Abstract
Aims:
This study was carried out to assess the protective and anti-oxidant activities of the methanolic extract of Tribulus terrestris fruits (METT) against sodium valproate (SVP)-induced testicular toxicity in rats.
Materials and Methods:
Fifty mature male rats were randomly divided into five equal groups (n = 10). Group 1 was used normal (negative) control, and the other four groups were intoxicated with SVP (500 mg/kg–1, orally) during the last week of the experiment. Group 2 was kept intoxicated (positive) control, and Groups 3, 4 and 5 were orally pre-treated with METT in daily doses 2.5, 5.0, and 10.0 mg/kg–1 for 60 days, respectively. Weights of sexual organs, serum testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels, semen picture, testicular anti-oxidant capacity and histopathology of testes were the parameters used in this study.
Results:
Oral pre-treatment with METT significantly increased weights of testes and seminal vesicles; serum testosterone, FSH and LH levels and sperm motility, count and viability in SVP-intoxicated rats. METT enhanced the activity of testicular anti-oxidant enzymes and partially alleviated degenerative changes induced by SVP in testes.
Conclusion:
The pre-treatment with METT has protective and anti-oxidant effects in SVP-intoxicated rats. Mechanisms of this protective effect against testicular toxicity may be due to the increased release of testosterone, FSH and LH and the enhanced tissue anti-oxidant capacity. These results affirm the traditional use of T. terrestris fruits as an aphrodisiac for treating male sexual impotency and erectile dysfunction in patients. The study recommends that T. terrestris fruits may be beneficial for male patients suffering from infertility.
KEY WORDS: Anti-oxidant, histopathology, sperm, testis, testosterone, Tribulus terrestris fruits
INTRODUCTION
Infertility is one of the major health problems in life, and approximately about 30% of this problem is due to male factors [1]. Several factors can interfere with the process of spermatogenesis, reduce sperm quantity and quality and decrease male fertility. Many diseases such as coronary heart diseases, diabetes mellitus and chronic liver diseases as well as insufficient vitamins intake have deleterious effects on spermatogenesis and production of normal sperm [2]. Oxidative stress is a major predisposing risk factor for many chronic diseases, including male infertility problem [3,4]. On the other side, intake of natural anti-oxidant agents from plants together with vitamins E and C can protect sperm DNA from oxidative stress in the testis of rats [5], and antagonize testicular toxicity in rabbits [6]. Medicinal plants are a promising source for safe, natural anti-oxidant agents as they contain many bioactive constituents, especially anti-oxidant flavonoids and polyphenol compounds [7].
Tribulus terrestris plant, popularly known as puncture vine, is a perennial creeping herb with a worldwide distribution. Since ancient times, T. terrestris has a long history as a powerful aphrodisiac [8,9]. In traditional medicine, T. terrestris roots are used for treating male infertility [10,11]. Moreover, T. terrestris roots extract and total saponins extracted from the roots have beneficial effects on various ailments such as urinary tract infections, inflammations, leucorrhea, edema, and ascites [11,12]. T. terrestris fruits extract has been successfully used in Europe and Asia to treat sexual dysfunction in males [13]. The different pharmacological effects of T. terrestris plant were reviewed by Chhatre et al. [14]. The present study was designed to evaluate the protective and anti-oxidant activities of the methanolic extract of T. terrestris fruits (METT) against testicular toxicity and oxidative stress induced sodium valproate (SVP) in rats, and to examine the possible mechanisms.
MATERIALS AND METHODS
Plant Material
The fruits of T. terrestris (Family Zygophyllaceae) plant were obtained from the company of medicinal herbs, seeds, agricultural products, Cairo, Egypt. These fruits were botanically authenticated by Dr. Abdelhalim Mohamed, Flora and Taxonomy Department, Agricultural Research Centre, Giza, Egypt. Herbal specimen was deposited at the Department of Pharmacology, Faculty of Veterinary medicine, Cairo University, Egypt.
Extraction of Plant Material
The dry fruits of T. terrestris plant were pulverized and freezing dried. Two hundred grams of powdered dried fruits were extracted with 2 l of 90% methanol (Sigma Aldrich for Chemicals, USA) by percolation for 72 h. The solvent was evaporated by vacuum distillation at 45°C using a rotatory evaporator (West Germany). The liquid extract yielded 10 g of gummy residue that used for preparing the different doses of the extract using tween 80 as a suspending agent.
Sodium Valproate (Depakin®)
It is one of products of Sanofi-Synthelabo Company, Paris, France. It was obtained as an oral solution packed in dark brown bottles each containing 40 ml. SVP is sold commercially under trade name Depakin® 200 mg/ml solution.
Rats and Husbandry
A total of 50 mature male Sprague Dawley rats with average body weight of 200-250 g and age of 10-13 weeks were used in this study. Rats were procured from Laboratory Animal Colony, Ministry of Public Health, Helwan, Egypt. The animals were housed in clean cages, kept under controlled hygienic conditions and maintained at room temperature at 25°C ± 2°C, relative humidity of 50% ± 5% and photoperiod of 12 h dark/12 h light cycles. Rats were fed on rat pellets, and free access of tap water was supplied. The experiment on animals was carried out according to the National Regulations on Animal Welfare and Institutional Animal Ethical Committee.
Experimental Design
The rats were randomized into five equal groups, of 10 animals each. Group 1 was normal (negative) control and administered diluted tween 80 (0.1 ml/rat). The other four groups were intoxicated by oral administration of SVP (500 mg/kg-1) during the last week of the experiment period for induction of testicular toxicity [15]. Group 2 was kept intoxicated positive (control) and Groups 3, 4, and 5 were pre-treated with the METT in daily doses of 2.5, 5.0 and 10.0 mg/kg-1, respectively. The extract was orally given once daily for 60 days to cover the period of the spermatogenic cycle in the rat [15]. Blood samples were withdrawn by puncture of retro-orbital plexus of veins in the eye using microcapillary tubes. The samples were kept standing for 15 min to clot then centrifuged at 10,000 rpm for 10 min to separate the serum which kept frozen at −70°C. The serum was used for estimation of testosterone, follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels. Rats were anesthetized by prolonged exposure to ether, and a longitudinal incision was made in the skin of scrotum and both testes were exposed. Semen samples were collected from cauda epididymis by cutting the tail of epididymis and squeezed it into a clean watch glass. The semen samples were used for semen analysis. The testes and accessory sexual organs were dissected out and weighed, and relative weights of sexual organs were calculated. The right testes were immediately taken on ice cooled bags and kept frozen at −70°C until the assessment of the activity anti-oxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT). The left testes were preserved in 10% neutral formalin solution till processed for histological examination.
Hormone Assay
Serum testosterone concentration was assayed according to the method described in the manufacturer’s directions. The method is based on the enzyme-linked immune absorbent assay (ELISA) as described by Tietz [16]. The assay kit of testosterone was obtained from Immunometrics Limited, London, UK. Serum testosterone concentrations were obtained by correlating the absorbance of the test sample at 550 nm with the corresponding absorbance on the standard curve. Serum levels of FSH and LH hormones were determined using ELISA kits (Amersham, Buckinghamshire, UK) according to Ballester et al. [17]. Assay kits of FSH and LH were supplied by Diagnostic Automation Inc., Calabasas, USA.
Semen Analysis
The seminal content of epididymis was obtained by cutting of cauda epididymis using surgical blades and squeezed into a sterile clean watch glass. This content was diluted 10 times with 2.9% sodium citrate solution and thoroughly mixed to estimate the percentage of sperm progressive motility and sperm count as described by Bearden and Fluquary [18]. Thereafter, one drop of sperm suspension was withdrawn, smeared on a glass slide and stained by eosin-nigrosin stain. The stained seminal smears were examined microscopically to determine the percentage of sperm viability (alive/dead) and morphological abnormalities [19].
Anti-oxidant Enzymes Assay
Tissue specimens of the right testes after thawing was homogenized in nine volumes of ice cooled buffered 0.9% saline solution. The homogenate was then centrifuged at 4000 rpm for 15 min at 4°C and the supernatant was used for anti-oxidant enzymes assay. The activity of SOD was determined as described by Nishikimi et al. [20] and expressed as unit/mg protein. GPx activity was determined as described by Paglia and Valentine [21] and expressed as nmol of glutathione utilized/min/mg protein. CAT activity was estimated according to Sinha [22]. The activity of CAT was expressed as nmol of H2O2 utilized/min/mg protein.
Histological Procedure
Testes, seminal vesicle and prostate glands were fixed in 10% neutral formalin solution. The fixed specimens were trimmed, washed and dehydrated in ascending grades of alcohol, then cleared in xylene, embedded in paraffin, sectioned at 4-6 microns thickness and stained with hematoxylen and eosin stain, then examined microscopically [23].
Statistical Analysis
Data were presented as means ± standard errors. Differences between means in the experimental groups were tested for significance using Student’s t-test. Differences were considered significant at P < 0.05 according to Snedecor and Cochran [24].
RESULTS
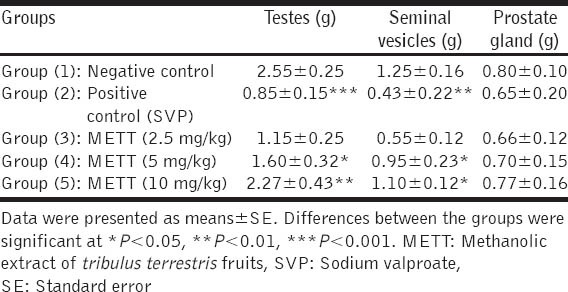
Oral administration of SVP to male rats in a dose of 500 mg/kg during the last week of the experiment induced significant (P < 0.05) decreases in weights of testes and seminal vesicles when compared to the normal control group. Rats pre-treated with oral administration of METT significantly normalized weights of testes and seminal vesicles, in a dose-dependent manner, when compared to SVP-intoxicated control group [Table 1].
Table 1.
Effect of methanolic extract of Tribulus terrestris fruits on weights of sexual organs in sodium valproate-intoxicated rats (n=10)
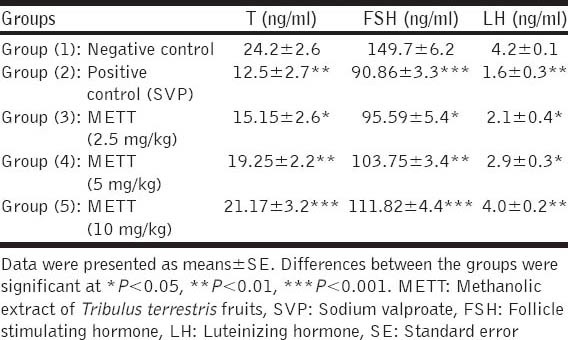
Oral administration of SVP (500 mg/kg) to rats during the last week of the experimental period significantly decreased serum testosterone, FSH and LH levels when compared with the normal control group. Pre-treatments of SVP-intoxicated rats with METT significantly increased serum testosterone, FSH and LH levels when compared with the intoxicated control group, in a dose dependent fashion [Table 2].
Table 2.
Effect of METT on serum testosterone, FSH and LH levels in SVP-intoxicated rats (n=10)
SVP when given orally to male rats (500 mg/kg) during the last week of the experiment induced significant decreases in sperm motility, viability and count and increased percentages of sperm abnormalities when compared with the normal control group. Pre-treatment with METT significantly increased sperm motility, viability and count and decreased percentages of sperm abnormalities, in a dose dependent manner, as recorded in Table 3. The most frequently seen sperm abnormalities were detached, double and circular heads; and bent and coiled tails and a detached neck as demonstrated in Figure 1.
Table 3.
Effect of METT fruits on sperm motility, viability, abnormality and count in SVP-intoxicated rats (n=10)
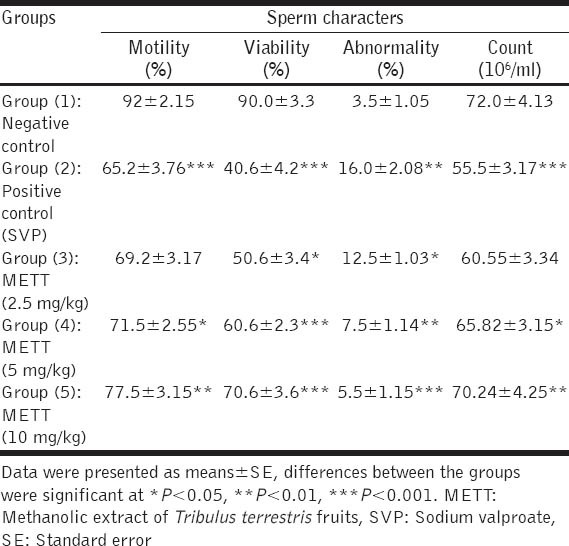
Figure 1.
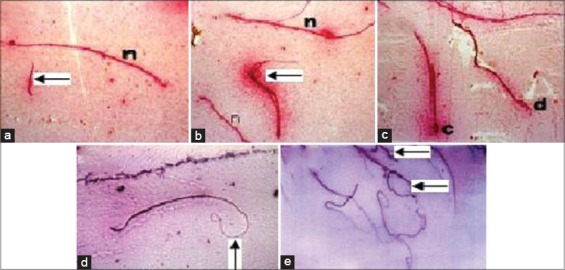
Seminal smears from cauda epididymis of the rat’s testes: (a) Normal mature sperm (n) and detached head of sperm (arrow), (b) double head of sperm (arrow), (c) circular head (c) and deformed head (d), (d) bent tail (arrow), (e) coiled tail of sperm (arrow) and detached neck (arrow)
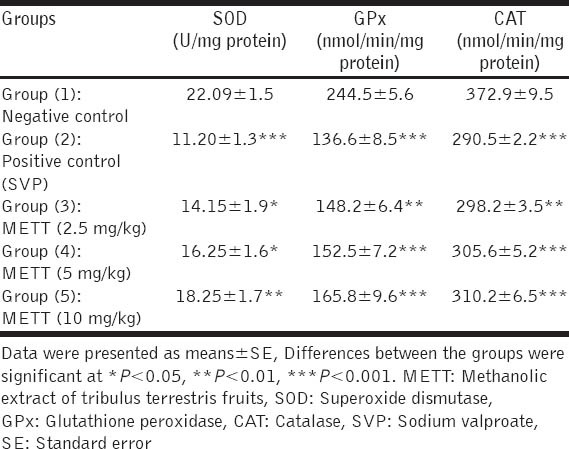
Data are shown in Table 4 revealed that intoxication of rats by SVP induced significant decreases in activities of testicular anti-oxidant enzymes SOD, GPx and CAT compared with normal control rats. The pre-treatment with METT with the three tested doses significantly increased the activity of testicular SOD, GPx and CAT, in a dose dependent fashion, when compared with positive intoxicated rats.
Table 4.
Effect of METT on activity of testicular SOD, GPx and CAT in SVP-intoxicated rats (n=10)
Histopathological Examination
Histopathological examination of testes of normal control rats revealed normal architecture with normal germinal epithelium and fully mature sperms filled the lumen of seminiferous tubules as shown in Figure 2a. The testes of rats orally given SVP in a dose of 500 mg/kg during the last week of the experiment period showed histopathological lesions characterized by edema and necrosis of the germinal epithelium with severe atrophy of seminiferous tubules [Figure 2b]. Some testicular sections showed complete absence of mature sperms (azoospermia) as demonstrated in Figure 2c. The testes of rats given the large dose (10 mg/kg) of METT showed partial improvement in the germinal epithelium of seminiferous tubules, and some mature sperms appeared in them, but the size of seminiferous tubules still atrophied [Figure 2d].
Figure 2.
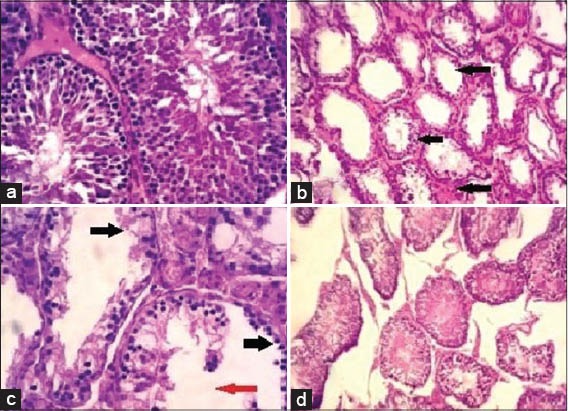
Photomicrographs of testes of: (a) Normal control rat showing normal histological structure of seminiferous tubules filled with mature sperms. Hematoxylen and eosin. (H and E × 400), (b) intoxicated rat by sodium valproate (SVP) showing atrophied seminiferous tubules and edema with absence of sperms (arrows). (H and E × 100), (c) intoxicated rat by SVP showing necrosis of the germinal epithelium of seminiferous tubules (black arrows) with absence of sperms (red arrow) (H and E × 400), (d) pre-treated rat by the large dose (10 mg/kg) of Tribulus terrestris fruits extract showing partial improvement of the germinal epithelium of seminiferous tubules and mature sperm were found in most of seminiferous tubules, but their sizes still atrophied. (H and E × 100)
DISSCUSION
The purposes of this study were to evaluate the protective and anti-oxidant activities of the METT against testicular toxicity induced by SVP in rats, and to examine its possible protective mechanisms.
Oral administration of SVP to rats induced male reproductive toxicity. The toxic effect of SPV characterized by decreased weights of the testes and seminal vesicles, low serum testosterone, FSH and LH levels, low semen quantity and quality, as well as incidence of testicular edema and necrosis with markedly atrophied seminiferous tubules. These effects were agreed with those previously reported [15,25-28]. These authors found that the weights of testes and epididymis as well as sperm count and viability were all decreased following SPV administration to rats and mice. In addition, serum levels of testosterone were dropped, and degeneration, edema necrosis and atrophy of most seminiferous tubules were seen upon histopathological examination of the testis. The previous authors attributed the toxic effect of SPV due to its direct cytotoxic effect on the testis and/or indirectly via decreasing serum testosterone level. Moreover, SVP is commonly used to induce male reproductive toxicity in rats and mice, and its toxic effect was found to a dose-dependent and of a reversible manner [27].
In this study, the oral pre-treatment with the METT at three dosage levels in SVP-intoxicated rats produced a protective effect against testicular toxicity. This protective effect characterized by increased weights of testes and seminal vesicles, improved semen quality and quantity, increased serum testosterone, FSH and LH levels as well as partial amelioration of testicular histopathological lesions seen. This protective effect seemed to be a dose-dependent. These findings were partially in agreement with those previously reported [8-11,14,29,30]. The previous authors concluded that T. terrestris plant acts as a powerful aphrodisiac in rats, mice and humans; improves semen quality and quantity and increases weights of the testis and epididymis. The reported increase in serum testosterone levels following administration of METT in this study was previously recorded by El-Tantawy et al. [31] using both chloroformic and ethanolic extracts of Tribulus alatus fruits in rats. However, a limited number of animal studies displayed a significant increase in serum testosterone levels after administration of METT, but this effect was only noted in humans. Moreover, Qureshi et al. [11] concluded that the release of nitric oxide after administration of T. terrestris extract to rats may offer a possible explanation for its protective activity on male reproductive dysfunction, independent of the serum testosterone level. The authors concluded that T. terrestris plant is successfully used in the management of sexual dysfunction, including erectile dysfunction in patients. In addition, the reported anti-oxidant activity of METT in this study was evident from the enhancement of activities of testicular anti-oxidant enzymes. The anti-oxidant effect of T. terrestris extract was similar to that previously demonstrated [32,33]. The anti-oxidant activity of T. terrestris fruits was attributed to the presence of active derivatives of 4,5-di-p-coumaroylquinic acid, which were isolated from the fruits and reported to have potent anti-oxidant activity [34].
The mechanism(s) underlying the protective effect of METT against testicular toxicity induced by SVP in rats could be attributed to the increased release of testosterone, FSH and LH serum levels. It was previously reported that abnormalities in the synthesis and release of androgens or their depletion by medical or surgical castration may suppress libido and decline erectile and ejaculatory functions [35,36]. The other possible mechanism of the protective effect of METT could be due to its potent anti-oxidant property, so reducing oxidative stress in the testis and improving reproductive function. The anti-oxidant activity of METT was previously attributed to the presence of 4,5-di-p-coumaroylquinic acid that previously isolated from T. terrestris fruits and proved to exhibit a potent anti-oxidant effect [34].
CONCLUSION
SVP induces testicular toxicity in male rats. The METT has protective and anti-oxidant effects against SVP-induced testicular toxicity. The protective effect of METT against SVP toxicity might be due to the increased release of testosterone, FSH and LH and the enhancement of activity anti-oxidant enzymes in testicular tissue by METT. These results affirm the traditional use of T. terrestris fruits as a potent aphrodisiac for treating male sexual impotency and erectile dysfunction in patients. Therefore, T. terrestris fruits may be beneficial for male patients suffering from infertility due to oxidative stress. Moreover, isolation of bioactive constituents of T. terrestris fruits is necessary to search for safe, natural anti-oxidant agents to be developed for the prevention of infertility in males.
ACKNOWLEDGMENT
The authors acknowledge Mr. Ahmed El-Shabrawy at Pharmacology Department, Faculty of Veterinary Medicine, Cairo University for his help in rearing and handling of rats. This work has been performed by the authors of their own interest.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Isidori AM, Pozza C, Gianfrilli D, Isidori A. Medical treatment to improve sperm quality. Reprod Biomed Online. 2006;12:704–14. doi: 10.1016/s1472-6483(10)61082-6. [DOI] [PubMed] [Google Scholar]
- 2.Bener A, Al-Ansari AA, Zirie M, Al-Hamaq AO. Is male fertility associated with type 2 diabetes mellitus? Int Urol Nephrol. 2009;41:777–84. doi: 10.1007/s11255-009-9565-6. [DOI] [PubMed] [Google Scholar]
- 3.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krajcovicova KM, Valachovicova M, Mislanova C, Pribiojova J. Antioxidative vitamins and oxidative lipid and DNA damage in relation to nutrition. Oxid Antioxid Med Sci. 2012;1:147–51. [Google Scholar]
- 5.Jedlinska-Krakowska M, Bomba G, Jakubowski K, Rotkiewicz T, Jana B, Penkowski A. Impact of oxidative stress and supplementation with vitamins E and C on testes morphology in rats. J Reprod Dev. 2006;52:203–9. doi: 10.1262/jrd.17028. [DOI] [PubMed] [Google Scholar]
- 6.Yousef MI. Vitamin E modulates reproductive toxicity of pyrethroid lambda-cyhalothrin in male rabbits. Food Chem Toxicol. 2010;48:1152–9. doi: 10.1016/j.fct.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Sebai H, Selmi S, Rtibi K, Souli A, Gharbi N, Sakly M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013;12:189. doi: 10.1186/1476-511X-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendar B, Bharavi K, Rao GS, Kishore PV, Kumar PR, Kumar CS, et al. Protective effect of an aphrodisiac herb Tribulus terrestris Linn on cadmium-induced testicular damage. Indian J Pharmacol. 2011;43:568–73. doi: 10.4103/0253-7613.84974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Nair V, Gupta YK. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J Pharmacol Pharmacother. 2012;3:43–7. doi: 10.4103/0976-500X.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, Huq AU, Singh R. Cypermethrin induced reproductive toxicity in male wistar rats: Protective role of Tribulus terrestris. J Environ Biol. 2013;34:857–62. [PubMed] [Google Scholar]
- 11.Qureshi A, Naughton DP, Petroczi A. A systematic review on the herbal extract Tribulus terrestris and the roots of its putative aphrodisiac and performance enhancing effect. J Diet Suppl. 2014;11:64–79. doi: 10.3109/19390211.2014.887602. [DOI] [PubMed] [Google Scholar]
- 12.Li M, Guan Y, Liu J, Zhai F, Zhang X, Guan L. Cellular and molecular mechanisms in vascular smooth muscle cells by which total saponin extracted from Tribulus terrestris protects against artherosclerosis. Cell Physiol Biochem. 2013;32:1299–308. doi: 10.1159/000354528. [DOI] [PubMed] [Google Scholar]
- 13.Elahi RK, Sirousrafiy A, Farhad S. Study on the effects of various doses of Tribulus terrestris fruits extract on epididymal sperm morphology and count in rat. Glob Vet. 2013;10:13–7. [Google Scholar]
- 14.Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev. 2014;8:45–51. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamza AA, Amin A. Apium graveolens modulates sodium valproate-induced reproductive toxicity in rats. J Exp Zool A Ecol Genet Physiol. 2007;307:199–206. doi: 10.1002/jez.357. [DOI] [PubMed] [Google Scholar]
- 16.Tietz NW. 3rd ed. Philadelphia, USA: W.B. Saunders Company; 1995. Clinical Guide to Laboratory Tests; pp. 134–8. [Google Scholar]
- 17.Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH-and LH-linked mechanisms. J Androl. 2004;25:706–19. doi: 10.1002/j.1939-4640.2004.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 18.Bearden HJ, Fluquary J. Reston, Virginia, USA: Restore Publishing Co. Inc; 1980. Applied Animal Reproduction; pp. 158–60. [Google Scholar]
- 19.Amann RP. Use of animal models for detecting specific alterations in reproduction. Fundam Appl Toxicol. 1982;2:13–26. doi: 10.1016/s0272-0590(82)80059-6. [DOI] [PubMed] [Google Scholar]
- 20.Nishikimi M, Rao NA, Yogi K. Colorimetric determination of superoxide dismutase in tissues. Biochem Biophys Res Common. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Determination of glutathione peroxidase in tissues by UV method. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 23.Carleton H. 4th ed. London, New York, Toronto: Oxford University Press; 1976. Carleton's Histological Technique; pp. 155–63. [Google Scholar]
- 24.Snedecor GW, Cochran WG. 7th ed. Ames, USA: Iowa State University Press; 1986. Statistical Methods; pp. 90–9. [Google Scholar]
- 25.Soliman GA, Abla Abd el-Meguid. Effects of antiepileptic drugs carbamazepine and sodium valproate on fertility of male rats. Dtsch Tierarztl Wochenschr. 1999;106:110–3. [PubMed] [Google Scholar]
- 26.Nishimura T, Sakai M, Yonezawa H. Effects of valproic acid on fertility and reproductive organs in male rats. J Toxicol Sci. 2000;25:85–93. doi: 10.2131/jts.25.85. [DOI] [PubMed] [Google Scholar]
- 27.Bairy L, Paul V, Rao Y. Reproductive toxicity of sodium valproate in male rats. Indian J Pharmacol. 2010;42:90–4. doi: 10.4103/0253-7613.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, Ahmad T, Parekh CV, Trivedi PP, Kushwaha S, Jena G. Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod Toxicol. 2011;32:385–94. doi: 10.1016/j.reprotox.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Ho CC, Tan HM. Rise of herbal and traditional medicine in erectile dysfunction management. Curr Urol Rep. 2011;12:470–8. doi: 10.1007/s11934-011-0217-x. [DOI] [PubMed] [Google Scholar]
- 30.Malviya N, Jain S, Gupta VB, Vyas S. Recent studies on aphrodisiac herbs for the management of male sexual dysfunction – A review. Acta Pol Pharm. 2011;68:3–8. [PubMed] [Google Scholar]
- 31.El-Tantawy WH, Temraz A, El-Gindi OD. Free serum testosterone level in male rats treated with Tribulus alatus extracts. Int Braz J Urol. 2007;33:554–8. doi: 10.1590/s1677-55382007000400015. [DOI] [PubMed] [Google Scholar]
- 32.Kadry H, Abou Basha L, El Gindi O, Temraz A. Antioxidant activity of aerial parts of Tribulus alatus in rats. Pak J Pharm Sci. 2010;23:59–62. [PubMed] [Google Scholar]
- 33.Kamboj P, Aggarwal M, Puri S, Singla SK. Effect of aqueous extract of Tribulus terrestris on oxalate-induced oxidative stress in rats. Indian J Nephrol. 2011;21:154–9. doi: 10.4103/0971-4065.83727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammoda HM, Ghazy NM, Harraz FM, Radwan MM, El Sohly M, Abdallah II. Chemical constituents from Tribulus terrestris fruits and screening of their antioxidant activity. Phytochemistry. 2013;92:153–9. doi: 10.1016/j.phytochem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Baskin LS, Sutherland RS, DiSandro MJ, Hayward SW, Lipschutz J, Cunha GR. The effect of testosterone on androgen receptors and human penile growth. J Urol. 1997;158:1113–8. doi: 10.1097/00005392-199709000-00108. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Gore AC. Sexual experience changes sex hormones but not hypothalamic steroid hormone receptor expression in young and middle-aged male rats. Horm Behav. 2009;56:299–308. doi: 10.1016/j.yhbeh.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


