Abstract
Background:
Essential oil components eugenol and carvacrol (ranging between 100 and 200 ppm for carvacrol and between 250 and 750 ppm for eugenol) were tested for antifungal activity against foodborne pathogenic fungal species Aspergillus carbonarius A1102 and Penicillium roqueforti PTFKK29 in in vitro and in situ conditions.
Materials and Methods:
In vitro antifungal activity of eugenol and carvacrol was evaluated by macrobroth method, while watermelon Citrullus lanatus L. Sorento slices were used for antifungal assays in situ.
Results:
Selected components, eugenol and carvacrol showed significant inhibitory effect against tested fungi (A. carbonarius A1102 and P. roqueforti PTFKK29) in yeast extract sucrose broth, as well as in in situ conditions. The minimal inhibitory concentration (MIC) of eugenol against A. carbonarius A1102 determined by macrobroth method was 2000 ppm, while against P. roqueforti PTFKK29 determined MIC was 1000 ppm. Carvacrol inhibited growth of A. carbonarius A1102 at minimal concentration of 500 ppm, while against P. roqueforti PTFKK29, MIC was 250 ppm. The assays in real food system watermelon slices for eugenol and carvacrol show that the inhibitory effect against both selected fungal species was concentration dependent. Furthermore, our results showed that antifungal effect of carvacrol as well as eugenol applied on watermelon slices in all concentrations was a result of effective synergy between an active antifungal compound and lower incubation temperature (15°C) in inhibition of A. carbonarius A1102.
Conclusion:
The present study suggests that the use of eugenol and carvacrol is promising natural alternative to the use of food chemical preservatives, in order to improve safety and quality of fresh-cut and ready-to-eat fruits.
KEY WORDS: Antifungal effect, Aspergillus carbonarius A1102, carvacrol, eugenol, Penicillium roqueforti PTFKK29, watermelon
INTRODUCTION
In recent years, consumers’ demands regarding food production and food safety have changed dramatically. Consumers are more aware of the influence food has on their health [1]. Fruits and vegetables represent an important part in the human diet providing a significant amount of essential vitamins, minerals and dietary fibers. Subsequently, minimally processed food like fresh-cut fruits and vegetables has become an important part of the diet due to its high nutritional value, freshness and practical use [2]. While most food processing technologies involve the stabilization of products and methods for prolonging their shelf life, minimal processing actually reduces the shelf life of food, which represents a potential microbiological risk for consumers, especially in case of improper hygienic conditions during distribution and processing [3].
Western society is facing a trend of “green” consumerism that sets a demand for food products preserved without synthetic additives, salt, as well as environmentally friendly food production technologies [4].
In order to follow this trend, one of the possible solutions in assuring food safety is the use of essential oils and their constituents as antimicrobial additives. Essential oils and their constituents like carvacrol and eugenol show antimicrobial activity against different toxicogenic and pathogenic microorganisms [5]. A number of studies have shown that they possess antibacterial [6,7], antiviral [8], antifungal [9-14], antiparasitic, insecticidal [15,16] and anticarcinogenic activity [17].
Constituents of essential oils like carvacrol and eugenol show antifungal activity against a wide range of fungi [18-20]. They could be used as a safer alternative to chemical fungicides in the food industry.
Carvacrol, (2-methyl-5-[1-methylethyl]-phenol), is a monoterpenoid phenol, a hydrophobic compound, soluble in ethanol, diethyl ether, carbon tetrachloride and acetone with a melting point at 1°C and boiling point at 237.7°C. It has been identified as the active constituent of essential oil of Origanum vulgare L. (oregano) and essential oil of Thymus vulgaris L. (thyme) that exhibits high antimicrobial and antioxidant activities [4].
Carvacrol has been shown to increase membrane fluidity and cause leakage of protons and potassium ions, resulting in a collapse of membrane potential and inhibition of adenosine triphosphate (ATP) synthesis [4]. Aside from the inhibition of the growth of vegetative bacterial cells, carvacrol is able to inhibit the production of diarrheal toxin by Bacillus cereus in broth and in real systems. Mode of action of toxin limitation includes two theories: If toxin excretion is an active process, there may be insufficient ATP or proton-motive force to export it from the cell. Alternatively, the lower specific growth rate may mean that the cells use all the available energy to sustain viability, leaving little over for toxin production [21].
Eugenol (2-methoxy-4-[2-propenyl]phenol) is an allyl chain substituted guaiacol, a major component of Syzygium aromaticum (clove) essential oil (approximately 85%), but also extracted from essential oils of Myristica fragrans Houtt. (nutmeg), Cinnamomum cassia Blume (cinnamon), Ocimum basilicum L. (basil) and Laurus nobilis L. (bay leaf). It is a clear to pale yellow oily liquid with a melting point at −7.5°C and boiling point at 254°C.
Sub-lethal concentrations of eugenol have been found to inhibit the production of amylase and proteases by B. cereus. Cell wall deterioration and a high-degree of cell lysis were also noted. The hydroxyl group on eugenol is thought to bind to proteins, preventing enzyme action in Enterobacter aerogenes [4].
Aspergillus carbonarius optimally grows at a temperature of 30°C, but it can grow even at 10°C. Water activity growth range is between 0.96 and 0.98, while the pH growth range is from 2 to 10 [22]. It is usually found in grapes, grape juice, wine, raisins, and sometimes on raw coffee beans [23]. Grape contamination can occur before harvest, during harvest or during processing [24].
A. carbonarius is the main producer of ochratoxin A, a nephrotoxin which afflicts all tested animal species, while its impact on human health is not easily determined [25]. The connection between ochratoxin A and Balcan endemic nephropathy has been the subject of numerous studies but is, as yet, not completely established [26]. Food products that could potentially be contaminated with ochratoxin A are jams and grape vinegar [24].
Penicillium roqueforti is a psychrothrophic mold that can grow well in cold temperatures and cause spoilage of refrigerated food products. However, the optimal growth temperature of P. roqueforti lies between 20°C and 30°C for water activity of 0.89-0.92. It can grow in a wide range of pH values (3-10) [22]. Furthermore, P. roqueforti is not susceptible to inhibiting activity of weak acids which are usually used as preservatives in the food industry [27].
Although known as a starter culture in “Roquefort” cheese production, P. roqueforti also produces certain toxins such as P. roqueforti toxin, roquefortin C, and mycophenolic acid. The latter two compounds have only limited toxicity [28].
The aim of this study was to determine the antifungal effect of essential oil constituents carvacrol and eugenol against the foodborne pathogenic molds A. carbonarius A1102 and P. roqueforti PTFKK29 in in vitro and in situ conditions on slices of watermelon Citrullus lanatus L. Sorento at different incubation temperatures.
MATERIALS AND METHODS
Fungal Cultures
Fungal cultures of A. carbonarius A1102 and P. roqueforti PTFKK29 species were obtained from the collection of fungi of the Faculty of Food Technology Osijek. Strains were maintained on slants of potato dextrose agar (PDA) (Biolife, Italy) at 4°C. Before experiments, cultures were grown on PDA slants at 25°C for 5 days. Spores were harvested and suspended homogeneously in sterile distilled water with 0.05% Tween 80. Afterwards, the spores in the suspension were counted (Bürker-Türk counting chamber) and their number was adjusted to 1 × 105 spores mL−1.
In vitro Antifungal Activity of Eugenol and Carvacrol
In vitro antifungal activity of eugenol and carvacrol was evaluated by macrobroth method [29] on fungal species of A. carbonarius A1102 and P. roqueforti PTFKK29.
Eugenol (concentrations applied were: 250, 500, and 750 ppm) and carvacrol (concentrations applied were: 100, 150 and 200 ppm) (Sigma, Germany) were dissolved in solution of 10% Tween 80 (Biolife, Italy), 96% ethanol (Kemika, Croatia) and distilled water, sterilized by filtration and used immediately.
Yeast extract sucrose broth (YESB) was used as growth medium for measurement of the inhibitory effect of eugenol and carvacrol. Medium was sterilized at 121°C for 15 min followed by cooling to 50°C in a water bath. After cooling, different concentrations of eugenol and carvacrol were added to sterile broth, homogenized and 5 mL of growth medium was dispensed in test tubes. Fungal spores were inoculated (105 CFU/mL) in YESB and incubated at 25 ± 1°C, and the minimal inhibitory concentration (MIC µg/mL) was recorded after 72 h of incubation. Suitable controls: Broth control (without fungal spores), growth controls (with fungal spores), solvent (Tween 80 and 96% ethanol) and eugenol or carvacrol controls were set under identical conditions. The last tube with no apparent growth of the organism represented the MIC of the compound. To test minimal fungicidal concentration (MFC), from last tube 100 µL was transferred to YESB without antifungal compounds. After 72 h of incubation at 25°C if no growth was observed, MFC was detected. The experiment was performed in duplicates and repeated twice.
In situ Antifungal Activity of Eugenol and Carvacrol
Antifungal activity of eugenol and carvacrol was tested in in situ conditions on watermelon C. lanatus L. Sorento. Watermelon was washed in tap water and dried with paper towels followed by surface sterilization with 70% ethanol. Fruit was cut (with sterile knife) on 5 mm thick slices, and additionally slices of ϕ = 20 mm, were cut with sterile tube cap. Slices were transferred in Petri plates (5 slices/dish) and left in laminar hood with ultraviolet lamps turned on for 20 min. Each watermelon slice was inoculated with 5 µL of fungal spore suspension (1 × 105/mL) followed by 5 µL of tested component solution. Slices were incubated at 25°C and 15°C. Every second day, fungal colony radii were measured, in 2 perpendicular directions, until 6th day of the incubation period. Experiment was performed in duplicates in 2 independent replications. Experiments were performed in July 2012 and July 2013.
Statistical Analyses
Results were analyzed by Microsoft® Office Excel 2003 (Microsoft Corporation, Redmond, USA) and GraphPad Prism version 5.0 for Windows (two-way ANOVA with multiple comparison and Bonferroni post-hoc test) (GraphPad Software, San Diego, USA).
RESULTS
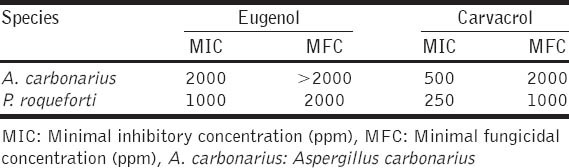
The antifungal activity of eugenol and carvacrol against fungal species A. carbonarius A1102 and P. roqueforti PTFKK29 expressed as MIC and MFC concentrations were presented in Table 1. Antifungal activity of tested compounds ranged from 1000 (MIC) to >2000 ppm (MFC) of eugenol or 250 (MIC) to 2000 ppm (MFC) of carvacrol.
Table 1.
Antifungal activity of eugenol and carvacrol on A. carbonarius and P. roqueforti
The activity of eugenol and carvacrol on watermelon slices (in situ conditions) at 25°C and 15°C was presented in Figures 1-4.
Figure 1.
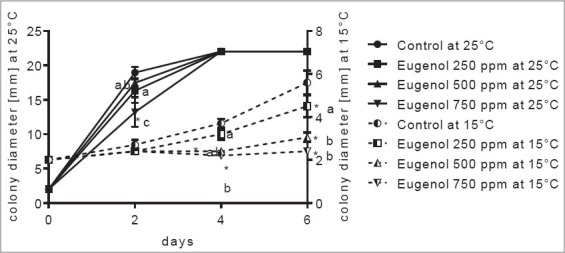
Aspergillus carbonarius colony growth inhibition by eugenol on watermelon slices at 25 and 15°C. *Significant difference compared to control (P ≤ 0.05). Letters (a, b, and ab): Significant difference between tested treatments (P ≤ 0.05)
Figure 4.
Penicillium roqueforti colony growth inhibition by carvacrol on watermelon slices at 25 and 15°C. *Significant difference compared to control (P ≤ 0.05). Letters (a, b, and ab): Significant difference between tested treatments (P ≤ 0.05)
DISCUSSION
Essential oils or their components like eugenol or carvacrol used for antifungal testing are becoming more interesting in modern food technology, although their effect is well-known. Nowadays, consumers concerned about their health are more interested in food produced with minimal processing or preservatives added. This represents a problem in food technology since food safety during the production process or storage is not easily attained. One of the most affected products is minimally processed food-fruit or vegetable salads. During processing minimally processed food, washing (with or without disinfectants), cutting and packaging are allowed. High temperature treatments are usually not permitted. These factors, together with cutting that cause cellular juice leakage, facilitate microbial growth. Fungi selected for this experimental study are important in the food industry for several reasons: A. carbonarius is widespread on fruits, produces a huge amount of contaminant conidia while P. roqueforti, although not common as a fruit detrimental fungus, is capable for growth at lower temperatures. Therefore, P. roqueforti goes in the group of important cold storage fungi. Watermelon, as well as other vegetables (melons, cantaloupes) and fruits (strawberries, apples, etc.) have become very popular ready-to-eat minimally processed salads for consumers worldwide. However, the absence of more intensive preservation methods increases their susceptibility to fungal (or bacterial) growth and besides spoilage, minimally processed salads are widely known as serious safety issue.
The most sensitive fungal species tested was P. roqueforti PTFKK29 [Table 1], since eugenol acted fungicidally at 2000 ppm on its growth. Carvacrol, at a concentration of 1000 ppm, was even more effective in fungicidal activity on tested fungi. Furthermore, MIC of carvacrol was, four times lower compared to eugenol. Since fungicidal effect of carvacrol is well-known, compound, as well as oregano or thyme essential oils, can be used as efficient preservatives in food processing technology. Similarly, A. carbonarius A1102 was more susceptible to carvacrol (MIC value of 500 ppm compared to 2000 ppm of eugenol) although this species, compared to P. roqueforti PTFKK29 is more resistant to tested chemicals. Interpretation of results obtained from antimicrobial assays is demanding, due to different methodology or different strains applied. In a similar experiment [30] authors observed MIC activity of carvacrol and eugenol on Penicillium expansum at a concentration of 262 and 500 ppm, respectively, while in our experiment (unpublished data) the same species was inhibited by 500 and 2000 ppm of antifungal compounds tested. Selection of different strains of the same species in antifungal assays is suggestible, since quite different results can be obtained.
The results of MIC activity of eugenol and carvacrol were used in in situ testing of antifungal efficacy of compounds against same species on watermelon slices incubated at 25°C and 15°C. The main idea of performing this assay on selected temperature regimes came from improper cold storage conditions, often observed in markets. Concentrations of compounds applied were ¼, ½, and ¾ of MIC of eugenol and carvacrol.
At a temperature of 25°C, A. carbonarius A1102 grew rapidly and at 4th day of incubation, all tested concentrations of eugenol, as well as control samples, reached 22 mm (diameter of watermelon slices). This species showed rapid growth on tested fruit slices, especially at higher storage temperature applied. Statistically significant difference, compared to control, was observed only at the highest concentration (750 ppm) applied at 2nd incubation day while among concentration of eugenol, 250 and 750 ppm were significantly different [Figure 1]. Lower growth rate at 15°C was observed where lag phase of fungal growth occurred even in the control sample until 2nd day, while 500 and 750 ppm prolonged this phase until 6th day (possibly even further, although this day was the final day of incubation). Higher concentrations applied, at 4th and 6th day were statistically significant. Carvacrol affected A. carbonarius growth in a similar way [Figure 2] where, at 25°C almost all samples reached the end of fruit slices. However, significant difference was observed between 200 ppm and 150 ppm, compared to control sample (2nd incubation day). At 15°C fungal growth was slower, reaching <6 mm at the end of the incubation period (control sample). Although almost no difference was observed at the start of incubation time, incubation at 4th and 6th day indicated a significant difference in activity of carvacrol, where 200 and 150 ppm were different compared to results obtained by 100 ppm, as well as a control sample. Both eugenol and carvacrol acted similarly on A. carbonarius during in situ experiments on watermelon slices.
Figure 2.
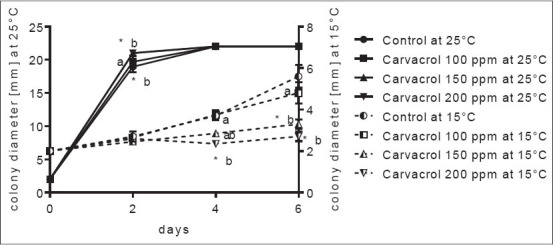
Aspergillus carbonarius colony growth inhibition by carvacrol on watermelon slices at 25 and 15°C. *Significant difference compared to control (P ≤ 0.05). Letters (a, b, and ab): Significant difference between tested treatments (P ≤ 0.05)
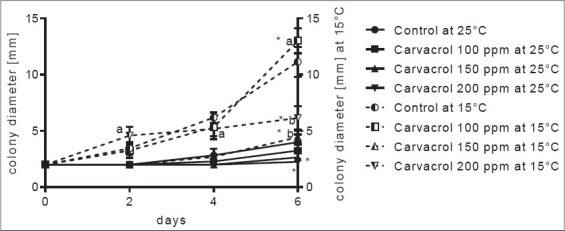
Compared to A. carbonarius, P. roqueforti showed slower growth rate at both selected temperature intervals [Figures 3 and 4]. Interestingly, this species grow faster at a lower temperature (15°C) which shows its psychrotrophic nature. Lag phase of fungal growth lasted until 4th incubation day for the control sample, while treatments prolonged this phase further to 6th day. Since the growth at 25°C was slow, differences between treatments and control are visible at 4th and 6th incubation day, although only difference between control and treatments was noticed. Further incubation would show differentiation between control and concentration of eugenol applied in this experimental setup. Higher growth rate with strong differentiation between concentrations applied was observed at 15°C incubation temperature where all treatments applied were significantly different compared to control sample. Although, considerably lower concentrations of carvacrol on slices were applied, similar results of fungal growth inhibition occurred at both temperatures applied [Figure 4]. Lag phase of P. roqueforti growth was shorter for 2 days compared to eugenol [Figure 3]. Although the growth rate of P. roqueforti treated with 100 ppm of carvacrol was faster, compared to control, as suspected since, if fungi are treated with chemicals applied in concentrations below their inhibitory concentration, their growth rate (and even mycotoxin production) can be even more pronounced. Differences are also possible due to fruit surface structure. Watermelon surface has pronounced hollows that make colony diameter assessment more complicated. This problem can be successfully resolved by an increasing number of fruit slices tested and with experiment repetitions.
Figure 3.
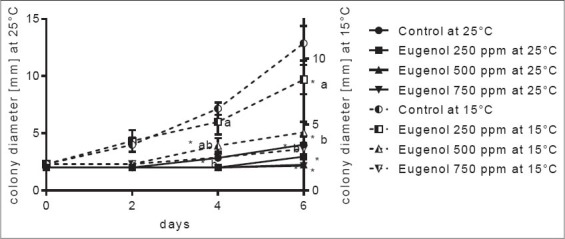
Penicillium roqueforti colony growth inhibition by eugenol on watermelon slices at 25 and 15°C. *Significant difference compared to control (P ≤ 0.05). Letters (a, b, and ab): Significant difference between tested treatments (P ≤ 0.05)
Potential disadvantage in the application of essential oils or their components in food systems is a modification of characteristic sensory profile of the food. In this experimental study, this specific issue was not detected, since small volume of tested compounds was applied (5 µL).
CONCLUSION
Both selected essential oil components, eugenol and carvacrol inhibited tested fungi (A. carbonarius A1102 and P. roqueforti PTFKK29) in YESB, as well as in in situ conditions (watermelon slices). Lag phase of the fungal colony growth on watermelon was prolonged in response to higher concentrations of components applied, for a longer period of time. Lower temperature (15°C) retarded the growth of A. carbonarius A1102, while this effect was not observed during the growth of P. roqueforti PTFKK29. Inhibitory effect of both tested compounds against fungal growth of selected fungal species on watermelon slices is concentration dependent.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Mollet B, Rowland I. Functional foods: At the frontier between food and pharma. Curr Opin Biotechnol. 2002;13:483–5. doi: 10.1016/s0958-1669(02)00375-0. [DOI] [PubMed] [Google Scholar]
- 2.de Sousa JP, de Azerêdo GA, de Araújo Torres R, da Silva Vasconcelos MA, da Conceição ML, de Souza EL. Synergies of carvacrol and 1, 8-cineole to inhibit bacteria associated with minimally processed vegetables. Int J Food Microbiol. 2012;154:145–51. doi: 10.1016/j.ijfoodmicro.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Gleeson E, Beirne DO. Effects of process severity on survival and growth of Escherichia coli and Listeria innocua on minimally processed vegetables. Food Control. 2005;16:677–85. [Google Scholar]
- 4.Burt S. Essential oils: Their antibacterial properties and potential applications in foods - A review. J Agric Food Chem. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Nguefack J, Dongmo JB, Dakole CD, Leth V, Vismer HF, Torp J, et al. Food preservative potential of essential oils and fractions from Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against mycotoxigenic fungi. Int J Food Microbiol. 2009;131:151–6. doi: 10.1016/j.ijfoodmicro.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Mourey A, Canillac N. Anti-Listeria monocytogenes activity of essential oils components of conifers. J Agric Food Chem. 2002;13:289–92. [Google Scholar]
- 7.Carson CF, Riley TV. Safety, efficacy and provenance of tea tree (Melaleuca alternifolia) oil. Contact Dermatitis. 2001;45:65–7. doi: 10.1034/j.1600-0536.2001.045002065.x. [DOI] [PubMed] [Google Scholar]
- 8.Lingk W. Health risk evaluation of pesticide contaminations in drinking water. Gesunde Pflangen. 1991;43:21–5. [Google Scholar]
- 9.Lis-Balchin M, Hart S, Deans SG, Eaglesham E. Comparison of the pharmacological and antimicrobial action of commercial plant essential oils. J Herbs Spices Med Plants. 1996;4:69–86. [Google Scholar]
- 10.Tolouee M, Alinezhad S, Saberi R, Eslamifar A, Zad SJ, Jaimand K, et al. Effect of Matricaria chamomilla L. flower essential oil on the growth and ultrastructure of Aspergillus niger van Tieghem. Int J Food Microbiol. 2010;139:127–33. doi: 10.1016/j.ijfoodmicro.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Bluma R, Amaiden MR, Etcheverry M. Screening of Argentine plant extracts: Impact on growth parameters and aflatoxin B1 accumulation by Aspergillus section Flavi. Int J Food Microbiol. 2008;122:114–25. doi: 10.1016/j.ijfoodmicro.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Unnikrishnan V, Nath BS. Hazardous chemicals in foods. Indian J Dairy Biosci. 2002;11:155–8. [Google Scholar]
- 13.Culter HG, Steverson RF, Cole PD, Jackson DM, Johnson AW. Washington, DC: ACS Symposium Series, American Chemical Society; 1986. Secondary metabolites from higher plants. Their possible role as biological control agents; pp. 178–96. [Google Scholar]
- 14.Tripathi P, Dubey NK. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol Technol. 2004;32:235–45. [Google Scholar]
- 15.Mihaliak CA, Gershenzo J, Croteau R. Lack of rapid monoterpene turnover in rooted plants, implications for theories of plant chemical defense. Oecologia. 2006;87:373–6. doi: 10.1007/BF00634594. [DOI] [PubMed] [Google Scholar]
- 16.Lin CM, Preston JF, 3rd, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–34. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- 17.Cardile V, Russo A, Formisano C, Rigano D, Senatore F, Arnold NA, et al. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J Ethnopharmacol. 2009;126:265–72. doi: 10.1016/j.jep.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Pawar VC, Thaker VS. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses. 2006;49:316–23. doi: 10.1111/j.1439-0507.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–218. [Google Scholar]
- 20.Pérez-Alfonso CO, Martínez-Romero D, Zapata PJ, Serrano M, Valero D, Castillo S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. Italicum involved in lemon decay. Int J Food Microbiol. 2012;158:101–6. doi: 10.1016/j.ijfoodmicro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ultee A, Smid EJ. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int J Food Microbiol. 2001;64:373–8. doi: 10.1016/s0168-1605(00)00480-3. [DOI] [PubMed] [Google Scholar]
- 22.Pitt JI, Hocking AD. 3rd ed. Dordrecht: Springer; 2009. Fungi and Food Spoilage; pp. 237–45. [Google Scholar]
- 23.Patiño B, González-Salgado A, González-Jaén M, Vázquez C. PCR detection assays for the ochratoxin-producing Aspergillus carbonarius and Aspergillus ochraceus species. Int J Food Microbiol. 2005;104:207–14. doi: 10.1016/j.ijfoodmicro.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Aydogdu H, Gucer Y. Microfungi and mycotoxins of grapes and grape products. Trakia J Sci. 2009;7:211–4. [Google Scholar]
- 25.Klich MA. Utrecht: Centraalbureau Voor Schimmelcultures; 2003. Identification of Common Aspergillus Species; pp. 46–57. [Google Scholar]
- 26.Frisvad JC, Thrane U, Samson RA. Mycotoxin producers. In: Dijksterhuis J, Samson RA, editors. Food Mycology a Multifaceted Approach to Fungi and Food. Boca Raton: CRC Press Taylor & Francis Group; 2007. pp. 135–63. [Google Scholar]
- 27.Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol. 2003;94:665–74. doi: 10.1046/j.1365-2672.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- 28.Duraković S, Duraković L. Zagreb: Kugler; 2003. Mikologija u Biotehnologiji; pp. 213–24. [Google Scholar]
- 29.Agarwal SK, Verma S, Singh SS, Tripathi AK, Khan ZK, Kumar S. Antifeedant and antifungal activity of chromene compounds isolated from Blepharispermum subsessile. J Ethnopharmacol. 2000;71:231–4. doi: 10.1016/s0378-8741(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 30.Zabka M, Pavela R. Antifungal efficacy of some natural phenolic compounds against significant pathogenic and toxinogenic filamentous fungi. Chemosphere. 2013;93:1051–6. doi: 10.1016/j.chemosphere.2013.05.076. [DOI] [PubMed] [Google Scholar]


