Abstract
Aim:
Plants used in the Far North Region of Cameroon by livestock farmers to manage foot and mouth disease (FMD) in cattle and the phytochemical composition and antioxidant potentials of two of them (Boscia senegalensis [BS] and Tapinanthus dodoneifolius [TD]) were investigated in this study.
Materials and Methods:
Ethno veterinary data were collected from 325 livestock farmers using semi-structured interviews from September 2011 to April 2012. The 2,2-diphenyl-picrylhydrazyl radical scavenging activity and total phenolic content (TPC) were first performed with five different solvents to choose the best extract of each plant based on these two factors. To achieve our aim, the ferric iron reducing activity, hydroxyl radical scavenging activity (HRSA), free radical scavenging activity (FRSA), vitamin E and iron content were analyzed on extracts selected using current techniques.
Results:
The results showed that 12 plants of 8 different families are regularly used by farmers to manage FMD. It also demonstrated that acetone extract of TD and methanolic extract of BS are the extracts which showed the best total antioxidant activity (AA) and the best TPC. In general, TD show the best AA during the HRSA and FRSA analysis compared with BS. Similarly, TD content more phenolic compounds and tannins than BS. Both plants contain proteins, saponins, tannins, phenols, alkaloid, and polyphenols which are known to have many biological activities.
Conclusion:
These results support the AA of both plants and can justify their use by herders to treat FMD which is often followed by many secondary diseases.
Keywords: Antioxidant activity, Boscia senegalensis, foot and mouth disease, Tapinanthus dodoneifolius
INTRODUCTION
In the Far North Region of Cameroon where the rate of extreme poverty is the highest in the country (41%), livestock production is one of the main activities and represents the second highest source of cash income to the rural populations after cotton [1,2]. In this region where breeding is a tradition for many native ethnic groups, infectious animal diseases including foot and mouth disease (FMD), constitute one of the mainhindrances to cattle productivity and production [3]. To treat this disease and to improve their yield, 69% of farmers use a variety of veterinary pharmaceutical medications and 25% of them use traditional medicine [4]. Herders who use veterinary drugs usually misuse them [4] and this would bring many consequences for animals and consumers [5,6].
Instead, the plants already very accessible, even used at uncertain doses are generally effective, easy to prepare and to administer, non-toxic, and little or no cost to herders [7]. They constitute a good alternative for the treatment of animals in this area. Indeed, in many countries in Africa, herbal medicines are more and more used to treat animal diseases [9,10]. Ethnobotanical documentation of medicinal plant used is generally an appropriate means of identifying potential sources of new drugs. Unfortunately, in this region of Cameroon, according to available literature, there is not yet information concerning the use of traditional medicine in animal clinics.
The aim of this study was to investigate the plants used for the treatment of FMD in cattle by livestock farmers in the Far North Region of Cameroon and to evaluate the phytochemical composition and antioxidant potentials of both of them (Boscia senegalensis [BS] Lam. and Tapinanthus dodoneifolius [TD] [DC.] Danser).
MATERIALS AND METHODS
Study Area
A survey was conducted in the Far North Region of Cameroon [Figure 1] from September 2011 to April 2012 as previous described [4]. The region lies between 9° and 13° north and 13° and 16° east with a total area of approximately 34,263 km² [10,11]. The mean annual rainfall is 700 mm and the temperature ranges between 27 and 41°C [12]. This area has a semi-arid climate with a single rainy season and one dry season of 8 months [9]. Two phytogeographic zones characterize the region: Sudanian in the southern grades and Sahelian in the Logon floodplain [13]. The plants mentioned during the survey by famers were directly collected with their help and then identified by M. Hamawa, a botanist in the University of Maroua. Phytochemical screening of all the plants collected were done and a literature were found to select the best plants to study further.
Figure 1.
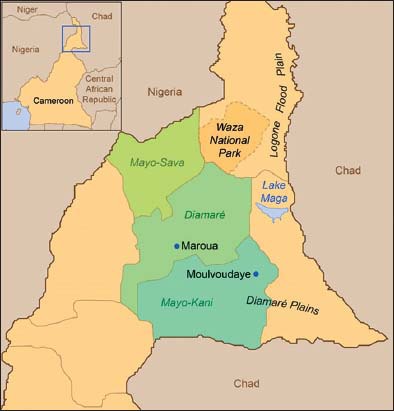
Study area in the Far North Region of Cameroon [4]
Plant Material
Plant materials used in this study were collected in June 2013 from Petté (leaves of BS) and Tokombéré (twigs of TD) districts in the Diamaré and Mayo Sava Divisions respectively in Far North Region of Cameroon. TD were collected from host plant Acacia albida. These plants were then identified and authenticated in the National Herbarium in Yaoundé were the Voucher specimens already existed under the reference numbers 23137 SRF/Cam and 50271 HNC respectively for BS and TD. The samples were collected in the morning (around 7 am), cleaned with tap water and air dried under a shade before grinding it to powder using a desk top mill fitted with a 500 µm sieve. The dried samples were then pulverized in a mill. The powder obtained was then sieved using a sieve of mesh 500 µ in diameter. The obtained powders were then put in polyethylene plastic, sealed and stored at 4°C in a refrigerator until when they were needed for analysis. The first phytochemical screening for choosing the plants for this study was done in the Veterinary Diagnostic Laboratory (Iowa State University, US), while the remainder analysis were performed in the National Advanced School of Agro-industrial Sciences (University of Ngaoundere, Cameroon) in July and August 2013.
Determination of an Optimum Solvent Partition Procedure
An analysis was first conducted to determine the solvent that would allow us to have the best antioxidant activity (AA) and the highest quantity of total phenolic compounds for both plants. To this effect, we considered the 2,2-diphenyl-picrylhydrazyl (DPPH) radical scavenging activity of extracts as the indicator of potential AA of the extracts [14].
Extraction
Five different solvents were used including: water, 70% acetone (v/v), methanol, 80% methanol (v/v) and dichloromethane. They were used due to their properties and because they were available in our laboratory [15]. To obtain each extract, 25 g of ground plant sample were extracted by stirring with 250 mL of solvent at room temperature (around 25°C) for 24 h and filtering through Whatman #1. The volume of the extract was removed by evaporation and the lot stored in a sealed tube at 4°C until when needed. For the dried extracts (pure methanol and dichloromethane), 0.02 g of powder obtained after evaporation were mixed with 100 mL of methanol and added to obtain a solution of 0.2 µg/µL. As against the other extracts which still contained water (80% methanol extracts and 70% acetone), volumes were complemented to 100 mL with distilled water. For aqueous extracts of which the volumes obtained were >100 mL, the volume was complemented to 200 mL with distilled water.
Determination of total phenol content (TPC) of the best extracts
TPC was determined using the Folin–Ciocalteu colorimetric method [16].
DPPH radical scavenging activity
The method described by Tepe et al. [17] was used. The AA of each extract was represented by IC50 parameter (substrate concentration required to cause the loss of 50% of the initial concentration of DPPH).
Determination of AA of the Best Extracts
Ferric iron reducing activity (FIRA)
The method described by Oyaizu [18] was used for this analysis.
Hydroxyl radical scavenging acti
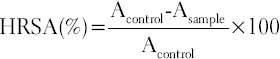
This was conducted according to the method of [19] with some modifications described below. In a test tube, 100 µL of extract, 500 µL of 5.6 mM 2-deoxy-D-ribose, 200 µL of a premixture of 100 µM FeCl3 and 100 mM ethylenediaminetetraacetic acid (1:1 v/v) solution, 100 µL of 1.0 mM H2O2, and 100 µL of 1.0 mM aqueous ascorbic acid were introduced. Tubes were well vortexed and incubated at 50°C for 30 min. Thereafter, 1 mL of 2.8% trichloroacetic acid and 1 mL of 1% thiobarbituric acid were added to each tube and vortexed mixture and heated in a water bath at 50°C for 30 min. The extent of oxidation (or deoxyribose degradation) was evaluated by measuring the absorbance of the solution at 532 nm. The percentage inhibition values were calculated from the absorbance of the control (Acontrol) and of the sample (Asample) using equation where the controls contained all the reaction reagents except the extract or positive control substance. Mannitol was used as standard. The AA of the extracts was expressed as equivalent mannitol (mg of manniol/g of powder) according to the following equation:
Free radical-scavenging activity (FRSA) by the use of a stable ABTS radical cation
The FRSA was determined by ABTS radical cation decolorization assay modified by Re et al. [20]. The results were corrected for dilution and expressed in mg trolox per 100 g dry weight.
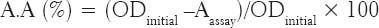
Determination of Total Flavonoid
The method of aluminum trichloride [21] was used to determine the total flavonoids content.
Determination of Total Tannins
This analysis was conducted following the method described by Makkar et al. [16].
Determination of Vitamin E
The vitamin E content was assessed by the spectrophotometric method as described by De Leenheer et al., and Milne et al. [22,23], with some modifications described below. Briefly, 1 g of powder was mixed with 30 mL of the solution of hexane/acetone (30/70). The mixture was successively heated to reflux for 1 h, cooled and filtered using the Whatman #1. The filtrate was washed with distilled water in a separating funnel and the lipid phase was collected in an Eppendorf.
For each sample, 0.1 g of oil were diluted in ethanol, transferred to tanks and their optical density were read at 522 nm. Trolox was used for calibration.
Determination of Iron Content
This assay was carried out according to the method of [24].
Phytochemical Screening
This analysis was performed using the best extracts selected after the previous analysis (TPC and FIRA) according to classic laboratory methods as described by [15,25-27].
Statistical Analysis
Data were presented as mean ± standard deviation. One-way analysis of variance followed by Duncan’s multiple range test was performed using Statgraphics 5.0. (Windows, www.statgraphics.com) to compare this data. A probability level of 0.05 was accepted as being significant.
RESULTS
Survey Result
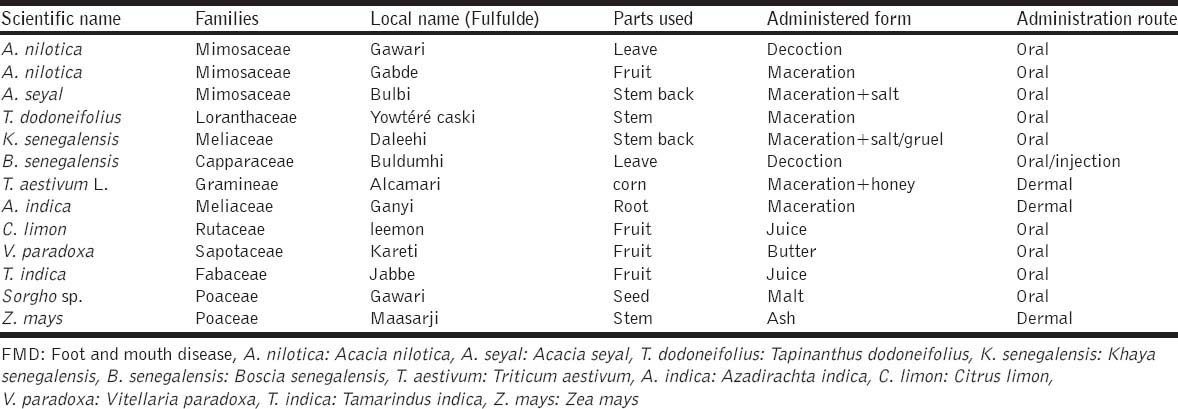
The results of the survey showed that 12 plants of 08 different families were used to treat FMD in the Far North Region of Cameroon. The parameters of use of each plant is summarize in Table 1. It is also important to note that the number of plants give here is less than the real number of plants used, because many farmers refused to tell us the local name of what they use. It revealed that the Mimosaceae family is the most widely used (23% or 3 of 13). The parts used are generally administered in macerated form (46% or 6 of 13) but may sometimes be given in association with salt. Administration to the animal is generally by the oral route (77% or 10 of 13). The doses are not generally defined and no undesirable secondary effects have been reported. During the survey, farmers said to use these plants alone or in combination with some elements like salt, urine of the animal that will be treated, honey, natron, tchoukouri, and kilbou (calcium carbonate). Among the 25% of herders who use the traditional medicine to manage FMD [4], 28%, 23%, 15% and 13% respectively use calcium carbonate, urine, salt and honey in association or not.
Table 1.
Overview of plants used for the treatment of cattle affected by FMD
Selection of the Best Solvent
As tabulated in Table 2, the TPC fluctuate from 0.207 to 8.578 g/100 g diabetes mellitus (DM). For the leaves, it is the extraction with methanol which gave the highest TPC (3.539 ± 0.258 g/100 g DM) while the dichloromethane had the lowest value of TPC (0.207 ± 0.01 g/100 g DM). Instead of TD the best TPC was recorded when using methanol (8.578 ± 0.532 g/100 g DM).
Table 2.
TPC and DPPH radical scavenging activity
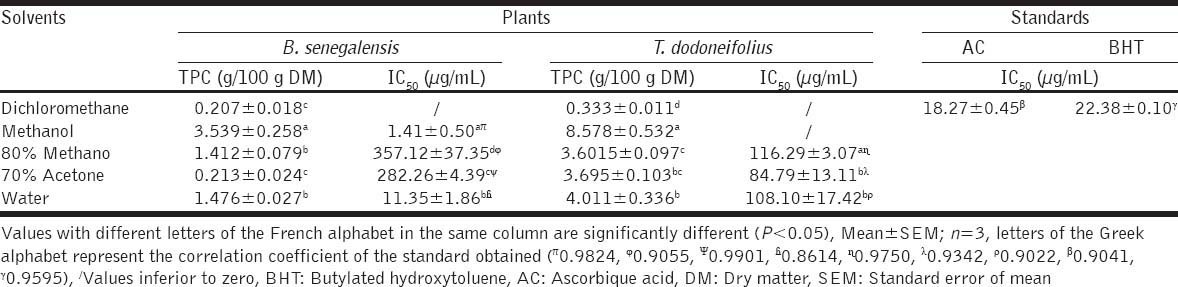
As the TPC, the IC50 were also significantly (P < 0.05) varied from one solvent to another with values that range from 1.41 ± 0.50 mg/mL (methanolic extract of BS) and 357.12 ± 37.35 mg/mL (80% methanolic extract of BS). The IC50 of the leaves methanolic extract was significantly lower than that of the water extract (11.35 ± 1.86 mg/mL). But for TD it is the 70% acetone extract which had an IC50 (84.79 ± 13.11 mg/mL) significantly lower than that of water (108.10 ±17.42 mg/mL) (P < 0.05).
AA
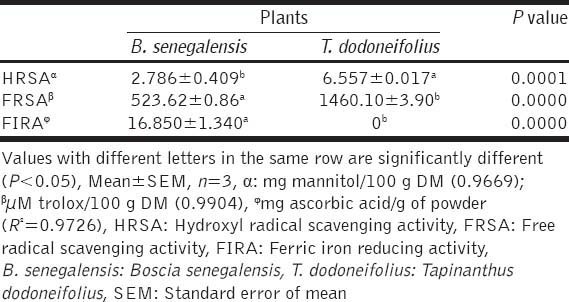
Table 3 shows the summary of the results of AA of both plants. The reduction of ferrous ion (Fe3+) to ferric ion (Fe2+) is measured by the intensity of the resultant blue-green solution which absorbs at 700 nm. It shows that the reducing power of methanol extract of BS is much more pronounced (16.850 ± 1.340 mg ascorbic acid/g of powder) than that of acetone extract of TD. On a contrary the ability of leaves to stabilize the radical cation ABTS0+ was significantly less (523.62 ± 0.86 µM Trolox/100 g DM) than the one of the acetone extract of TD (1460.10 ± 3.90 µM Trolox/100 g DM). It also came out that deoxyribose was significantly more protected by the acetone extract of TD (6.557 ± 0.017 mg mannitol/100 g DM) than methanol extract of BS (2.786 ± 0.409 mg mannitol/100 g DM). We also noted a significative difference between the different types of techniques used to evaluate the AA of the plants studied. Indeed, for both plants, the best activity was noted when using the trolox equivalent antioxidant capacity.
Table 3.
Antioxidant activities of plants
Some Important Elements Content of BS and TD
The levels of flavonoids measured was 305.463 ± 4.453 mg ethoxyquin (EQ) of quercetin/100 g DM for BS and 80.894 ± 1.684 mg EQ of quercetin/100 g DM for TD but this plant contained significantly more tannins (8.429±0.011 g EQ tannic acid/100 g DM) [Table 4].
Table 4.
Total tannins, flavonoid, Iron and vitamins E content of B. senegalensis and T. dodoneifolius
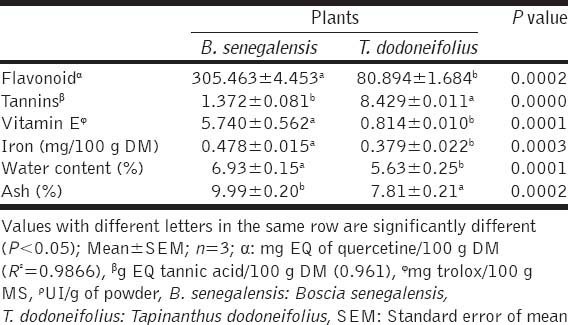
Our results also showed that the methanol extract of BS contained more vitamin E (5.740 ± 0.562 mg trolox/100 g MS) and iron (0.478 ± 0.015 mg/100 g DM) than the acetone extract of TD.
Phytochemical Screening
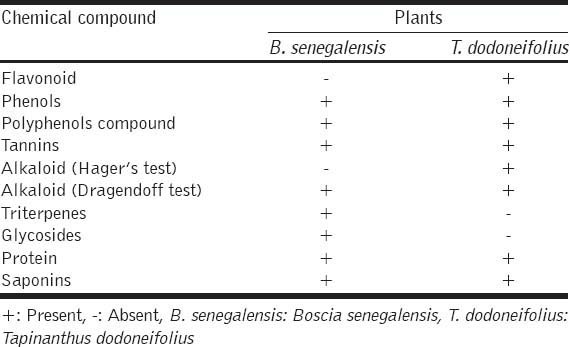
Phytochemical analysis showed that extracts of both plants contain flavonoids, tannins, phenol, polyphenols compounds, glycosides, proteins, triterpenes and alkaloids [Table 5].
Table 5.
Phytochemical composition of the alcoholic extracts of B. senegalensis and T. dodoneifolius
DISCUSSION
It became clear from the survey conducted that many plants are used to treat FMD. This can be explained by the fact that, FMD which is a viral disease is generally followed by many secondary diseases [28]. Thus, the plants used by farmers of our study may help on treating secondary infections or symptoms or to boost the immune system because the majority said they saw an improvement on the health of the animals treated. Indeed, one plant can be a source of different biological active compounds, this is why one plant can be used to treat many diseases [29]. The knowledge of medicinal plant use among the livestock farmers is said to have been developed gradually over a period of practical experience and are generally transmitted from one generation to another.
The results showed that for BS, it is the methanol extract that showed the best total AA and the highest amount of TPC. This could be explained by the fact that generally methanol allows the extraction of anthocyanins, flavones, simple phenols, polyphenols, etc. [30], which are groups of compounds that the quantities in a plant were correlated to the AA [31-34]. The results also show that, when TPC increases, IC50 increases also. This shows that for this plant, it is the phenolic compounds that are responsible for the total AA. This confirms the findings of [33]. In the case of TD there is no correlation between TPC and total AA. It is the 70% acetone extract that shows the best AA and yet it has lower TPC than methanol and water extract. This could indicate that for this species it is not only phenols that are responsible for its AA. Indeed, acetone in addition to extract phenols, can also extract flavonols [30] for which the AA is well documented [33]. In addition, being mixed with water, the acetone may also allow removal of tannins and other phenolic compounds [35].
The results showed that the parts of the plants study have a good activity against free radical in comparison with some plants already used for the FMD and its secondary diseases treatment [36,37]. It also came out that, the best activity of both plants is noted when testing the FRSA by use of a stable ABTS radical cation. This may suggest that, the best mechanism of the AA of the plant extracts is the capacity to pick up free radical and stabilize the radical cation. Therefore, both plant extracts may contain more antioxidant compounds of first class which are the compounds which convert highly reactive radicals into stable molecules [38]. They can completely stop a reaction until the radicals are fully consumed. The antioxidants of this class are phenolic compounds [39]. Hence, our extracts may contain the phenolic compounds responsible for their AA, which is also verified by the results of Table 4. These polyphenols which act via the trapping of reactive oxygen species by reduction and the formation of inert complexes with iron and copper ions are capable to protect polyunsaturated lipids against oxidation phenomenon, generators of radicals and lipid aldehydes responsible for the development of several diseases [40].
The analysis obtained revealed the high concentration of both the tannins and flavonoid contents. The beneficial effects of several flavonol glycosides and various flavonoid-rich extracts of various plants are already known to have AA [41]. These suggest that in the present study, there may also be a correlation between the rich tannins and flavonoid contents of the extract and its potent AA.
Tannins form a group of phenolics compounds resulting from polymerization of flavonoids units [42]. The tannin content of both plants can be considered higher when compared with plant materials used by herders to treat animal and human diseases [43-46]. These compounds are also known for their antioxidant and antitumor [47-49] which may help to treat the signs of FMD.
Similarly, the flavonoids which constitute the most important groups of phenols in plants [50,51] were found in high quantity in leaves. The amount of flavonoids contained in the extracts studied is very high compared to some plants used for the treatment of inter-digital lesions in animals [52]. In fact this symptom is one of the main characteristics of FMD when animals are affected. Studies made in 1986 by Selway [53] and 2 years later by Thomas [54] already show that several flavonoids have antiviral effects, this makes us think that these group of compounds may contribute in the mechanisms of recovery of health of animals affected by FMD. They are also a good antioxidants, antimicrobial, antibacterial, anti-inflammatory, antiviral, antitumor, etc. [55-59].
The amount of vitamin E and iron obtained from plant extracts are not negligible since by definition, vitamins and trace elements are needed just in few quantities in the body. In addition, vitamin E is known among all the other vitamins as the best antioxidant with vitamin C [60]. The quantity found may contributed to improve the health of the cattle treated because it is well k that a low vitamin E status in cows reduce the immune response and likely increase the incidence of certain diseases. This prove it importance for the contribution to animal health [61]. Its AA are generally done by inhibiting lipid peroxidation in biological membranes [62]. Iron is also known to have AA [63].
The ash content ranged from 8 to 10%, which is significant and shows that our extracts could be a potential source of other minerals. These minerals, which according to several studies are however very involved in maintaining the health of the animal [64,65], could contribute for the treatment of cattle affected by FMD.
CONCLUSION
It appears form this study that 12 plants of 8 different families are regularly used by livestock farmers. Both the plants analyzed have a good AA and contain phenolic compounds, flavonoid, tannins, vitamin E and iron in non-negligible quantity. In addition, the screening shown also that they contain alkaloid, triterpenes, proteins and saponins. All these compound are known to have many biological activities. This can justify their use by herders to treat animal disease. The plants studied here can be seen as a potential source of useful drugs. Further studies on these plants are ongoing in order to isolate, identify, characterize and elucidate the structure of the bioactive compounds.
ACKNOWLEDGMENTS
This study was supported by the US. National Science Foundation (DEB-1015908) via a fellowship from the Disease Ecology and Computer Modeling Laboratory (DECML) of the Ohio State University. Authors would like to thank Drs. Armand B. Abdou, Prs. Douglas Kinghorn, Andre P. Zoli and Mark Moritz and Mr. Hamawa Youkouda for their scientific contribution and Tiedja Tomdieu and Laya Alphonse for their help for collecting data in the field.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ziebe R, Thys E, De Deken R. Analysis of livestock production systems on the scale of a canton: The case of Boboyo in the Far north Cameroon. Rev Elev Méd Vét Pays Trop. 2005;58:159–65. [Google Scholar]
- 2.Yaoundé: National Government Publication; 2009. Cameroon. Strategy Document for Growth and Employment (ECSD). Reference Framework For The Governmental Action from 2010 to 2020; p. 168. [Google Scholar]
- 3.Yaya A. PhD thesis. National Polytechnic Institute of Toulouse; 2008. Genomic polymorphism in Mycoplasma mycoides subsp. Mycoides SC applications in molecular epidemiology and validation of a model of subcutaneous inoculation to study the virulence of the strains; p. 175. [Google Scholar]
- 4.Vougat NRRB, Tiédja T, Ziébé R, Foyet HS, Moritz M, Vondou L, Schrunk DE, Imerman PM, Rumbeiha WK, Garabed R. Quality and use of veterinary pharmaceuticals by cattle farmers in the Far North Region of Cameroon. No published. [Google Scholar]
- 5.Babapour A, Azami L, Fartashmehr J. Overview of antibiotic residues in beef and mutton in Ardebil, North West of Iran. World Appl Sci J. 2012;19:1417–22. [Google Scholar]
- 6.Donkor ES, Newman MJ, Tay SC, Dayie NT, Bannerman E, Olu-Taiwo M. Investigation into the risk of exposure to antibiotic residues contaminating meat and egg in Ghana. Food Control. 2011;22:869–73. [Google Scholar]
- 7.Jabbarm A, Akhtar MS, Muhammad G, Lateef M. Possible role of ethnoveterinary medicine in poverty reduction in Pakistan: Use of botanical anthelmintics as an example. J Agric Soc Sci. 2005;1:187–95. [Google Scholar]
- 8.Yirga G, Teferi M, Gidey G, Zerabruk S. An ethnoveterinary survey of medicinal plants used to treat livestock diseases in Seharti-Samre District, Northern Ethiopia. Afr J Plant Sci. 2012;6:113–9. [Google Scholar]
- 9.Moritz M, Ewing D, Garabed RB. On Not knowing zoonotic diseases: Pastoralists’ ethnoveterinary knowledge in the far north region of Cameroon. Hum Organ. 2013;72:1–11. doi: 10.17730/humo.72.1.72672642576gw247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annuaire Statistique du Cameroun: Deuxième Partie; 2010. Institut Nationale de la Statistique (INS). Population et Affaires Sociales; pp. 39–62. [Google Scholar]
- 11.Ministry of Economy, Planning and Development of territory; 2011. MIDIMA. Diagnostic assessment 2008-2009, to update the regional director of planning and development scheme Sustainable Planning (SDRADDT) in the region of the Far North conducted in 2011. Final report (main document) in November 2009; p. 36. [Google Scholar]
- 12.Koulaouna LB. Thesis. University of Maroua; 2013. Sustainable management of pastoral resources to face hydro-climatic constraints in dry areas: the case of the floodplain of logon (Far North Cameroon) p. 145. [Google Scholar]
- 13.Moritz M, Kitchey K, Saidou K. The social context of herding contracts in the far north region of Cameroon. J Mod Afr Stud. 2011;49:263–85. [Google Scholar]
- 14.Ling LT, Palanisamy UD, Cheng HM. Prooxidant/antioxidant ratio (ProAntidex) as a better index of net free radical scavenging potential. Molecules. 2010;15:7884–92. doi: 10.3390/molecules15117884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prashant T, Bimlesh K, Mandeep K, Gurpreet K, Harleen K. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 16.Makkar HP, Blummel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric. 1993;61:161–5. [Google Scholar]
- 17.Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2005;90:333–40. [Google Scholar]
- 18.Oyaizu M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn J Nutr. 1986;40:307–15. [Google Scholar]
- 19.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 20.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 21.Yi ZB, Yu Y, Liang YZ, Zeng B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium citri reticulatae of a new citrus cultivar and its main flavonoids- LWT. Food Sci Technol. 2007;4:1000–16. [Google Scholar]
- 22.De Leenheer AP, Nelis HJ, Lambert WE, Bauwens RM. Chromatography of fat-soluble vitamins in clinical chemistry. J Chromatogr. 1988;429:3–58. doi: 10.1016/s0378-4347(00)83866-9. [DOI] [PubMed] [Google Scholar]
- 23.Milne DB, Botnen J. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin Chem. 1986;32:874–6. [PubMed] [Google Scholar]
- 24.Rodier J. Paris. France: Dunod Technique; 1978. L’analyse de l’eau: Chimie, Physico-Chimie, Bactériologie, Biologie; p. 240. [Google Scholar]
- 25.N’guessan K, Kadja B, Guédé NZ, Dossahoua T, Aké-Assi L. Screening phytochimique de quelques plantes médicinales ivoiriennes utilisées en pays Krobou (Agboville, Côte-d’Ivoire) Sci Nat. 2009;6:1–15. [Google Scholar]
- 26.Harbone JB. 2nd ed. London: Chapman and Hall; 1973. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 49–188. [Google Scholar]
- 27.Ghani A. Dhaka: Asiatic Society of Bangladesh; 1998. Medicinal Plants of Bangladesh; pp. 78–83. [Google Scholar]
- 28.Di Nardo A, Knowles NJ, Paton DJ. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev Sci Tech. 2011;30:63–85. doi: 10.20506/rst.30.1.2022. [DOI] [PubMed] [Google Scholar]
- 29.Pan L, Yong Y, Deng Y, Lantvit DD, Ninh TN, Chai H, et al. Isolation, structure elucidation, and biological evaluation of 16,23-epoxycucurbitacin constituents from Eleaocarpus chinensis. J Nat Prod. 2012;75:444–52. doi: 10.1021/np200879p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117:112–9. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Sasikumar JM, Jinu U, Shamna R. Antioxidant activity and HPTLC analysis of Pandanus odoratissimus L. Root. Eur J Biol Sci. 2009;1:17–22. [Google Scholar]
- 34.Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. Afr J Biotechnol. 2008;7:1797–806. [Google Scholar]
- 35.Elhefian EA, Ayman AJ, Adam MM, Omer AA, Abdul HY. A preliminary qualitative study of two common Acacia species in Sudan. EJ Chem. 2012;9:851–6. [Google Scholar]
- 36.Karou D, Dicko MH, Simpore J, Traore SA. Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr J Biotechnol. 2005;4:823–8. [Google Scholar]
- 37.Olajide O, Idowu D, Okolo S, Orishadipe A, Sunday T. Phytochemical and antioxidant properties of some Nigerian medicinal plants. Am J Sci Ind Res. 2013;4:328–32. [Google Scholar]
- 38.Demanze C, Karliskind A. Analyses des antioxydants dans les corps gras alimentaires, n 28, APRIA. Actualités Scientifiques et Techniques en Agroindustrielles. 1980:127. [Google Scholar]
- 39.Kortenska VD, Yanishlieva NV, Kasaikina OT, Totzeva IR, Boneva MI, Russina IF. Phenol antioxydant efficiency in various lipid substrates containing hydroxyl compounds. Eur J Lipid Sci Technol. 2002;104:513–9. [Google Scholar]
- 40.Nkhili E. Thèse de Doctorat. France: Université D’Avignon-Montpellier; 2009. Polyphénols de l’Alimentation: Extraction, Interactions avec les ions du Fer et du Cuivre, Oxydation et Pouvoir antioxydant; p. 378. [Google Scholar]
- 41.Amic D, Davidovic-Amic D, Beclo D, Trinajstic N. Structure-radical scavenging activity relationships of flavonoids. Croat Chem Acta. 2003;76:55–61. [Google Scholar]
- 42.Abdou BA, Njintang YN, Scher J, Mbofung CM. Phenolic compounds and radical scavenging potential of twenty Cameroonian spices. Agric Biol J North Am. 2010;1:213–24. [Google Scholar]
- 43.Abdou BA, Vroumsia T, Nkouam GB, Njintang YN, Mang YD, Scher J, et al. Effect of solar and electric drying on the content of the phenolic compounds and antioxidant activity of three varieties of onion (Allium cepa L) Int J Biol Pharm Allied Sci. 2012;1:204–20. [Google Scholar]
- 44.Kabore A, Traore A, Pare S, Sawadogo BC, Kalkoumdo G, Tamboura HH, et al. Ethno-medicinal study of plants used in ectoparasites infections of ruminant livestock in sahelian region of Burkina Faso, West Africa. J Nat Prod Plant Resour. 2012;2:611–6. [Google Scholar]
- 45.Edeoga HO, Okwu DE, Mbaeble BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 46.Ndip RN, Malange Tarkang AE, Mbullah SM, Luma HN, Malongue A, Ndip LM, et al. In vitro anti-Helicobacter pylori activity of extracts of selected medicinal plants from North West Cameroon. J Ethnopharmacol. 2007;114:452–7. doi: 10.1016/j.jep.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 47.Yoshizawa S, Horiuchi T, Fujiki H, Yoshida T, Okuda T, Sugimura T. Antitumor promoting activity of (-)-epigallocatechin gallate, the main constituent of “Tannin” in green tea. Phytother Res. 1987;1:44. [Google Scholar]
- 48.Okuda T, Yoshida T, Hatano T. Polyphenols from Asian plants, structural diversity and antitumor and antiviral activities. In: Huang MT, Ho CT, Lee CY, editors. Phenolic Compounds in Food and Their Effects on Health II, Antioxidants and Cancer Prevention. Washington, DC, USA: American Chemical Society; 1992. pp. 160–83. [Google Scholar]
- 49.Okuda T, Ito H. Tannins of constant structure in medicinal and food plants-hydrolyzable tannins and polyphenols related to tannins. Molecules. 2011;16:2191–217. [Google Scholar]
- 50.Abdou BA. PhD thesis. University of Ngaoundere; 2009. Contribution to the study of the development of a food based functional spices from Cameroon: Physicochemical characterization and functional; p. 228. [Google Scholar]
- 51.Cíz M, Cízová H, Denev P, Kratchanova M, Slavov A, Lojek A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control. 2010;21:518–23. [Google Scholar]
- 52.Saha P, Mazumder UK, Haldar PK, Sen SK, Naskar S. Antihyperglycemic activity of Lagenaria siceraria aerial parts on streptozotocin induced diabetes in rats. Diabetol Croat. 2011;40:49–60. [Google Scholar]
- 53.Selway JW. Antiviral activity of flavones and flavans. In: Cody V, Middleton E, Harborne JB, editors. Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological and Structure Activity Relationships. New York: Alan R Liss, Inc; 1986. pp. 521–36. [Google Scholar]
- 54.Thomas PR, Nash GB, Dormandy JA. White cell accumulation in dependent legs of patients with venous hypertension: a possible mechanism for trophic changes in the skin. Br Med J (Clin Res Ed) 1988;296:1693–5. doi: 10.1136/bmj.296.6638.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fatty Acids. 1998;58:17–24. doi: 10.1016/s0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- 56.Wang HK, Xia Y, Yang ZY, Natschke SL, Lee KH. Recent advances in the discovery and development of flavonoids and their analogues as antitumor and anti-HIV agents. Adv Exp Med Biol. 1998;439:191–225. doi: 10.1007/978-1-4615-5335-9_15. [DOI] [PubMed] [Google Scholar]
- 57.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 58.Jachak SM. Natural products: Potential source of COX inhibitors. CRIPS. 2001;2:12–5. [Google Scholar]
- 59.Agrawal AD. Pharmacological activities of flavonoids: A review. Int J Pharm Sci Nanotechnol. 2011;4:1–5. [Google Scholar]
- 60.Zhao B, Tham SY, Lu J, Lai MH, Lee LK, Moochhala SM. Simultaneous determination of vitamins C, E and beta-carotene in human plasma by high-performance liquid chromatography with photodiode-array detection. J Pharm Pharm Sci. 2004;7:200–4. [PubMed] [Google Scholar]
- 61.Spears JW. Role of mineral and vitamin status on health of cows and calves. WCDS Adv Dairy Technol. 2011;23:287–97. [Google Scholar]
- 62.Panfili G, Fratianni A, Irano M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J Agric Food Chem. 2003;51:3940–4. doi: 10.1021/jf030009v. [DOI] [PubMed] [Google Scholar]
- 63.Ahola JK, Engle TE, Whittier JC. Western Beef Resource Committee, Cattle Producer's Library, Nutrition Section, CL315; 2010. Trace Minerals and the Immune System in Cattle; pp. 315–1. 5. [Google Scholar]
- 64.Traore AO. Paris: L’Harmattan; 2009. Manuel D’Alimentation Animale; p. 164. [Google Scholar]
- 65.Smith T. Short Round Article Reprint. The Official Publication of CBR; 2011. Importance of Trace Minerals for Cattle Health and Fertility; pp. 1–4. [Google Scholar]


