Abstract
Aim:
Antioxidant activity is one of the important indexes for estimating medicinal value for the traditional Chinese medicine. The aim of this study is to investigate the antioxidant activity of 11 flavonoids mainly revealing luteolin as mother nucleus isolated from Pyrethrum tatsienense.
Materials and Methods:
The antioxidant activity of 11 flavonoids was measured in vitro using the classical 1,1-diphenyl-2-picrylhydrazyl removal method. The percentages of scavenging activity of 11 flavonoids were analyzed by taking the choice of a-tocopherol as positive drugs, and the scavenging activity was plotted against the sample concentration to obtain the IC50 values.
Results:
Ten flavonoids containing phenolic hydroxyl groups have different levels of antioxidant activity. Antioxidant activity mainly depends on the numbers and the substitutional positions of phenolic hydroxyls in B ring. When C-3', 4' positions in B ring of flavonoids are replaced by hydroxyl groups, the antioxidant activity improved remarkably. Phenolic hydroxyl groups in A ring contribute some to antioxidant activity because of the electrophilic effect of C ring, and the numbers and substitutional positions of methoxyl and glycosyl have a little effect on the antioxidant activity.
Conclusion:
Structure-activity relationships of antioxidant activity about flavonoids isolated from P. tatsienense are concluded, which will be beneficial to deep understanding the pharmacological functions of this Tibetan medicine in vivo from the point of antioxidation.
KEY WORDS: Antioxidant activity, flavonoids, Pyrethrum tatsienense, structure-activity relationships
INTRODUCTION
Overproduced free radicals or a weakened natural antioxidant system can often lead to the oxidative stress that may eventually result in oxidative injury and diseases. Modern medical studies have shown that a variety of free radicals caused by oxidation in various tissues is one of the important factors of organic damage and pathological changes. Some serious diseases such as cardiovascular disease, diabetes, acquired immune deficiency syndrome, neurodegenerative diseases, inflammation, cancer, and aging process are related to oxidative damage caused by free radicals. It has been widely recognized nowadays that lowering oxidative stress can provide clinical benefits to a variety of pathological conditions, and antioxidant treatment as research focus has thus been regarded as a viable therapy to alleviate the oxidative injury in these disorders [1,2]. Under physiological conditions, a number of free radical scavengers in human body can effectively scavenge free radicals, which keeps the organisms healthy under the normal concentrations of free radicals. The free radical scavengers are usually divided into enzymes and non-enzymes. Enzymes scavengers comprise superoxide dismutase, catalase, glutathione peroxidase, and so on. Natural non-enzymatic scavengers contain Vitamin C, Vitamin E, tea polyphenols, and phenolic acid compounds, which exist widely in natural Chinese herbal medicines and foods [3,4].
Pyrethrum tatsienense (Bur. et Franch.) Ling [Figure 1], an herb of genus Pyrethrum in composite family, is mainly distributed in Qinghai, Sichuan, Yunnan and Tibet eastern part in China. P. tatsienense spends cold in nature and bitter taste, which has the functions of clearing away the heat-evil, expelling superficial evils, dispelling wind, eliminating dampness, anti-inflammatory and analgesia. Thus P. tatsienense has been widely used in folk and clinic in Tibet for the notable therapeutic effects of treating hepatitis, headaches, head injuries, ulcers, wounds, etc. [5]. The early phytochemical studies by our group showed that flavonoids mainly revealing luteolin as mother nucleus were present in considerable amounts in P. tatsienense, and most of flavonoids exist in the forms of glucoside. As we all know, therapeutic properties of flavonoids are diverse as antioxidant [6-9], anti-inflammatory, anti-neoplastic, hepatoprotective, neuroprotective activities [10]. Recently, ethanol extracts of P. tatsienense were found to have the hepatoprotective activity in D-galactosamine liver injury models [11], which reveals that flavonoids in P. tatsienense may be the active ingredients of hepatoprotective combined with the outcomes of phytochemical studies. The important clinical impacts of oxidative stress on liver disease mainly include changes in gene expression, inflammation trigger, liver fibrosis, cancer, and apoptosis. Reactive oxygen free radicals as the starting and regulating factors of liver cell damage may cause necrosis or apoptosis through direct or immune mechanism and hence the antioxidant activity is of great significance to protect liver cell damage.
Figure 1.

Pyrethrum tatsienense (Bur. et Franch.) ling
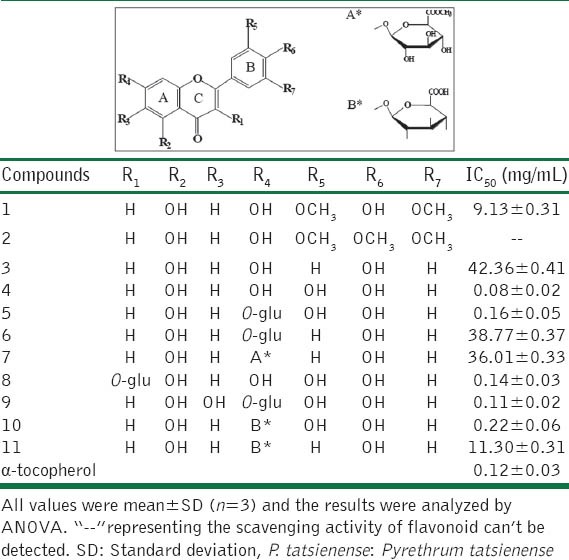
Antioxidant activities of the 11 flavonoids isolated from P. tatsienense [Table 1], named as tricin (1), 4′-methoxy-tricin (2), apigenin (3), luteolin (4), luteolin-7-O-β-D-glucoside (5), apiolin-7-O-β-D-glucoside (6), apiolin-7-O-β-D-glucuronic acid methyl ester (7), quercetin-7-O-β-D-glucoside (8), 6-hydroxy-luteolin-7-O-b-D-glucoside (9), luteolin-7-O-β-D-glucoside aid (10), and apigenin-7-O-β-D-glucoside acid (11), were studied in some detail by the classic antioxidant method of 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay in vitro in this experiment, and meaningful conclusions are drawn from the point of structure-activity relationships.
Table 1.
The structures and antioxidant activity of 11 flavonoids isolated from P. tatsienense
MATERIALS AND METHODS
Plant Material
The inflorescence of P. tatsienense (Bur. et Franch.) Ling were collected in Tibet Province in China, and authenticated by Prof. Chen-Chen Zhu (Institute of Clinical Pharmacology, Guangzhou University of Chinese Medicine). A voucher specimen (PT-201102) was deposited in Herbarium of Institute of Clinical Pharmacology, Guangzhou University of Chinese Medicine.
Materials and Apparatus
DPPH radical and a-tocopherol were obtained from Sigma Chemical Company (USA). The 11 flavonoids were isolated from ethanol extracts of P. tatsienense by the measures of normal phase, reversed phase, sephadex LH20 and molecular sieve column chromatograph. Their purities were over 98 % by normalization of the peak areas detected by high-performance liquid chromatography-ultra violet (UV) (Elite, China). All other reagents were of analytical grade.
Absorbance measurements were performed with a UV1000 UV-vis spectrophoto meter (Techcomp, China).
Sample Preparation
A total of 8.3 mg DPPH·was resolved in 200 mL of methanol to obtain 0.043 mg/mL stock solution. The 11 flavonoids and a-tocopherol were resolved in methanol to obtain 1.0 mg/mL stock solution, and then diluted by methanol to get 0.1, 0.3, 0.5, 0.8, and 1.0 mg/mL solutions, respectively.
Measurement of Antioxidant Activity
Each mixture contained 1.95 mL of DPPH solution and 50 µL of sample solutions, which reacted at room temperature for 20 min. Later, the absorbance values were measured at 517 nm. The absorbance value of methanol as blank control is A0, and the absorbance value of reaction solution is A. Lower absorbance of the reaction mixture indicates higher radical scavenging activity. a-tocopherol was taken as positive control and the percentage DPPH· inhibition of the test samples was calculated as:
Inhibition % = (1 – A/A0)× 100 %
Where A is the absorbance of the tested sample or positive control, A0 is the absorbance of the reaction mixture without sample.
Each measurement was carried out three times in parallel.
Statistical Analyses
The measurements were performed in triplicate and the data were recorded as mean ± standard deviation. The IC50 value was defined as the final concentration of 50% free radical inhibition and was calculated by linear regression analysis. All values are shown with confidence interval and the P < 0.05. Statistical calculations were carried out using the SPSS (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
DPPH· assay is the classics method to evaluate free radical scavenging activity of natural products. DPPH· are the stable purple free radicals with maximum absorption wavelength at 517 nm, which can accept an electron or hydrogen radical to become a stable diamagnetic molecule. The percentages of scavenging activity of 11 flavonoids at different concentrations were analyzed by taking a-tocopherol as the positive drug. The percentages of scavenging activity were plotted against the different sample concentration to obtain the IC50 values. The smaller the clearance rate IC50 value is, the stronger the antioxidant activity of flavonoid is.
Experimental results showed that DPPH free radical scavenging ability of flavonoids increased with the adding concentration of sample, which indicated that flavonoids could effectively scavenge free radicals with certain dose-effect relationships. As shown in Table 1, Comparing the IC50 values of flavonoid 1 with 3, antioxidant activity of the former was obviously larger than the latter although the numbers and substitutional positions of phenolic hydroxyls of these two compounds are the same. It is speculated that the ortho-positions of 4’ hydroxyl are substituted by the two methoxyls as electron donors, which are favorable for the increase of conjugative effect of semi-quinonoid skeletons after hydrogen abstraction. In other word, methoxyl groups remaining at the ortho-positions of phenolic hydroxyl in aromatic ring play a synergistic effect on the antioxidant activity without considering its steric effect. The IC50 value of flavonoid 2 could not be detected, which showed this compound had no DPPH· scavenging activity owing to not containing phenolic hydroxyls in its structure. When C-7 position of flavonoid 4 is replaced by glucose, flavonoid 5 is obtained. The IC50 value of the latter was larger than that of the former, which indicated that antioxidant activity of flavonoid 5 decreased due to the reduced phenolic hydroxyls after glycosidation. Although glucose ring has several hydroxyls, it does not have the ability to delocalize electrons owing to not having conjugated system in its structure. Thus hydroxyls of glucose ring can not be viewed as active sites to scavenge DPPH free radicals. The antioxidant activity will decrease after phenolic hydroxyls glycosidation, the absorbance and distribution of flavonoids in vivo will change because of the increase of volume and polarity of the compounds. The same antioxidant effect will be obtained if flavonoid glycosides can form aglycone under the action of hydrolases in vivo.
On the other hand, free radicals clearance rate IC50 value of flavonoid 4, 5, 8, 9, and 10 are comparable with a-tocopherol, and the antioxidant activity of these five flavonoids are significantly stronger than the other six flavonoids. The reasons should be that the positions of C-3’, 4’ in B ring of these five flavonoids were all replaced by two hydroxyls, which lead to the significant increase of antioxidant activity. In other word, the catechol hydroxyls at C-3’, 4’ positions in flavonoids are the most important active sites. It is presumably that intramolecular hydrogen bond is formed by semi-quinonoid free radical with 4’ hydroxyl after the first hydrogen abstraction, and further quinone is formed after the second hydrogen abstraction occurring at 4’ hydroxyl [Figure 2][12]. Thus the o-dihydroxyls at C-3’, 4’ positions in flavonoids are more advantageous to the high delocalization effect of electrons. Although flavonoid 10 has two sets of o-dihydroxyls at C-3’,4’ positions in B ring and at C-5,6 positions in A ring respectively, the IC50 value of flavonoid 10 was a little lower than that of flavonoid 9. This result indicated that the catechol hydroxyls at C-5, 6 positions in A ring of flavonoid had an insignificant effect on the antioxidant activity. The reason probably is that the intramolecule hydrogen bond are formed by the hydroxyl at C-5 position in A ring with the carbonyl oxygen at C-4 position in C ring, which decrease the ability of hydrogen abstraction of o-dihydroxyls at C-5,6 positions in flavonoid 9. In addition, antioxidant activity of phenolic hydroxyls in A ring of flavonoid decreased significantly because of electrophilic effect of C ring.
Figure 2.
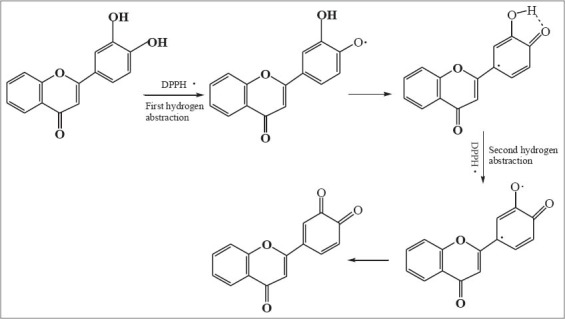
The possible scavenging 1,1-diphenyl-2-picrylhydrazyl mechanism occurring at C-3’, 4’ positions of fl avonoids 4, 5, 8, 9, and 10
CONCLUSIONS
The degree of antioxidant activity depends mainly on the ability of compounds providing protons to neutralize free radicals at the initial stage of oxidation or terminating the radical chain reaction [13]. The main process of flavonoids scavenging free radicals is that phenolic hydroxyls of flavonoids can react with free radicals to terminate chain reaction. Two related mechanisms to eliminate free radicals contain hydrogen abstraction reaction and electron transfer [14]. The ability of flavonoids scavenging free radicals depends mainly on the ease or complexity of the breakdown of phenolic hydroxyls and the stability of semi-quinonoid free radicals after hydrogen abstraction reaction. The bond dissociation energy of phenolic hydroxyl in the aromatic ring is a key mark to measure the strength of phenolic hydroxyl. The lower the dissociation energy is, the more active phenolic hydroxyl is. Thus the easier hydrogen abstraction reaction is, the stronger antioxidant activity is [15,16].
The experimental results of DPPH· methods show that flavonoids have strong antioxidant capacity. The relationships between the structures of flavonoid and antioxidant activity are as follows: (1) The increase of phenolic hydroxyls among flavonoids is favorable for the antioxidant activity, (2) antioxidant activity will increase remarkably when the C-3’, 4’ positions in B ring of flavonoids are replaced by hydroxyls, (3) the ortho position of phenolic hydroxyl containing methoxyl (electron donor group) will be favorable for the improvement of antioxidant capacity, (4) antioxidant activity decreases when flavonoids are glycosylated, which reflects that antioxidant capacity mainly depends on the number of phenolic hydroxyls and nearly has nothing to do with the hydroxyl in sugar ring, (5) the substitutional positions of phenolic hydroxyls have more effects on antioxidant activity than the numbers of phenolic hydroxyls have. This result is agreement with theoretical calculation results which combined phenolic bond dissociation enthalpy with radical scavenging ability [17].
The above studies indicate that flavonoids mainly revealing luteolin as mother nucleus isolated from P. tatsienense can effectively eliminate free radicals, which provide valuable clues for further clarifying the mechanism of preventing or treating hepatic injury induced by free radicals, especially for deeply analyzing the pharmacological functions of P. tatsienense in vivo from the point of antioxidation.
ACKNOWLEDGEMENTS
This work was financially supported by National Natural Science Foundation of China (30960530).
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Chen BC, Cai GM, Yuan Y, Li TT, He Q, He JF. Chemical constituents in hemp pectin I. Chin J Exp Tradit Med Form. 2012;18:98–100. [Google Scholar]
- 2.Mitra I, Saha A, Roy K. Development of multiple QSAR models for consensus predictions and unified mechanistic interpretations of the free-radical scavenging activities of chromone derivatives. J Mol Model. 2012;18:1819–40. doi: 10.1007/s00894-011-1198-x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao BL. Beijing: Science Press; 1999. Oxygen free radicals and natural antioxidant. [Google Scholar]
- 4.Jing P, Zhao SJ, Jian WJ, Qian BJ, Dong Y, Pang J. Quantitative studies on structure-DPPH•scavenging activity relationships of food phenolic acids. Molecules. 2012;17:12910–24. doi: 10.3390/molecules171112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xining: Qinghai People's Press; 1991. Northwest Plateau Institute of Biology Tibetan Medicine. [Google Scholar]
- 6.Lu XX. Research progress in antioxidant mechanism of flavonoids. Food Res Dev. 2012;33:220–4. [Google Scholar]
- 7.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhong JQ, Li B, Jia Q, Li YM, Zhu WL, Chen KX. Advances in the structure-activity relationship study of natural flavonoids and its derivatives. Yao Xue Xue Bao. 2011;46:622–30. [PubMed] [Google Scholar]
- 9.Bubols GB, Vianna Dda R, Medina-Remon A, von Poser G, Lamuela-Raventos RM, Eifler-Lima VL, et al. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13:318–34. doi: 10.2174/138955713804999775. [DOI] [PubMed] [Google Scholar]
- 10.Chen QR. Pharmacological study of flavonoids. China Pract Med. 2012;7:254–5. [Google Scholar]
- 11.Lin CZ, Chen DJ, Bei Ri ZR, Hu M, Li XH, Zhu CC, et al. Protective effect of Pyrethrum tatsienense (Bur.et Franch.) Ling on acute hepatic injury induced by D-galactosamine in rats. Pharm Clin Chin Mater Med. 2011;27:79–81. [Google Scholar]
- 12.Amić A, Marković Z, Dimitrić Marković JM, Stepanić V, Lučić B, Amić D. Towards an improved prediction of the free radical scavenging potency of flavonoids: The significance of double PCET mechanisms. Food Chem. 2014;152:578–85. doi: 10.1016/j.foodchem.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Wu AZ, Lin CZ, Zhu CC. Progress in structure-activity relationship of phenylethanoid glycosides. Nat Prod Res Dev. 2013;25:862–5. [Google Scholar]
- 14.Nagaoka SI, Kuranaka A, Tsuboi H, Nagashima U, Mukai K. Mechanism of antioxidant reaction of vitamin E. charge transfer and tunneling effect in proton-transfer reaction. J Phys Chem. 1992;96:2754–61. [Google Scholar]
- 15.Zhang X, Yang YJ, Lv QZ. Density functional theory calculations on antioxidation activity of the isoflavone compounds from astragalus. Chem Res Appl. 2012;24:1662–9. [Google Scholar]
- 16.Ponomarenko J, Trouillas P, Martin N, Dizhbite T, Krasilnikova J, Telysheva G. Elucidation of antioxidant properties of wood bark derived saturated diarylheptanoids: A comprehensive (DFT-supported) understanding. Phytochemistry. 2014;103:178–87. doi: 10.1016/j.phytochem.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Wright JS, Johnson ER, DiLabio GA. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc. 2001;123:1173–83. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]


