level.
Conclusions. Because missed clinic visits contributed to observed differences in viral load outcomes among Black and IDU patients, achieving an improved understanding of differential visit attendance is imperative to reducing disparities in HIV.
Disparities in HIV prevalence and health outcomes are well described for racial and ethnic minority populations and among persons with reported injection drug use (IDU) in the United States.1–4 Among individuals diagnosed with HIV infection, these disparities are principally evident with respect to differential viral suppression (or virological failure), higher prevalence of AIDS, and increased mortality.1,5,6 Although recent literature indicates that 72% to 77% of all HIV-positive persons in the United States are linked to HIV primary care within 4 months of initial HIV diagnosis, only 35% of persons living with HIV are virally suppressed, attributable in part to challenges with longitudinal retention in care and inconsistent adherence to antiretroviral therapy (ART).3,7 Moreover, at a population level, surveillance data indicate that fewer Black persons have suppressed viral loads relative to both White and Hispanic/Latino populations.7 Similar disparities have been observed for patients who report IDU, with lower documented clinical service use, higher viral load burden, and increased mortality.4,8,9
In recent years, considerable focus has been placed on the role of retention in HIV primary care as a critical determinant of HIV outcomes.10,11 Suboptimal retention in care is associated with lower ART receipt and worse biological outcomes, including higher viral load, which can contribute to increased infectivity and transmission.10,12,13 Disparate health care access and socioeconomic barriers may also have an impact on both prevalence and mortality rates in HIV.6,14–16 In addition, published literature has established that having a greater number of missed or “no-show” primary care HIV visits, as a measure of care retention, is associated with increased mortality among both new and established clinic patients in HIV primary care settings in the United States and abroad.15–17 Finally, among persons living with HIV/AIDS in the United States, poor retention rates have been observed in Black persons and those who report IDU.4,9,17,18
Although studies have identified suboptimal retention in care and inferior viral load responses among Black persons and persons who report IDU, few investigations have explicitly examined the role of poor retention patterns as they relate to observed disparities in HIV biological outcomes. In this study, we used a large cohort of established HIV primary care patients to examine the role of retention behavior, as measured by cumulative no-show visits, in contributing to disparities in HIV viral load outcomes among Black persons and persons who report IDU. We hypothesized that differential frequency of no-show clinic visits would contribute to the disparate occurrence of virological failure (nonsuppression) among race and risk transmission groups. As improving health outcomes and attenuating disparities are principal objectives of the US National HIV/AIDS Strategy,19 investigating the contribution of retention in HIV care to observed disparities in outcomes may help establish a modifiable intermediary intervention target on the continuum of HIV care.
METHODS
The study sample included 10 053 patients at 6 clinical sites before the implementation of the Centers for Disease Control and Prevention and Health Resources and Services Administration–sponsored Retention in Care (RIC) Intervention Study (ClinicalTrials.gov: CDCHRSA9272007); details from the parent study have been described in detail previously.20,21 The 6 university-affiliated HIV Outpatient Primary Care Clinics were in mid- to large-size metropolitan areas in the United States: Baltimore, Maryland; Birmingham, Alabama; Boston, Massachusetts; Brooklyn, New York; Houston, Texas; and Miami, Florida. Data from each site were stripped of individual identifiers.
Inclusion Criteria
To ensure that the study population comprised established clinic patients, we employed 2 specific inclusion criteria for analyses. First, patients must have arrived for at least 1 scheduled HIV primary care visit at the participating clinic during the 12-month period before the study measurement period of May 1, 2008, through April 30, 2009 (the year preceding implementation of the RIC intervention).
Second, each of the patients who met the first criterion had to have a scheduled HIV primary care appointment during the first 6 months of the study measurement period. As this group had greater opportunities to receive ART and achieve viral suppression, we elected to use data from established clinic patients versus new clinic patients, who may have had insufficient time to achieve viral suppression, the primary outcome of interest.
Finally, we chose the 12-month period before the intervention implementation as the measurement period to avoid potential confounding by phase 1 of the subsequent RIC intervention, which began in May 2009.20 Because we included all patients meeting the eligibility criteria, analyses are reflective of clinic-wide evaluation of established patients in 6 study sites.
Variables of Interest
We collected patient-level sociodemographic factors from records data. These included gender, race, ethnicity, age, site, and HIV risk factor. Risk factor categories were heterosexual, men who have sex with men (MSM), IDU, and other or unknown; we categorized records listing both MSM and IDU risk factors as IDU.
We defined baseline viral load and CD4 count as the measure nearest the observation period start date, May 1, 2008, within a plus-or-minus-120-day window. We defined the principal exposure of interest, retention in HIV primary care, as number of missed HIV primary care clinic visits, with consistent coding employed across all participating sites. We defined missed clinic visits as scheduled HIV primary care appointments that were not cancelled by the patient or by the clinic for which the patient did not arrive, also called “no-show” visits.22
We conducted sensitivity analyses by using visit adherence (proportion of arrived HIV primary care visits) in place of missed visit count as the measure of retention in HIV primary care, with comparable findings observed (data not shown).
The primary outcome measure was nonsuppressed plasma viral load, defined as a viral load greater than 400 copies per milliliter (c/mL), because of limits of viral load detection at this level with assays used by laboratories during the study period (2008–2009). Viral load measures were captured as part of routine care, with the measure closest to the end of the 12-month observation period (±120 days) used for analytic purposes.
Statistical Analyses
We computed descriptive statistics including means, standard deviations, frequencies, and percentages for all study variables to assess distributions. To investigate the relationship among missed visits, the sociodemographic variables of Black race and IDU, and virological nonsuppression at 12 months (> 400 c/mL), we fit staged multivariable logistic regression models. First, we used multivariable models to evaluate relationships between sociodemographic variables (including race and IDU) and virological nonsuppression at the end of 12 months. Next, we added the count of no-show visits that occurred during the 12-month observation period, our designated measure of retention in care, to the model to assess the impact of missed visits on the parameter estimates and statistical significance of the race and HIV transmission risk categories. Consistent with previous studies,23 we employed this staged modeling approach to evaluate the putative role of differential retention in care, operationalized as missed visit count, as a mediator on the causal pathway between Black race and IDU with virological failure.
Next, we employed separate multivariable models to assess associations between patient characteristics and virological nonsuppression among patients stratified by frequency of no-show visits. These models used patients’ retention classified as zero, 1, 2, 3, or 4 or more no-show visits during the measurement period. We used these multivariable models stratified by missed visit count to evaluate the presence of disparities in virological nonsuppression across sociodemographic groups exhibiting similar patterns of HIV care retention (i.e., according to the same number of no-show visits accrued over 12 months). Because we hypothesized that missed visits would be a mediator on the casual pathway between race and risk factor with laboratory indicators,10,24 we evaluated these stratified models to provide additional evidence, beyond the staged modeling approach, of the putative mediating role of missed visits in contributing to disparities in virological nonsuppression among Black and IDU patients.
For patients with missing 12-month viral load measures (n = 1818), we imputed values with SAS’s multiple imputation procedure (version 9.3, SAS Institute Inc, Cary, NC), a fully conditional specification multiple imputation method. Multiple imputation is a Monte Carlo technique in which missing values are replaced by m simulated versions. According to recommendations,25 we used m = 10 simulated versions for primary analyses, and also ran m = 20 simulated versions as sensitivity analyses, with highly consistent results observed (data not shown). Variables included in the imputation process were site, gender, race, ethnicity, HIV risk factor classification, age at baseline, baseline viral load, baseline CD4, 12-month viral load (continuous), and the number of no-show visits (continuous). The imputed missing values (1818 × 10 simulations) yielded 42% with viral loads less than or equal to 400 c/mL (n = 7603), and 58% at greater than 400 c/mL (n = 10 577). We analyzed each of the simulated complete data sets by standard methods, and we combined the results by using SAS’s MIANALYZE procedure to produce estimates and confidence intervals that incorporated missing-data uncertainty.
RESULTS
Among 10 053 study participants, the majority were male (65%), Black (64%), approximately 19% Hispanic ethnicity, and the mean age was 46 years (Table 1). Baseline viral load for the sample was 2.59 (±1.17) log10 c/mL, and baseline CD4 cell count was 456 c/mL (±296; Table 1). Just over one quarter of the sample had a transmission risk category of MSM (28.2%), with approximately half with heterosexual risk behavior (49.2%), and an additional 13.1% had a risk factor of IDU (Table 1). At 12-month follow up, more than one third of the total sample (37%) exhibited a nonsuppressed viral load (> 400 c/mL), or had a missing 12-month viral load value.
TABLE 1—
Sociodemographic Characteristics: Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care Preintervention, 6 US Metropolitan Areas, May 2008–April 2009
| Characteristic | Mean ±SD or Frequency (%) |
| Site | |
| Boston University Medical Center | 1053 (10.5) |
| Johns Hopkins University | 1883 (18.7) |
| Baylor College of Medicine/Thomas Street Health Center | 2904 (28.9) |
| University of Miami | 1984 (19.7) |
| SUNY Downstate Medical Center | 922 (9.2) |
| University of Alabama at Birmingham | 1307 (13.0) |
| Age, y | 45.95 ±10.0 |
| Gendera | |
| Female | 3465 (34.6) |
| Male | 6549 (65.4) |
| Race | |
| Black | 6435 (64.0) |
| White | 3004 (29.9) |
| Other | 614 (6.1) |
| Ethnicitya | |
| Hispanic | 1880 (18.9) |
| Non-Hispanic | 8066 (81.1) |
| HIV risk factor | |
| Heterosexual | 4947 (49.2) |
| IDU | 1548 (15.4) |
| MSM | 2837 (28.2) |
| Other or unknown | 721 (7.2) |
| Baseline CD4 | 456.3 ±295.7 |
| Baseline plasma HIV RNA (log10 c/mL) | 2.59 ±1.17 |
| Mean number of no-show visits | 1.51 ±1.70 |
| 12-mo virological failure (> 400 c/mL) or missinga | 3749 (37.3) |
Note. IDU = intravenous drug use; MSM = men who have sex with men; SUNY = State University of New York. The metropolitan areas surveyed were in Baltimore, MD; Birmingham, AL; Boston, MA; Brooklyn, NY; Houston, TX; and Miami, FL. The sample size was n = 10 053.
Missing values: gender = 39; ethnicity = 107; viral load at 12 months = 1818.
As shown in Table 1, the mean number of no-shows for HIV primary care visits for the overall sample was 1.51 during the 12-month observation period. A graphic comparison of the overall no-show count patterns for Black and White patients is available in Figures 1 and 2; the mean number of no-show visits differed by race group (1.69 Black vs 1.13 White), and by HIV transmission risk classification, specifically IDU (1.96 IDU vs 1.20 MSM; data not shown).
FIGURE 1—
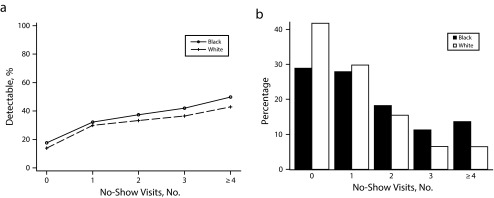
Percentage of (a) virological failure by race stratified by frequency of no-show visits and (b) no-show visits by race: Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care preintervention, 6 US metropolitan areas, May 2008 to April 2009.
Note. The metropolitan areas surveyed were in Baltimore, MD; Birmingham, AL; Boston, MA; Brooklyn, NY; Houston, TX; and Miami, FL.
FIGURE 2—
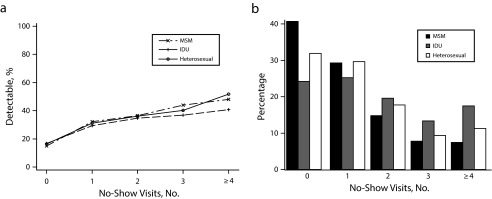
Percentage of (a) virological failure by risk factor stratified by frequency of no-show visits and (b) no-show visits by risk factor: Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care preintervention, 6 US metropolitan areas, May 2008 to April 2009.
Note. IDU = intravenous drug use; MSM = men who have sex with men. The metropolitan areas surveyed were in Baltimore, MD; Birmingham, AL; Boston, MA; Brooklyn, NY; Houston, TX; and Miami, FL.
In the initial multivariable model (Table 2), older age (odds ratio [OR] = 0.83 per 10 years; 95% confidence interval [CI] = 0.78, 0.88) and Hispanic ethnicity (OR = 0.81; 95% CI = 0.67, 0.98) were associated with more favorable viral load outcomes (i.e., a decreased odds of a nonsuppressed viral load). Black race (OR = 1.19; 95% CI = 1.01, 1.40), IDU (OR = 1.23; 95% CI = 1.03, 1.47), baseline CD4 less than 200 c/mL (OR = 1.24; 95% CI = 1.04, 1.47), and higher baseline viral load (OR = 2.06; 95% CI = 1.96, 2.17) were associated with statistically significantly greater odds of having a nonsuppressed 12-month viral load.
TABLE 2—
Odds of Virological Failure in Multivariable Analyses for Characteristics With Patient Variables and No-Show Visit Count: Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care Preintervention, 6 US Metropolitan Areas, May 2008–April 2009
| Odds of VF, Patient Variables Only |
Odds of VF, With No-Show Count |
|||
| Characteristic | OR (95% CI) | P | OR (95% CI) | P |
| Gender: male vs female | 1.05 (0.91, 1.21) | .526 | 1.07 (0.92, 1.23) | .371 |
| Ethnicity: Hispanic vs non-Hispanic | 0.81 (0.67, 0.98) | .03 | 0.79 (0.65, 0.96) | .015 |
| Race | ||||
| Black vs White | 1.19 (1.01, 1.40) | .039 | 1.11 (0.94, 1.32) | .202 |
| Other vs White | 1.24 (0.89, 1.73) | .198 | 1.20 (0.85, 1.68) | .283 |
| Risk factor | ||||
| IDU vs heterosexual | 1.23 (1.03, 1.47) | .025 | 1.10 (0.91, 1.32) | .324 |
| MSM vs heterosexual | 0.91 (0.76, 1.08) | .263 | 0.91 (0.76, 1.08) | .266 |
| Age, baseline (per 10 y) | 0.83 (0.78, 0.88) | < .001 | 0.86 (0.81, 0.91) | < .001 |
| Baseline CD4 | ||||
| < 200 vs > 350 | 1.24 (1.04, 1.47) | .015 | 1.14 (0.96, 1.36) | .127 |
| 200–350 vs > 350 | 1.04 (0.90, 1.20) | .612 | 0.99 (0.85, 1.14) | .87 |
| Baseline viral load (log10 c/mL) | 2.06 (1.96, 2.17) | < .001 | 1.98 (1.88, 2.09) | < .001 |
| No-show frequency (per no-show visit) | 1.20 (1.16, 1.25) | < .001 | ||
Note. CI = confidence interval; IDU = intravenous drug use; MSM = men who have sex with men; OR = odds ratio; VF = virological failure. The metropolitan areas surveyed were in Baltimore, MD; Birmingham, AL; Boston, MA; Brooklyn, NY; Houston, TX; and Miami, FL. The sample size was n = 10 053.
After we added the no-show visit count variable to the initial multivariable model, the odds of having a nonsuppressed viral load remained unchanged with respect to age, ethnicity, and baseline viral load. However, we observed attenuated parameter estimates for patient characteristics that were significant predictors of virological nonsuppression in the initial model (Table 2). With respect to race, the original odds for virological failure for Black patients decreased from 1.19 in the initial model to 1.11 (95% CI = 0.94, 1.32), and the contribution of race was no longer statistically significant (P = .039 vs .202). Similarly, for IDU versus heterosexual risk group, odds of virological failure were attenuated from 1.23 in the initial model to 1.10 (95% CI = 0.97, 1.32), and was no longer statistically significant (P = .025 vs .324) with the inclusion of no-show frequency (Table 2). A 20% increased odds for virological nonsuppression at 12 months was observed for each additional missed visit accrued during this time period.
Next, we used separate multivariable models to assess associations between sociodemographic characteristics (including race and IDU) and virological failure stratified by frequency of no-show visits (Table 3). The contribution of race and IDU to odds of nonsuppressed viral load was not statistically significant in any models for any level of the no-show groups (Table 3). A visual representation of this relationship is presented in Figure 1a for race, where the proportion of nonsuppressed viral load for Black and White race subgroups is depicted according to number of no-show visits alongside Figure 1b, illustrating percentage of Black and White patients by number of missed visits; similar visual results are shown for IDU risk factor (Figure 2).
TABLE 3—
Odds of Virological Failure in Multivariable Analysis by No-Show Visit Frequency: Centers for Disease Control and Prevention and Health Resources and Services Administration Retention in Care Preintervention, 6 US Metropolitan Areas, May 2008–April 2009
| Odds of VF No-Show Count = 0 |
Odds of VF No-Show Count = 1 |
Odds of VF No-Show Count = 2 |
Odds of VF No-Show Count = 3 |
Odds of VF No-Show Count ≥4 |
||||||
| Characteristic | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Gender: male vs female | 1.03 (0.76, 1.38) | .857 | 1.06 (0.83, 1.37) | .638 | 1.13 (0.79, 1.62) | .487 | 1.05 (0.71, 1.55) | .796 | 1.01 (0.74, 1.39) | .93 |
| Ethnicity: Hispanic vs non-Hispanic | 0.72 (0.46, 1.14) | .16 | 0.84 (0.60, 1.17) | .302 | 0.73 (0.47, 1.16) | .183 | 0.76 (0.39, 1.46) | .402 | 0.70 (0.39, 1.28) | .248 |
| Race | ||||||||||
| Black vs White | 1.15 (0.82, 1.61) | .406 | 1.00 (0.74, 1.35) | .994 | 1.10 (0.78, 1.55) | .603 | 1.25 (0.72, 2.18) | .424 | 1.09 (0.67, 1.76) | .723 |
| Other vs White | 1.22 (0.68, 2.20) | .496 | 1.19 (0.67, 2.10) | .548 | 1.32 (0.67, 2.60) | .419 | 0.90 (0.37, 2.20) | .825 | 1.20 (0.53, 2.72) | .663 |
| Risk factor | ||||||||||
| IDU vs heterosexual | 1.31 (0.84, 2.05) | .235 | 1.16 (0.86, 1.57) | .34 | 1.15 (0.77, 1.70) | .488 | 1.30 (0.79, 2.15) | .294 | 0.74 (0.50, 1.09) | .127 |
| MSM vs heterosexual | 0.92 (0.66, 1.30) | .642 | 0.97 (0.74, 1.28) | .84 | 0.87 (0.58, 1.31) | .509 | 1.09 (0.70, 1.70) | .707 | 0.73 (0.47, 1.15) | .177 |
| Age, baseline (per 10 y increased age) | 0.86 (0.76, 0.97) | .013 | 0.89 (0.80, 0.99) | .025 | 0.86 (0.74, 0.99) | .032 | 0.83 (0.69, 1.00) | .056 | 0.88 (0.76, 1.03) | .114 |
| Baseline CD4 | ||||||||||
| < 200 vs > 350 | 0.89 (0.58, 1.36) | .578 | 1.11 (0.82, 1.48) | .498 | 1.40 (0.96, 2.06) | .081 | 0.95 (0.61, 1.48) | .807 | 1.55 (1.07, 2.24) | .02 |
| 200–350 vs > 350 | 0.95 (0.72, 1.26) | .718 | 1.02 (0.79, 1.33) | .854 | 0.97 (0.72, 1.30) | .824 | 0.90 (0.56, 1.44) | .658 | 1.08 (0.73, 1.61) | .699 |
| Baseline viral load (log10 c/mL) | 2.42 (2.14, 2.73) | < .001 | 1.93 (1.75, 2.12) | < .001 | 1.83 (1.62, 2.05) | < .001 | 1.88 (1.63, 2.16) | < .001 | 1.80 (1.59, 2.05) | < .001 |
Note. CI = confidence interval; IDU = intravenous drug use; MSM = men who have sex with men; OR = odds ratio; VF = virological failure. The metropolitan areas surveyed were in Baltimore, MD; Birmingham, AL; Boston, MA; Brooklyn, NY; Houston, TX; and Miami, FL. The sample size was n = 10 053.
DISCUSSION
This study has important implications, particularly for illustrating the relationship between retention patterns, as measured by missed clinic visits, and viral load outcomes for groups with traditionally disparate HIV outcomes. This is especially noteworthy with respect to racial subgroup disparities, as a primary objective of the US National HIV/AIDS Strategy is to reduce HIV-related inequities specifically by increasing the proportion of HIV-diagnosed Black patients with undetectable viral load by at least 20%.19 Race alone cannot be specifically targeted for change in interventions to improve viral suppression, and Black race could heretofore only be identified as a priority population for focused interventions. However, our finding of a comparable frequency of virological nonsuppression across racial groups when stratified by missed visit frequency suggests that no-show events represent a specific target behavior for potential intervention. Although future investigations will determine whether interventions to equalize no-show visit frequencies between racial groups will attenuate and overcome disparities in HIV viral suppression, our findings provide empirical evidence supporting the potential of this approach.
Because the association between poor retention, particularly no-show behavior, and poorer biological outcomes evidenced by virological failure and mortality is established in the literature,10,11,15,26 we employed no-show frequency as a measure of overall retention in primary care. Study findings were consistent with our original hypothesis that no-show clinic visits contribute to the association between patient race and HIV risk transmission behavior and virological nonsuppression. Our results suggest that the frequency of no-show clinic events influences the relationship between disparities in race and risk subgroups and virological nonsuppression for established HIV patients, which is consistent with the limited number of investigations of this type.23 As analyses using a dichotomous measure of no-show visit events (yes or no) produced similar results (data not shown), it is essential to further evaluate missed clinic visits and no-show events as modifiable behavioral factors, and use them as intervention targets for HIV clinical and public health programming.
Reduced service use and no-show behaviors are prevalent for persons who report IDU,8,15,27 as replicated in this study. As observed for Black patients, the greater frequency of viral nonsuppression observed among persons with reported IDU was markedly attenuated when we controlled for missed visits. Moreover, we observed comparable frequencies of viral nonsuppression between IDU and heterosexual risk factor when stratified by missed visit count. This is not unexpected, as reduced ancillary service use and decreased uptake of clinical and laboratory services have also been shown for persons who inject drugs and for persons with drug abuse histories.4 Limits to availability and scope of HIV education and services28 have also been cited as barriers for those who report IDU that may contribute to the frequency of missed visits and likelihood of nonsuppressed viral load.
When one is considering potential intervention approaches, proactive clinical scheduling systems and sophisticated surveillance technologies may prove beneficial for both identifying and subsequently preventing no-show behavior. Success has been shown for automated and live text or voice appointment reminders alone or in combination with coordinated messages20,29 or missed visit notifications to improve attendance at health care visits. In addition, the evidence for employing telemedicine services for improving HIV outcomes in rural populations in the United States is limited, but poses promising evidence for reducing missed visits that are the result of transportation limitations.
Barriers to accessing care, including time, distance to clinic, and insurance or payment limitations, have been shown to influence retention negatively,18,30,31 and may be indicators of important social determinants fueling disparities and serving as intervention targets. Lack of insurance is described as a contributor to gaps in HIV care, poor retention, and staying out of care entirely for specific risk subgroups such as persons with drug or alcohol use.30,32,33 Level of social support and social capital10,34–37 can influence visit adherence and gaps in HIV primary care, particularly among persons who report IDU,35 persons of younger age,33 and racial/ethnic minority MSM.38 Access barriers, particularly with respect to ART, have been shown to contribute to HIV prevalence and overall mortality in the United States.6,39,40 Recent literature posits an association between poverty and worse viral suppression and mortality in persons with HIV.18,41 It is important to note, however, that several recent investigations indicated that racial and sociodemographic disparities were reduced when access to HIV services was similar among heterogeneous cohorts of HIV-positive individuals.42,43
Other intervention targets include psychological and attitudinal factors, which have also shown an association with no-show events, and with retention behavior patterns in general. Perceived and anticipated stigma are documented barriers to HIV service use10,44 and present a distinct barrier to self-management behaviors in HIV-infected female populations.45 HIV self-care behaviors, including medication adherence and visit attendance for infected subgroups, are also shown to be influenced by cultural factors, mistrust, and conspiracy beliefs.46–48 Emerging system- and individual-level literature that describes the influence of patient satisfaction, health care empowerment, patient–provider relationships, and clinical interactions on retention in care also shows promise for improving appointment attendance, and for implementation of retention interventions at multiple levels.10,49,50
This study helps to reiterate the need to address suboptimal retention behavior across all HIV-positive subgroups to improve virological outcomes. The increased use of evidence-based intervention approaches to prevent or reduce no-show events, including peer support, education initiatives, structured strengths-based case management, addressing substance use, and frequent personal contact with clients from clinical facilities,15,20,51–53 may help to lessen known race and risk transmission group disparities in HIV, particularly with respect to retention and virological suppression.
Limitations
Limitations of this study included use of an observational cohort study design over a relatively short 12-month follow-up period. Second, exposure to ART, the major contributor to virological suppression, was not systematically captured and available for inclusion in these analyses. As a greater proportion of Black patients had 4 or more missed visits (Figure 1b), poor ART adherence or delayed ART initiation in this subgroup may have contributed to the observed association between low baseline CD4 count and viral nonsuppression. In addition, although mechanisms for recording patient-level insurance status and type were in place for billing purposes at participating clinical sites during the observation period, these were not reported uniformly or coded consistently across all sites for comparison in this analysis. Therefore, we did not systematically explore insurance status and type, though they are potential socioeconomic indicators of health care access or poverty.31,37
We elected to focus on missed visit count as the primary measure of retention as missed visits are readily captured in real time and immediately actionable, although variability between patients in total scheduled visits has an impact on this measure. The robustness of study findings in sensitivity analyses using visit adherence, which further accounts for total number of scheduled visits, is noteworthy. Finally, these findings may not be applicable for persons who are newly diagnosed or new to HIV outpatient primary care or for individuals who have been out of care for more than 12 months, as we focused exclusively on established clinic patients. Notably, studies of patients newly initiating outpatient HIV medical care or ART have demonstrated a lower average frequency of missed visits over a 12-month observation period (1.11 to 1.23) than seen in the sample of established patients (1.51) in the current study.54,55
Conclusions
With a large multisite observational US cohort drawn from HIV outpatient clinics, our analyses showed differences consistent with described disparities in virological nonsuppression for a number of patient subgroups. By using a staged logistic regression approach and a series of multivariable models stratified by retention behavior, specifically no-show visit count, we made innovative observations regarding nonsuppressed viral load for Black race and IDU risk factor. Our findings indicate that the frequency of missed HIV primary care visits meaningfully contribute to observed differences in viral load outcomes for Black persons and persons who report IDU, a novel and noteworthy finding within a cohort of this size. These results indicate a need for efforts that emphasize the development, investigation, and implementation of interventions to target factors that influence no-show behavior, in an effort to overcome observed disparities and to improve outcomes for all persons living with HIV.
Acknowledgments
The Retention in Care (RIC) Intervention study was funded by the Centers for Disease Control and Prevention and Health Resources and Services Administration (ClinicalTrials.gov: CDCHRSA9272007).
Findings were presented in part at the 8th International Conference on HIV Treatment and Prevention Adherence; June 3, 2013; Miami, FL.
The authors wish to thank the entire RIC Study Group.
Human Participant Protection
All participating clinical sites received institutional review board approvals to use clinic-wide, patient-level data in this investigation.
References
- 1.An Q, Prejean J, Hall HI. Racial disparity in US diagnoses of acquired immune deficiency syndrome, 2000–2009. Am J Prev Med. 2012;43(5):461–466. doi: 10.1016/j.amepre.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Frazier EL, Rhodes P et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 3.Lansky A, Brooks JT, DiNenno E, Heffelfinger J, Hall HI, Mermin J. Epidemiology of HIV in the United States. J Acquir Immune Defic Syndr. 2010;55(suppl 2):S64–S68. doi: 10.1097/QAI.0b013e3181fbbe15. [DOI] [PubMed] [Google Scholar]
- 4.Barash ET, Hanson DL, Buskin SE, Teshale E. HIV-infected injection drug users: health care utilization and morbidity. J Health Care Poor Underserved. 2007;18(3):675–686. doi: 10.1353/hpu.2007.0053. [DOI] [PubMed] [Google Scholar]
- 5.Des Jarlais DC, McCarty D, Vega WA, Bramson H. HIV infection among people who inject drugs: the challenge of racial/ethnic disparities. Am Psychol. 2013;68(4):274–285. doi: 10.1037/a0032745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin MS, Colen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100(6):1053–1059. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618–1623. [PubMed] [Google Scholar]
- 8.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 US areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 9.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis. 2012;55(9):1242–1251. doi: 10.1093/cid/cis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walburn A, Swindells S, Fisher C, High R, Islam KM. Missed visits and decline in CD4 cell count among HIV-infected patients: a mixed method study. Int J Infect Dis. 2012;16(11):e779–e785. doi: 10.1016/j.ijid.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Crawford TN, Sanderson WT, Thornton A. Impact of poor retention in HIV medical care on time to viral load suppression. J Int Assoc Provid AIDS Care. 2014;13(3):242–249. doi: 10.1177/2325957413491431. [DOI] [PubMed] [Google Scholar]
- 12.Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev. 2013;4 doi: 10.1002/14651858.CD009153.pub3. CD009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 14.Colubi MM, Pérez-Elías M, Elías L et al. Missing scheduled visits in the outpatient clinic as a marker of short-term admissions and death. HIV Clin Trials. 2012;13(5):289–295. doi: 10.1310/hct1305-289. [DOI] [PubMed] [Google Scholar]
- 15.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDS. 2013;27(8):442–449. doi: 10.1089/apc.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugavero MJ, Hui-Yi L, Willig JH et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rebeiro P, Althoff KN, Buchacz K et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62(3):356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberhart MG, Yehia BR, Hillier A et al. Behind the cascade: analyzing spatial patterns along the HIV care continuum. J Acquir Immune Defic Syndr. 2013;64(suppl 1):S42–S51. doi: 10.1097/QAI.0b013e3182a90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Office of National AIDS Policy. Washington, DC: The White House; 2010. National HIV/AIDS Strategy. [Google Scholar]
- 20.Gardner LI, Marks G, Craw JA et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124–1134. doi: 10.1093/cid/cis623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugavero MJ, Westfall AO, Zinski A et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–580. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugavero MJ, Lin HY, Allison JJ et al. Racial disparities in HIV virologic failure: do missed visits matter? J Acquir Immune Defic Syndr. 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keruly JC, Conviser R, Moore RD. Association of medical insurance and other factors with receipt of antiretroviral therapy. Am J Public Health. 2002;92(5):852–857. doi: 10.2105/ajph.92.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley and Sons Inc; 1987. Introduction. [Google Scholar]
- 26.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27(7):751–758. doi: 10.1089/AID.2010.0268. [DOI] [PubMed] [Google Scholar]
- 27.Knowlton AR, Hoover DR, Chung SE, Celentano DD, Vlahov D, Latkin CA. Access to medical care and service utilization among injection drug users with HIV/AIDS. Drug Alcohol Depend. 2001;64(1):55–62. doi: 10.1016/s0376-8716(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 28.Williams C, Eisenberg M, Becher J, Davis-Vogel A, Fiore D, Metzger D. Racial disparities in HIV prevalence and risk behaviors among injection drug users and members of their risk networks. J Acquir Immune Defic Syndr. 2013;63(suppl 1):S90–S94. doi: 10.1097/QAI.0b013e3182921506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, Atun R, Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev. 2013;12 doi: 10.1002/14651858.CD007458.pub3. CD007458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthulingam D, Chin J, Hsu L, Scheer S, Schwarcz S. Disparities in engagement in care and viral suppression among persons with HIV. J Acquir Immune Defic Syndr. 2013;63(1):112–119. doi: 10.1097/QAI.0b013e3182894555. [DOI] [PubMed] [Google Scholar]
- 31.Riley ED, Moore KL, Haber S, Neilands TB, Cohen J, Kral AH. Population-level effects of uninterrupted health insurance on services use among HIV-positive unstably housed adults. AIDS Care. 2011;23(7):822–830. doi: 10.1080/09540121.2010.538660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pecoraro A, Royer-Malvestuto C, Rosenwasser B et al. Factors contributing to dropping out from and returning to HIV treatment in an inner city primary care HIV clinic in the United States. AIDS Care. 2013;25(11):1399–1406. doi: 10.1080/09540121.2013.772273. [DOI] [PubMed] [Google Scholar]
- 33.Paz-Bailey G, Pham H, Oster A et al. Engagement in HIV care among HIV-positive men who have sex with men from 21 cities in the United States. AIDS Behav. 2014;18(suppl 3):348–358. doi: 10.1007/s10461-013-0605-y. [DOI] [PubMed] [Google Scholar]
- 34.Latkin CA, Davey-Rothwell MA, Knowlton AR, Alexander KA, Williams CT, Boodram B. Social network approaches to recruitment, HIV prevention, medical care, and medication adherence. J Acquir Immune Defic Syndr. 2013;63(suppl 1):S54–S58. doi: 10.1097/QAI.0b013e3182928e2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramaswamy M, Kelly PJ, Li X, Berg KM, Litwin AH, Arnsten JH. Social support networks and primary care use by HIV-infected drug users. J Assoc Nurses AIDS Care. 2013;24(2):135–144. doi: 10.1016/j.jana.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webel AR, Cuca Y, Okonsky JG, Asher AK, Kaihura A, Salata RA. The impact of social context on self-management in women living with HIV. Soc Sci Med. 2013;87:147–154. doi: 10.1016/j.socscimed.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiser SD, Tsai AC, Gupta R et al. Food insecurity is associated with morbidity and patterns of healthcare utilization among HIV-infected individuals in a resource-poor setting. AIDS. 2012;26(1):67–75. doi: 10.1097/QAD.0b013e32834cad37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traeger L, O’Cleirigh C, Skeer M, Mayer K, Safren S. Risk factors for missed HIV primary care visits among men who have sex with men. J Behav Med. 2012;35(5):548–556. doi: 10.1007/s10865-011-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol. 2013;68(4):197–209. doi: 10.1037/a0032694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano TP, Bartsch G, Zhang Y et al. Disparities in outcomes for African American and Latino subjects in the Flexible Initial Retrovirus Suppressive Therapies (FIRST) trial. AIDS Patient Care STDS. 2010;24(5):287–295. doi: 10.1089/apc.2009.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon J, Wanke C, Terrin N, Skinner S, Knox T. Poverty, hunger, education, and residential status impact survival in HIV. AIDS Behav. 2011;15(7):1503–1511. doi: 10.1007/s10461-010-9759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guest JL, Weintrob AC, Rimland D et al. A comparison of HAART outcomes between the US military HIV Natural History Study (NHS) and HIV Atlanta Veterans Affairs Cohort Study (HAVACS) PLoS One. 2013;8(5):e62273. doi: 10.1371/journal.pone.0062273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giordano TP, Morgan RO, Kramer JR et al. Is there a race-based disparity in the survival of veterans with HIV? J Gen Intern Med. 2006;21(6):613–617. doi: 10.1111/j.1525-1497.2006.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21(10):1321–1334. doi: 10.1080/09540120902803158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webel AR, Higgins PA. The relationship between social roles and self-management behavior in women living with HIV/AIDS. Womens Health Issues. 2012;22(1):e27–e33. doi: 10.1016/j.whi.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaston GB, Alleyne-Green B. The impact of African Americans’ beliefs about HIV medical care on treatment adherence: a systematic review and recommendations for interventions. AIDS Behav. 2013;17(1):31–40. doi: 10.1007/s10461-012-0323-x. [DOI] [PubMed] [Google Scholar]
- 47.Gillman J, Davila J, Sansgiry S et al. The effect of conspiracy beliefs and trust on HIV diagnosis, linkage, and retention in young MSM with HIV. J Health Care Poor Underserved. 2013;24(1):36–45. doi: 10.1353/hpu.2013.0012. [DOI] [PubMed] [Google Scholar]
- 48.Thames AD, Moizel J, Panos SE et al. Differential predictors of medication adherence in HIV: findings from a sample of African American and Caucasian HIV-positive drug-using adults. AIDS Patient Care STDS. 2012;26(10):621–630. doi: 10.1089/apc.2012.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dang BN, Westbrook RA, Black WC, Rodriguez-Barradas MC, Giordano TP. Examining the link between patient satisfaction and adherence to HIV care: a structural equation model. PLoS ONE. 2013;8(1):e54729. doi: 10.1371/journal.pone.0054729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson MO, Sevelius JM, Dilworth SE, Saberi P, Neilands TB. Preliminary support for the construct of health care empowerment in the context of treatment for human immunodeficiency virus. Patient Prefer Adherence. 2012;6:395–404. doi: 10.2147/PPA.S30040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Institute of Medicine. Monitoring HIV Care in the United States: Indicators and Data Systems. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 52.Higa DH, Marks G, Crepaz N, Liau A, Lyles C. Interventions to improve retention in HIV primary care: a systematic review of US studies. Curr HIV/AIDS Rep. 2012;9(4):313–325. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin EG, Wang KH. Integrating substance abuse treatment into HIV care: missed opportunities in the AIDS Drug Assistance Program. J Acquir Immune Defic Syndr. 2013;62(4):421–429. doi: 10.1097/QAI.0b013e31827ee56c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mugavero MJ, Amico KR, Westfall AO et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mugavero MJ, Westfall AO, Cole SR et al. Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis. 2014;59(10):1471–1479. doi: 10.1093/cid/ciu603. [DOI] [PMC free article] [PubMed] [Google Scholar]


