Abstract
Background:
Aiming the continuity of the studies of Austroplenckia populnea, Brazilian species of the Celastraceae family, in the present study, it was investigated the effect of crude extracts obtained with ethanol, ethyl acetate and chloroform and two purified constituents, proanthocyanidin A and 4’-O-methylepigallocatechin, both isolated from its samaras, on cancer cell proliferation assays.
Materials and Methods:
The human cancer cells lines MCF-7 (ductal breast carcinoma), A549 (lung cancer), HS578T (ductal breast carcinoma) and non-cancer HEK293 (embryonic kidney cells) were treated with different concentrations of extracts and constituents and the effect was observed through the acid phosphatase method. The chemical structures of the purified compounds were identified by the respective IR and 1H and 13C nuclear magnetic resonance spectral data.
Results:
While crude extracts from samaras of the folk medicine A. populnea can trigger cell proliferative effects in human cell lines, the purified compounds (proanthocyanidin A and 4’-O-methyl-epigallocatechin) isolated from the same extracts can have an opposite (anti-proliferative) effect.
Conclusion:
Based on the results, it was possible to suggest that extracts from samaras of A. populnea should be further investigated for possible cancer-promoting activities; and the active extracts can also represent a source of compounds that have anti-cancer properties.
Keywords: Austroplenckia populnea, cancer cell proliferation, Celastraceae, samaras
INTRODUCTION
In general, a plant extract contains low concentrations of active compounds and a large number of promising but undiscovered compounds [1]. A widely observed and accepted fact about the medicinal value of plants is mainly the bioactivity of the phytocomponents present in it. Although a major effort towards of the elucidation of bioactive phytochemicals is from purification of plant materials, very few screening programs have been initiated in crude plant materials, which could yield a significant number of active extracts. Also, there is a need for developing new easy to use sensitive bioassays for screening these bioactive phytocompounds [2].
Some studies with species of the Celastraceae family show positive results for the anticancer activity [3,4]. Constituents isolated from species of the Celastraceae family such as sesquiterpenes, agarofurans and pentacyclic triterpenes present proven antitumoral properties [5-7]. Among these triterpenes, it has been reported that tingenone and pristimerin, frequently found in different species of the Celastraceae family, present potential properties against cancer cell lines [3].
Austroplenckia populnea (Reissek) Lundell is a Brazilian tropical tree that belongs to Celastraceae family. This plant, popularly known as “Mangabarana,” “Marmelo-do-campo” or “Mangabeira-brava,” can be found in a big tropical ecoregion known as “Cerrado” mainly in the State of Minas Gerais, Brazil, and has been of great interest to researchers in function of its chemical constituents, and larvicidal and molluscicidal properties [8,9]. The decoct from leaves and roots of A. populnea have been used in traditional medicine to treat gastrointestinal disorders [10] and rheumatism [11]. During continued phytochemical investigation of extracts from leaves, stems and roots of A. populnea an extensive number of constituents have been isolated, finding the main compounds as different series of pentacyclic triterpenes, such as friedelanes, agarofurans and others [8,9]. When subjected to in vitro assays, some constituents of A. populnea presented properties such as antibacterial [9,12], antitrypanosomal [13], anti-inflammatory, antinociceptive [14], antiulcerogenic, analgesic [15] and male contraceptive effect [16,17]. In accordance to Monache et al. [18], antitumoral property was also attributed to A. populnea.
Considering that the bioactive property of samaras of A. populnea was not been reported so far and aiming to contribute to the knowledge of phytotherapeutic potential of the A. populnea, in the present study it was investigated the effect of crude extracts and two purified constituents from samaras on the following human cancer cell lines: MCF-7 (ductal breast carcinoma), A549 (lung cancer), HS578T (ductal breast carcinoma) and a non-cancer HEK293 (embryonic kidney cells) through acid phosphatase method.
MATERIALS AND METHODS
Plant Material and Extracts Preparation
The mature samaras of A. populnea were collected during the months of June and July in Nova Lima Region, Minas Gerais State, Brazil. A sample of the collected material was compared and identified with a voucher specimen (No. 10473) deposited at the Herbarium of the Museu de Historia Natural, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. The samaras were dried at room temperature and then fragmented on a knife mill. The powdered material (286.97 g) was submitted to continuous extraction process in a Soxhlet apparatus using hexane, chloroform, ethyl acetate and ethanol as solvent extractors. Afterwards, each solvent was recovered in a rotatory evaporator providing the dry hexane (SAPEH, 45.19 g), chloroform (SAPEC, 4.23 g), ethyl acetate (SAPEAE 5.83 g), and finally ethanol (SAPEE, 33.66 g) extracts.
Isolation and Structure Identification of Proanthocyanidin A and 4’-O-Methylepigallocatechin
To determine the principal chemical groups, each extract from samaras of A. populnea was analyzed following methodology suggested by Wagner et al. [19] and Matos [20]. Proanthocyanidin A and 4’-O-methylepigallocatechin were isolated from polar extract (SAPEE) of samaras through silicagel column chromatography eluted with mixtures of hexane, chloroform, ethyl acetate and methanol in order of increased polarities. These compounds were purified by preparative thin layer chromatography using mixtures of ethyl acetate and methanol.
The structure elucidation of proanthocyanidin A was based on the assignments of its infrared and 1H and 13C (with distortionless enhancement by polarization transfer [DEPT] experiment) nuclear magnetic resonance (NMR) spectral analysis, including comparison with the NMR data already published [21]. The compound 4’-O-methylepigallocatechin was identified by 1H and 13C NMR including 2D experiments followed by comparison with NMR spectral data previously reported [22,23].
Cell Culture and Treatment
The human tumor cells MCF-7 (ductal breast carcinoma); A549 (lung adenocarcinoma); HS578T (ductal breast carcinoma); and non-cancerous cells HEK293 (embryonic kidney cells) provide by American Type Culture Collection (Rockville, MD, USA) were maintained in Dulbecco’s Modified Eagle’s medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin/streptomycin (Sigma). The cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were treated ×1 with trypsin ethylenediaminetetraacetic acid (Sigma) and washed once with phosphate buffer saline (PBS) for maintenance before transfections and cell harvesting.
The extracts were dissolved in sterile dimethylsulfoxide (DMSO) and added to the culture medium aiming to reach maximum concentration of 1% DMSO (v/v) [24]. The isolated compounds were dissolved in growth medium. The sample solutions of substances isolated from samaras of A. populnea were individually added to the wells (96-well microtiter plate) at four different concentrations: 125, 250, 500 and 1000 µg/mL for extracts and 50, 100, 250 and 500 µg/mL, for constituents. For each concentration of the extract or purified constituent from samaras, the assays were made in triplicate. Cells maintained in growth medium only with DMSO (1%, v/v) were used as a solvent control.
Cell Proliferation Assay
The effect of extracts and constituents from samaras of A. populnea on cell proliferation was evaluated through acid phosphatase method [25]. This colorimetric method is based on the conversion of substrate p-nitrophenyl phosphate to p-nitrophenol, which absorbs light at 405 nm (yellow). The amount of p-nitrophenol produced is proportional to the number of living cells present in the culture medium. Cells were previously plated in wells of 96 microtiter plates (100 µL of 1 × 104 cell/mL solution in each well) for 24 h. After removing the medium from microplates, the cells were rinsed twice with PBS. Freshly prepared (100 µL) of phosphatase substrate (10 mM p-nitrophenol phosphate [sigma] in 0.1 M sodium acetate [sigma], 0.1% triton X-100 [BDH], pH 5.5) was added to each well and the microplates were wrapped with aluminium foil and incubated in the dark at 37°C for 2 h. An aliquot (50 µL) of NaOH (1.0 mol/L) was added to each well to stop the enzymatic reaction and followed by measuring the absorbance at 405 nm with a reference wavelength of 620 nm using a Microplater Reader (Modulus Microplate Multimode Reader for Luminescence, Fluorescence and Absorbance).
Statistics
All the experiments were conducted in triplicate. To evaluate the statistical significance, the results were analyzed by means of Student’s t-test. Results were expressed as mean ± standard error of the mean (error bars in graphs). Differences were considered to be significant at P < 0.05.
RESULTS AND DISCUSSION
Phytochemical Analyses
In accordance with methodology suggested by Wagner et al. (1984) [19] and Matos (1997) [20] were identified in the extracts isolated from samaras of A. populnea the presence of long chain hydrocarbons (the most part in SAPEH), triterpenes, flavonoids and catechins. This result is in agreement with previous works in which were isolated mainly pentacyclic triterpenes [9,12].
Structure Elucidation
Proanthocyanidin A and 4’-O-methyl epigallocatechin [Figure 1] were isolated and identified from samaras of A. populnea. These two compounds were previously isolated from other parts of A. populnea [13] and Maytenus truncata, another member of the Celastraceae family, by Meléndez-Salazar [26].
Figure 1.
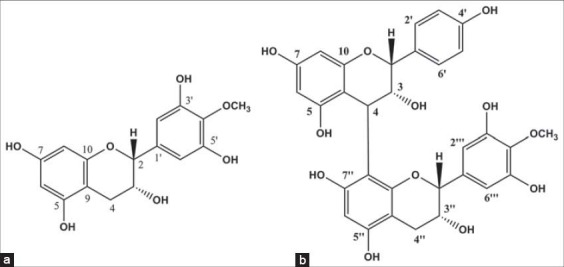
Chemical structures of 4’-O-methyl epigallocatechin (a) and proanthocyanidin A (b)
4’-O-Methylepigalocatechin [Figure 1a] was isolated as an amorphous yellowish solid (m.p. 172.0-174.0 °C). In the 1H NMR spectra (CD3OD, 400 MHz) were observed signals at dH 6.53 (2H, s, H-2’; H-6’), dH 5.95 (1H, d, J = 2.0 Hz, H-8) and dH 5.93 (1H, d, J = 2.4 Hz, H-6) associated to hydrogen of aromatic ring. This signals together those at dH 2.85 (1H, dd, J = 4.2; 16.4 Hz, H-4a) and dH 2.73 (1H, dd, J = 4.2; 16.4 Hz, H-4b) which were attributed to hydrogen of methylene group suggested a flavane structure of the epigalocatechin type [22]. The signal at dH 3.79 (3H, s) were attributed to methoxyl group and the signal at dH 4.78 (1H, s, H-2) and at dH 4.19 (1H, s (large), H-3) were related to carbinol hydrogen atoms. Through the 13C NMR spectrum and DEPT-135 (CD3OD, 100 MHz) were observed the signals at δC: 79.8 (C2), 67.5 (C3), 29.3 (C4), 157.3 (C5), 96.6 (C6), 157.8 (C7), 96.1 (C8), 100.1 (C9), 158.1 (C10), 136.7 (C1′), 107.3 (C6′), 151.5 (C3′ and C5′), 136.3 (C4′) and 60.9 (OMe) [21-23].
Proanthocyanidin A [Figure 1b] was isolated as a brownish amorphous solid (m.p. 227.2-230.0°C). In its 1H NMR spectrum (CD3OD, 400 MHz) were observed signals at dH 7.21, 6.72, 6.59 and at dH 5.93 related to hydrogen of aromatic ring. The signal at dH 3.75 was attributed to hydrogen atoms of the methoxyl group and the signal at dH 2.89 to methylene hydrogen (H-4″). The signal at dH 5.14 (H-3) and dH 3.89 (H-3″) were associated to methinic hydrogen neighboring to C-O and bonded to a hydroxyl group. By the 13C NMR spectrum (CD3OD, 100 MHz) and DEPT-135 were observed the signals at δC: 77.5 (C2), 73.7 (C3), 37.4 (C4), 157.8 (C5), 97.2 (C6), 157.8 (C7), 96.3 (C8), 100.5 (C9), 156.5 (C10), 132.1 (C1′), 129.2 (C2′), 115.8 (C3′ and C5′), 157.8 (C4′), 129.3 (C6′), 80.0 (C2″), 67.0 (C3″), 29.8 (C4″), 157.8 (C5″), 96.3 (C6″), 157.8 (C7″), 107.5 (C8″), 100.5 (C9″), 155.8 (C10‴), 132.1 (C1‴), 107.5 (C2‴ and C6‴), 151.4 (C3‴ and C5‴), 136.0 (C4‴) and 60.8 (OMe) [21].
Cell Proliferation Assay
In order to evaluate the phytotherapeutic nature of samaras of A. populnea, three crude extracts and two purified compounds were respectively added to the culture medium of three different human tumor cells: MCF-7 (ductal breast carcinoma); A549 (lung adenocarcinoma); HS578T (ductal breast carcinoma); and in culture medium of non-cancerous HEK293 (embryonic kidney cells). These cancer cell lines were used because they are highly studied in the research involving cytotoxic analysis and could be easily used for comparison with different compounds [27]. The biological effect of the samples from samaras of A. populnea on cell proliferation was evaluated through the acid phosphatase method.
After 24 and 48 h of treatment, it was observed that the extracts induced an enhancement in the cell proliferation [Figure 2], and there was a significant variation within and between cell lines over time. At 24 h post-treatment of MCF-7 cells with chloroform extract, significant increase in cell proliferation was observed at concentration of 250, 500 and 1000 µg/mL and no effect with 125 µg/mL [Figure 2e]. A further increase in proliferation at 250 and 1000 µg/mL and no significant effect at 125 and 500 µg/mL were verified after 48 h incubation time [Figure 2f]. However, in relation to A549 cell line a significantly decrease in cell proliferation was observed for all concentrations (125, 250, 500 and 1000 µg/mL) of crude chloroform extract from samaras [Figure 2e]. After 24 h of the treatment, the chloroform extract only induced a small cell proliferative effect. For this reason, this type of effect was considered after 48 h incubation time [Figure 2f]. The impact of chloroform extract (250 µg/mL) after 24 h treatment, on HS578T cell proliferation, was considered significant. After 48 h incubation, it was observed that this extract induce cell proliferation at all concentration used in the experiments. In relation to HEK293 embryonic cells were observed a consistent increase in cell proliferation after 24 and 48 h of treatments, for all concentrations [Figure 2e and f]. Ethyl acetate extract induced an increase in the cell proliferation in all four cell lines and with all four concentrations, both after 24 or 48 h [Figure 2c and d]. It was also observed a significantly increased in cell proliferation induced by the four concentrations of ethanol extract (125, 250, 500 and 1000 µg/mL), after 24 or 48 h of treatment of the cells MCF-7, A549, HS578T or HEK293 [Figure 2a and b].
Figure 2.
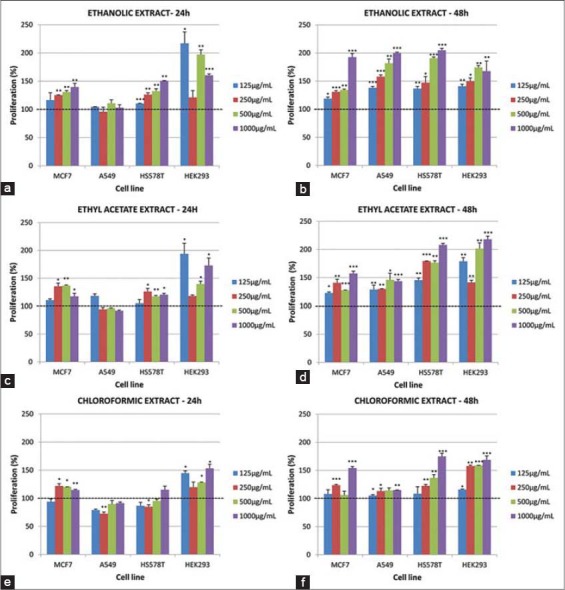
Impact on cell proliferation after 24 and 48 h treatment with three crude extracts isolated from samaras of Austroplenckia populnea, on four cell lines: MCF-7, A549, HS578T and HEK293. Treatment of chloroform extract (a) (24 h) and (b) (48 h), ethyl acetate extract (c) (24 h) and (d) (48 h), ethanol extract € (24 h) and (f) (48 h). Final median concentration of extracts was 125 µg/mL (light blue), 250 µg/mL (red), 500 µg/mL (green) and 1000 µg/mL (dark blue). Data are represented as mean relative percentage (%) and standard deviation of three replicates. Significant differences based on the control were established using Student’s t-test and are represented by asterisks (P < 0.05: *, P ≤ 0.01: **, P ≤ 0.001: ***)
Two constituents isolated from samaras of A. populnea were also subjected to assays to determine its effect on the proliferation of the above cited cells lines. After 24 h the treatment with the proanthocyanidin A, in all concentrations, they were not observed induction of the proliferation of cells MCF-7, A549 or HS578T. In the other hand, it was observed a significant decrease in proliferation of HEK293 cells [Figure 3a]. After 48 h incubation time, it was not observed effect on MCF-7 cells. Proanthocyanidin A at concentrations of 100 and 250 µg/mL decreased the proliferation of A549 cells [Figure 3a]. A significant decrease in HS578T cell proliferation was observed with 50, 100 and 250 µg/mL and no effect with 500 µg/mL [Figure 3a and b]. A significant increase in proliferation was observed at 48 h with HEK293 relative to 24 h treatments; however, the increase was below the control treatments. The other constituent isolated from samaras, 4’-O-methylepigallocatechin, post 24 h treatment had no significant impact on MCF-7 cell proliferation across the concentrations [Figure 3c]. Although, in A549 a significant decrease in cell proliferation at 24 h with 50 and 250 µg/mL concentration was observed and post 48 h there was further decrease in the proliferation [Figure 3c and d]. A significant decrease in proliferation of HS578T at 24 h at 100 µg/mL and further significant decrease in proliferation at 48 h at 50, 100 and 250 µg/mL was observed [Figure 3c and d]. For HEK293 cells, it was verified a significant decrease in proliferation at 24 h with 50, 100 and 250 µg/mL but increased proliferation at 48 h was observed relative to 24 h treatments but the increase was below the relative control treatments.
Figure 3.
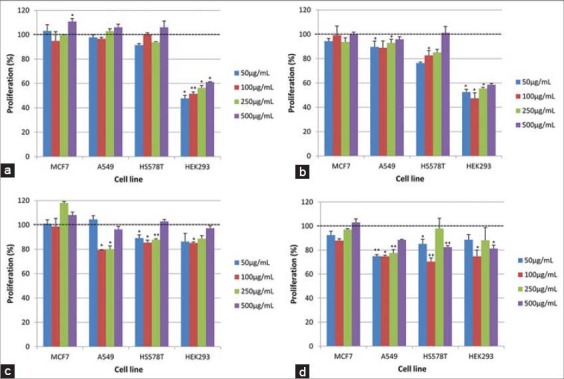
Impact on cell proliferation post 24 and 48 h treatment of two purified compounds from samaras of Austroplenckia populnea on four cell lines MCF-7, A549, HS578T and HEK293. Treatment of proanthocyanidin A (24 h) (a) and (48 h) (b), 4’-O-methylepigallocatechin (24 h) (c) and (48 h) (d). Final concentration of extracts in media was 50 µg/mL (light blue), 100 µg/mL (red), 250 µg/mL (green) and 500 µg/mL (dark blue). Data are shown as relative percentage (%) mean and one standard deviation of three replicates. Significant differences to the control were analyzed using Student’s sssss-test and are represented by asterisks (P < 0.05: *, P ≤ 0.01: **)
There was no real information on the total bioactive compounds present in samaras of this plant and the biological impact produced by each one. Thus, in the present work three crude extracts from samaras of A. populnea were used, obtained with ethanol, ethyl acetate and chloroform, and two purified constituents proanthocyanidin A and 4’-O-methyepigallocatechin to evaluate the impact on cell proliferation on three different human cancer cell lines MCF-7, A549, HS578T and non-cancer HEK293. It was observed from the preliminary cell proliferation data that all the three crude extracts seem to promote an increase on cell proliferation over time in all four model cell lines.
Using only crude extract, it was observed a significantly increased in cell proliferation in most of the cells used. It might reflect of an interaction of a group of compounds with different action on the cell metabolic pathway which has been acting in the growth cell regulation. The elucidation of which specific pathway in being affected by these extracts in the tumor and normal cell lines was not performed because this analysis did not focus in molecular analysis and/or gene expression analysis. The importance of the extracts in growth regulation of the normal cell lines is the main focus because parts of A. populnea, such as leaves and roots have been used in traditional medicine to treat ulcers, dysenteries and rheumatism. It might be dangerous for the population that make this uses because these plant parts (even in small quantity) could cause the increase of cell proliferation without cell-repair, leading to a possible increase the risk of inducing a cancer.
Conversely, the purified compounds were found to significantly decrease the cell proliferation over time in A549, HS578T and HEK293, and did not have any effect on MCF-7. Proanthocyanidin A and 4’-O-methylepigallocatechin belong to the group of flavonoids and are known for their antioxidant properties and anti-proliferative effect in cancer cell line [28,29].
CONCLUSION
From the preliminary data, crude extracts from samaras of A. populnea could be useful in cure for various diseases as rheumatism and bones injuries because of its positive impact on cell growth, but it revokes a question of how safe it is to be taken orally. The samaras extract may contain bioactive phytocomponents that need further investigation.
ACKNOWLEDGMENT
The authors do thank the Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG) for financial support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–13. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 2.Kupchan SM, Maruyama M, Hemingway RJ, Hemingway JC, Shibuya S, Fujita T, et al. Eupacunin, a novel antileukemic sesquiterpene lactone from Eupatorium cuneifolium. J Am Chem Soc. 1971;93:4914–6. doi: 10.1021/ja00748a048. [DOI] [PubMed] [Google Scholar]
- 3.Gomes JP, Cardoso CR, Varanda EA, Molina J, Fernandez MF, Olea N, et al. Antitumoral, mutagenic and (anti) estrogenic activities of tingenone and pristimerin. Braz J Pharmacogn. 2011;21:963–71. [Google Scholar]
- 4.Niero R, de Andrade SF, Cechinel Filho V. A review of the ethnopharmacology, phytochemistry and pharmacology of plants of the Maytenus genus. Curr Pharm Des. 2011;17:1851–71. doi: 10.2174/138161211796391029. [DOI] [PubMed] [Google Scholar]
- 5.Shirota O, Morita H, Takeya K, Itokawa H. Cytotoxic aromatic triterpenes from Maytenus ilicifolia and Maytenus chuchuhuasca. JNat Prod. 1994;57:1675–81. doi: 10.1021/np50114a009. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Tian X, Pan Y. Antitumor sesquiterpenes from ss. Helv Chim Acta. 2003;86:3320–5. [Google Scholar]
- 7.Buffa W, Bolzani VD, Furlan M, Pereira SI, Pereira MA, França SC. In vitro propagation of Maytenus ilicifolia (Celastraceae) as potential source for antitumoral and antioxidant quinomethide triterpenes production. A rapid quantitative method for their analysis by reverse-phase high-performance liquid chromatography. Arkivoc. 2004;6:137–46. [Google Scholar]
- 8.Vichnewski W, Prasad JS, Herz W. Polyhydroxyagarofuran derivatives from Austroplenckia polpunea. Phytochemistry. 1984;23:1655–7. [Google Scholar]
- 9.Vieira Filho SA, Duarte LP, Paes HC, Silva GD, Sousa JR, Lanna MC. Antibacterial activity of pentacyclic triterpenes from Austroplenckia populnea. Acta Hortic. 1999;501:199–03. [Google Scholar]
- 10.Correa MP, Penna LA. 6 Illustrated Vols. Vol. 4. Rio de Janeiro: Ministério da Agricultura. Instituto Brasileiro de Desenvolvimento Florestal 1926-1984; Dicionário de Plantas Úteis do Brasil e das Exóticas Cultivadas; p. 152. [Google Scholar]
- 11.Gonzalez JG, delle Monache G, delle Monache F, Marini-Bettolò GB. Chuchuhuasha - A drug used in folk medicine in the Amazonian and Andean areas. A chemical study of Maytenus laevis. J Ethnopharmacol. 1982;5:73–7. doi: 10.1016/0378-8741(82)90022-8. [DOI] [PubMed] [Google Scholar]
- 12.Miranda RR, Duarte LP, Silva GD, Vieira Filho SA, Carvalho PB, Messas AC. Evaluation of antibacterial activity of “Mangabarana” Austroplenckia populnea Reissek (Celastraceae) Rev Bras Farmacogn. 2009;19:370–5. [Google Scholar]
- 13.Duarte LP, Vieira Filho SA, Silva GDF, Pinto AS. Antitrypanosomal activity of pentacyclic triterpenes isolated from Austroplenckia polpunea (Celastraceae) Rev Inst Med Trop São Paulo. 2002;44:109–12. doi: 10.1590/s0036-46652002000200010. [DOI] [PubMed] [Google Scholar]
- 14.Andrade SF, Cardoso LG, Carvalho JC, Bastos JK. Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of Austroplenckia populnea. J Ethnopharmacol. 2007;109:464–71. doi: 10.1016/j.jep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Seito LN, Mazaro R, Di Stasi LC. Antiulcerogenic and analgesic effects of the Austroplenckia populnea extracts in mice. Phytother Res. 2002;16:193–6. doi: 10.1002/ptr.932. [DOI] [PubMed] [Google Scholar]
- 16.Mazaro R, Di Stasi LC, Filho SA, De Grava Kempinas W. Decrease in sperm number after treatment of rats with Austroplenckia populnea. Contraception. 2000;62:45–50. doi: 10.1016/s0010-7824(00)00135-9. [DOI] [PubMed] [Google Scholar]
- 17.Mazaro R, Di Stasi L, De Grava Kempinas W. Effects of the hydromethanolic extract of Austroplenckia populnea (Celastraceae) on reproductive parameters of male rats. Contraception. 2002;66:205–9. doi: 10.1016/s0010-7824(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 18.Monache FD. Populnonic acid, a new triterpenic acid with friedelane carbon skeleton. Gazz Chim Ital. 1972;102:636–46. [Google Scholar]
- 19.Wagner H, Blat S, Zgainsky EM. Berlin: Springer; 1984. Plant Drug Analysis. [Google Scholar]
- 20.Matos FJ. 2nd ed. Fortaleza, Ceará: Editora UFC; 1997. Introduction to Experimental Phytochemistry. [Google Scholar]
- 21.Weeratunga G, Bohlin L, Verpoorte R, Kumar V. Flavonoids from Elaeodendron Balae root Bark. Phytochemistry. 1985;24:2093–5. [Google Scholar]
- 22.Meng X, Lee MJ, Li C, Sheng S, Zhu N, Sang S, et al. Formation and identification of 4’-O-methyl-(-)-epigallocatechin in humans. Drug Metab Dispos. 2001;29:789–93. [PubMed] [Google Scholar]
- 23.Medeiros FA, Medeiros AA, Tavares JF, Barbosa Filho JM, Lima EO, Silva MS. Licanol, um novo flavanol, e outros constituintes de Licania macrophylla Benth. Quim Nova. 2012;35:1179–83. [Google Scholar]
- 24.Rocha FD, Soares AR, Houghton PJ, Pereira RC, Kaplan MA, Teixeira VL. Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytother Res. 2007;21:170–5. doi: 10.1002/ptr.2038. [DOI] [PubMed] [Google Scholar]
- 25.Muniyappa MK, Dowling P, Henry M, Meleady P, Doolan P, Gammell P, et al. MiRNA-29a regulates the expression of numerous proteins and reduces the invasiveness and proliferation of human carcinoma cell lines. Eur J Cancer. 2009;45:3104–18. doi: 10.1016/j.ejca.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Meléndez-Salazar GD. Belo Horizonte, PhD Thesis. Depto de Química, ICEx, UFMG; 2005. Maytenus truncata Reissek: Phytochemical study, hystological analysis and sorptive capacity of leaves and biological evaluation of extracts; p. 235. [Google Scholar]
- 27.Liu JM, Haroun-Bouhedja F, Boisson-Vidal C. Analysis of the in vitro inhibition of mammary adenocarcinoma cell adhesion by sulphated polysaccharides. Anticancer Res. 2000;20:3265–71. [PubMed] [Google Scholar]
- 28.Touriño S, Selga A, Jiménez A, Juliá L, Lozano C, Lizárraga D, et al. Procyanidin fractions from pine (Pinus pinaster) bark: radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J Agric Food Chem. 2005;53:4728–35. doi: 10.1021/jf050262q. [DOI] [PubMed] [Google Scholar]
- 29.García-Pérez ME, Royer M, Duque-Fernandez A, Diouf PN, Stevanovic T, Pouliot R. Antioxidant, toxicological and antiproliferative properties of Canadian polyphenolic extracts on normal and psoriatic keratinocytes. J Ethnopharmacol. 2010;132:251–8. doi: 10.1016/j.jep.2010.08.030. [DOI] [PubMed] [Google Scholar]


