Abstract
Background:
The leaves of Hibiscus rosa-sinensis L. (Malvaceae) are used for the treatment of dysentery and diarrhea, to promote draining of abscesses and as analgesic agent in the traditional medicine of Cook Islands, Haiti, Japan and Mexico.
Aim:
The present study investigated the oral acute and subacute toxicity of methanol leaf extract of H. rosa-sinensis in mice.
Materials and Methods:
In the acute toxicity study, a single oral dose of 2000 mg/kg of extract was given to five mice at 48 h intervals. Animals were observed individually for any clinical signs of toxicity or mortality for 14 days. In the sub-acute toxicity study, mice were treated with 400 mg/kg and 800 mg/kg doses of the extract for 14 days. The hematological and biochemical parameters and histopathology of liver and kidneys of animals were studied at the end of the experiment.
Results:
For acute treatment, the extract did not reveal any signs of toxicity or mortality in any animal, during the 14 days observation period. The LD50 of extract was estimated to be greater than 2000 mg/kg. In the sub-acute toxicity study, administration of 400 mg/kg and 800 mg/kg doses of extract to mice for two weeks did not reveal any marked adverse effects on hematological, biochemical parameters and histopathology of liver and kidney in the 400 mg/kg group. However, hepato-renal toxicity as evidenced by elevated levels of alanine aminotransferase, aspartate aminotransferase, total and indirect bilirubin, urea and creatinine was seen in the animals that received 800 mg/kg dose of extract for 14 days. In addition, in the same group of animals, the histological assessments of liver and kidney also showed various adverse effects viz. dilated sinusoids, apoptotic nuclei and inflammatory infiltrate inside sinusoidal capillaries in the liver, and marked the disorganization of tubules and glomeruli, and enlarged interstitial spaces in the kidney.
Conclusion:
The results of this study suggest that for traditional medicinal purpose, only a low dose of H. rosa-sinensis leaf extract (i.e., 400 mg/kg) should be considered as safe.
Keywords: Acute toxicity, Hibiscus rosa-sinensis L, histopathology, Malvaceae, subacute toxicity
INTRODUCTION
The majority of the people in developing countries use various traditional herbal medicines to treat a number of diseases and ailments [1]. Although, many studies have been undertaken in the past to investigate the pharmacological potential of such remedies, however, rather little work has been done to assess the potential toxicities of such products. There is now growing evidence that many herbal medicines do cause serious toxicity to their users [2,3]. Therefore, much more scientific attention is now being given to assess the potential toxicity of herbal medicines than before.
Hibiscus rosa-sinensis L. (Malvaceae) is a perennial shrub distributed in tropical and subtropical regions of the world. In traditional medicine, the leaves of this plant are used against dysentery and diarrhea, to promote draining of abscesses, against gonorrhea and as analgesic agent in Cook Islands, Guam, Haiti, Japan and Mexico, etc. [4]. The petroleum ether extracts of the leaves and flowers of this plant have been shown to possess the antibacterial activities [5]. The mucilage from the leaf of this plant has also been shown to possess considerable anti-complementary activity [6]. In addition, Sachdewa et al. have reported the hypoglycemic effects of leaf extract of H. rosa-sinensis in rats [7]. A recent study has also shown that the leaf extract of this plant possesses significant in vitro and in vivo activity against Hymenolepis diminuta, a zoonotic helminth parasite [8]. Although, the pharmacological properties of H. rosa-sinensis leaf extract are widely known, but there is insufficient data about its potential toxicity. Therefore, the present study was undertaken to assess the acute and sub-acute oral toxicity of H. rosa-sinensis leaf extract in mice.
MATERIALS AND METHODS
Experimental Animals
Healthy Swiss albino mice of either sex, weighing between 25 and 30 g, were used. The animals were housed individually in acrylic cages and maintained in a standard laboratory environment. They were fed with standard rodent pellets and water ad libitum. All the experimental protocols related to use of mice were approved by the Institutional Ethics Committee (Animal Models) of North-Eastern Hill University, Shillong.
Plant Material
The leaves of H. rosa-sinensis were collected from North Tripura district of Tripura, India in August, 2010 and identified by a plant taxonomist. A voucher specimen (AKY-11882) of plant material has been deposited in the Parasitology and Ethnopharmacology Lab., Department of Zoology, NEHU, Shillong. The leaves were dried under shade, powdered and extracted in methanol, using a Soxhlet extractor. The final yield (w/w) of plant extract was about 18%.
Acute Toxicity Study
This study was performed as per the up-and-down-procedure of Organization for Economic Cooperation and Development (OECD) guidelines 425 [9]. A limit dose of 2000 mg/kg of extract was used involving five mice. Each mouse was treated with a single oral dose of 2000 mg/kg of extract in sequence at 48 h intervals. Animals were observed individually at least once during the first 30 min after dosing, periodically during the first 24 h, and daily thereafter, for a total of 14 days for any clinical signs of toxicity or mortality.
Sub-acute Toxicity Study
The sub-acute toxicity study on plant extract was performed as per the OECD guidelines 407 [10], with slight modifications. Based on the findings of the acute toxicity test, two different doses of extract, i.e. 400 mg/kg (low dose) and 800 mg/kg (high dose), were selected and administrated orally daily for 14 days to two different groups of mice (n = 10). The third group of mice (n = 10) was included as a control and received only vehicle for the same duration. After extract treatments, all the experimental animals were observed daily for any abnormal clinical signs and mortality for 14 days. At the end of 14 day’s observation period, the animals were anaesthetized, and their blood samples were collected through cardiac puncture with and without anticoagulant (EDTA), for hematological and biochemical studies, respectively. In hematological studies, red blood cell (RBC), white blood cell (WBC), and platelet counts, hemoglobin, mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration were determined using a hematology analyzer (Nihon Kohden Celltac MEK 6410 K Cell Counter). For biochemical analysis, blood without additive was centrifuged at 3000 × g at 4°C for 10 min, serum was separated and alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, direct and indirect bilirubin, urea and creatinine were estimated using a semi-automated Biochemical Analyzer (Bayer RA-50). For histopathological studies, the liver and kidney of animals were excised and examined macroscopically. These organs were then preserved in 10% buffered formalin for histopathological examinations by standard techniques.
Statistical Analysis
Data are expressed as mean ± standard errors of the mean. Evaluations were performed using Student’s t-test, and by one-way analysis of variance, followed by Bonferroni test. P < 0.05 was considered as statistically significant.
RESULTS
The acute toxicity test revealed that oral administration of a single 2000 mg/kg dose of H. rosa-sinensis methanol leaf extract to five mice did not brings out any signs of toxicity or mortality in treated animals during the 14 days observation period. In the subacute toxicity tests, administration of 400 and 800 mg/kg doses of H. rosa-sinensis leaf extract to two groups of mice did not showed any visual symptoms of toxicity or mortality in animals during the entire 14-days observation period. Furthermore, the haematological and biochemical parameters and histopathology of liver and kidneys of mice that received the 400 mg/kg dose of plant extract did not show any noticeable adverse effects [Table 1]. However, various treatment-related adverse effects were noticeable in biochemical and hematological parameters and in the histopathogy of liver and kidney of animals that received the 800 mg/kg dose of extract for 14 days [Tables 1 and 2, Figure 1]. The biochemical analysis revealed a significant increase in ALT, AST, total bilirubin, urea and creatinine in 800 mg/kg extract-treated animals [Table 1]. Whereas, the hematological analysis showed only a slight increase in the RBC and WBC counts in the 800 mg/kg-extract treated group of animals [Table 2].
Table 1.
Effects of oral administration of H. rosa-sinensis methanol leaf extract for 2 weeks on biochemical parameters of mice (n=10)
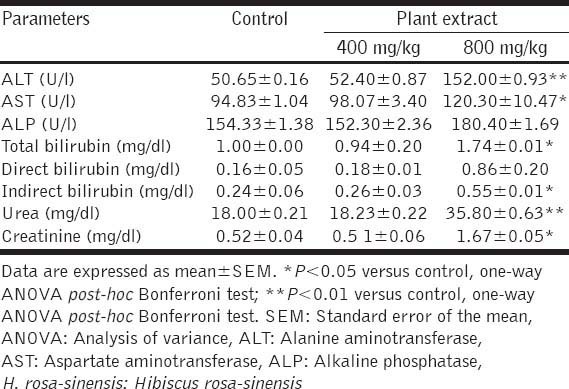
Table 2.
Effects of oral administration of H. rosa-sinensis leaves extract for 2 weeks on hematological parameters of mice (n=10)

Figure 1.
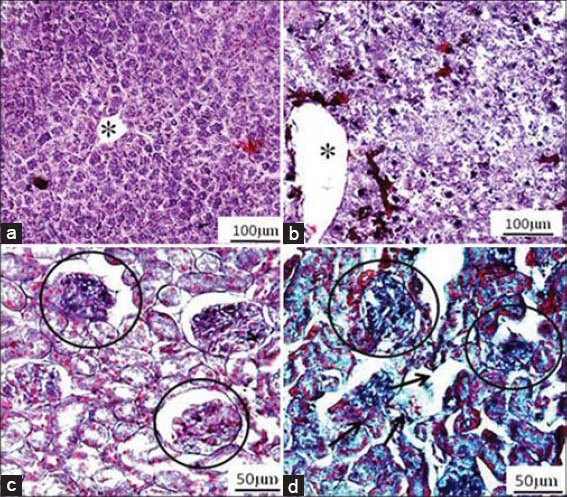
(a) Cross section of the liver of a control mouse, showing well-preserved liver plates and central vein (*). (b) Cross section of the liver of a mouse treated with Hibiscus rosa-sinensis leaf extract (800 mg/kg) by oral route for 14 days, showing dilated sinusoids and central vein (*). (c) Cross section of the kidney of a control mouse, showing conserved glomeruli (encircled areas) and tubules. (d) Cross section of the kidney of a mouse treated with H. rosa-sinensis leaf extract (800 mg/kg) by oral route for 14 days, showing disorganization of tubules (regular arrow) and glomerulus (encircled area) (haematoxylin-eosin)
In the histopathological investigations, the microanatomy of liver and kidneys did not present any treatment-related adverse effects in the animals that received the 400 mg/kg dose of plant extract (photomicrographs not shown). However, the histological assessments of liver and kidney of animals that received the 800 mg/kg dose of extract for 14 days showed various abnormalities in tissues viz. dilated sinusoids, apoptotic nuclei and inflammatory infiltrate inside sinusoidal capillaries in the liver and disorganization of tubules and glomeruli, enlarged interstitial spaces, etc. in the kidneys, which were not present in the control group [Figure 1].
DISCUSSION
In general, the safety studies on herbal medicines have been carried out by performing acute and sub-acute toxicity tests in laboratory animals (e.g. rodents and non-human primates) [3]. In the present study, we investigated the acute and sub-acute oral toxicity of H. rosa-sinensis leaf extract in Swiss albino mice. The acute and subacute toxic effects differ principally from each other with respect to the amount of test agent involved and the time intervening before the effects are observed. While, the acute effects are normally observed soon after a single exposure of test agent, the sub-chronic effects are usually monitored over an extended period during which there is repeated exposure of test agent.
In the present acute toxicity study, administration of a single 2000 mg/kg dose of plant extract to five mice did not reveal any signs of toxicity or mortality in any animal during the entire observation period. Therefore, the LD50 of extract may be considered to be greater than 2000 mg/kg. According to the Globally Harmonized System (GHS) of Classification and Labelling of Chemicals, the substances having an LD50 value greater than 2000 mg/kg are considered as relatively safe [11]. In some related studies, the LD50 values of therapeutic herbal extracts have been found to be greater 2000mg/kg, and as per the GHS criterion, these extracts have been considered to be reasonably safe on acute exposure [12].
In the sub-acute toxicity study, two groups of mice were treated with 400 and 800 mg/kg doses of extract for 14 days and the effects were monitored on biochemical and hematological parameters and histopathological features of liver and kidneys of treated animals. Interestingly, none of the studied parameters showed any evidence of adverse effects in 400 mg/kg treated group of mice. However, various treatment-related adverse effects were visible in the studied parameters in the 800 mg/kg treated group of mice. For example, a significant increase was observed in ALT, AST and total bilirubin in 800 mg/kg extract-treated animals. Since, ALT is a cytoplasmic enzyme found in very high concentration in the liver, an increase of this specific enzyme suggests a possible hepatocellular damage due to the extract treatment in mice. Similar findings were reported by Rhiouani et al. in case of the Moroccan traditional medicinal plant, Herniaria glabra, where the highest dose of extract caused a significant increase in ALT [13]. Similarly, the finding of an elevated level of total bilirubin in 800 mg/kg group may be due to the retention of bile subsequent to intrahepatic or extrahepatic bile flow in treated animals [13]. In addition to these parameters, the urea and creatinine also showed a significant rise in 800 mg/kg group. According to Satyanarayana et al. a concomitant rise of creatinine and urea provides a positive indication towards adverse effects in kidney functions [14]. Therefore, a concurrent increase in the levels of urea and creatinine, as noticed in the present study, indicates that 800 mg/kg dose of extract also brings out certain adverse effects on kidney functions in experimental animals. Of the various hematological parameters analyzed in this study, only some slight increase was noticeable in the RBC and WBC counts in 800-mg/kg extract treated group of mice. It is assumed that this increase in erythrocytes and leukocytes may be due to an increase in the rate of hematopoiesis. In general, in such clinical conditions, the body produces more cells to meet out the demand for more mature cells, for example in conditions such as dehydration, blood loss, etc. [15]. Therefore, it is likely that any such factors might be responsible for the rise of RBC and WBC counts in 800-mg/kg group. It may be mentioned here that in many toxicity tests, the rapid rate of renewal of hematopoiesis has been considered as a sensitive target for toxicity [15].
Another important finding of this study was this that the histological assessments of liver and kidneys of animals that received the 800 mg/kg dose of extract for 14 days showed various treatment-related toxicological changes viz. dilated sinusoids, apoptotic nuclei and inflammatory infiltrate inside sinusoidal capillaries in the liver and disorganization of tubules and glomeruli, enlarged interstitial spaces, etc. in the kidney. This observed hepato-renal toxicity of the high dose of plant extract is also supported by marked elevations of ALT, AST, creatinine, and urea in biochemical tests, which are good indicators of liver and kidney functions [14,16]. Therefore, these two findings together suggest that administration of the extract at 800 mg/kg dose for two weeks duration induces significant damage to the liver and kidneys of treated animals.
CONCLUSION
In conclusion, the acute toxicity study on H. rosa-sinensis leaf extract suggests that a single 2000 mg/kg limit dose of extract is devoid of any adverse effects in mice. However, the subacute toxicity study indicates that repeated intake of extract in high doses (800 mg/kg) for 14 days may cause liver and kidney toxicity in mice. Hence, this study suggests that a low dose of extract (i.e. 400 mg/kg) of this plant should be considered as safe in traditional medicinal use.
ACKNOWLEDGMENTS
This study was supported, in part, by a grant under the Departmental Special Assistance Program of the University Grants Commission (UGC), New Delhi in the Department of Zoology, NEHU, Shillong. PN was recipient of a Fellowship by the UGC, New Delhi. We thank the editor and the anonymous reviewer for their constructive comments to revise the paper.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Liu WJ. Introduction to traditional herbal medicines and their study. In: Liu JH, editor. Traditional Herbal Medicine Research Methods. New Jersey: John Wiley & Sons, Inc; 2011. pp. 1–26. [Google Scholar]
- 2.Wojcikowski K, Johnson DW, Gobé G. Medicinal herbal extracts – renal friend or foe? Part one: The toxicities of medicinal herbs. Nephrology (Carlton) 2004;9:313–8. doi: 10.1111/j.1440-1797.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 3.Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, et al. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J Ethnopharmacol. 2004;94:205–17. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Jadhav VM, Thorat RM, Kadam VJ, Sathe NS. Traditional medicinal uses of Hibiscus rosa-sinensis. J Pharm Res. 2009;2:1220–2. [Google Scholar]
- 5.Arullappan S, Zakaria Z, Basri DF. Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa-sinensis. Trop Life Sci Res. 2009;20:109–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu N, Tomoda M, Suzuki I, Takada K. Plant mucilages. XLIII. A representative mucilage with biological activity from the leaves of Hibiscus rosa-sinensis. Biol Pharm Bull. 1993;16:735–9. doi: 10.1248/bpb.16.735. [DOI] [PubMed] [Google Scholar]
- 7.Sachdewa A, Nigam R, Khemani LD. Hypoglycemic effect of Hibiscus rosa sinensis L. leaf extract in glucose and streptozotocin induced hyperglycemic rats. Indian J Exp Biol. 2001;39:284–6. [PubMed] [Google Scholar]
- 8.Nath P. Ph.D. Thesis, Department of Zoology. Shillong: North Eastern Hill University; 2014. Evaluation of anthelmintic activity of some medicinal plants used in the folk-lore medicine system of Riang tribe in Tripura; p. 284. [Google Scholar]
- 9.OECD. OECD Guidelines for the testing of chemicals, repeated dose 28-day oral toxicity study in rodents. 407. 1995:8. Adopted 1995 Jul 27. [Google Scholar]
- 10.OECD. OECD Guidelines for the Testing of Chemicals, Acute Oral Toxicity- up-and-down-procedure (UDP). 425. 2008:27. Adopted 2008 Oct 03. [Google Scholar]
- 11.The Purple Book. New York, Geneva: United Nations Economic Commission for Europe; 2005. Anonymous. Globally Harmonized System of Classification and Labelling of Chemicals (GHS) p. 60. [Google Scholar]
- 12.Konan NA, Bacchi EM, Lincopan N, Varela SD, Varanda EA. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.) J Ethnopharmacol. 2007;110:30–8. doi: 10.1016/j.jep.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Rhiouani H, El-Hilaly J, Israili ZH, Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J Ethnopharmacol. 2008;118:378–86. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Satyanarayana PS, Singh D, Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol. 2001;23:175–81. doi: 10.1358/mf.2001.23.4.634641. [DOI] [PubMed] [Google Scholar]
- 15.d’Yvoire MB, Bremer S, Casati S, Ceridono M, Coecke S, Corvi R, et al. ECVAM and new technologies for toxicity testing. In: Balls M, Combes RD, Bhogal N, editors. New Technologies for Toxicity Testing. Advances in Experimental Medicine and Biology. Vol. 745. New York: Landes Bio Science and Springer Science+Buisness Media, Inc; 2012. p. 171. [Google Scholar]
- 16.Hall RL. Principles of clinical pathology for toxicology studies. In: Hayes WA, editor. Principles and Methods of Toxicology. 4th ed. Philadelphia: Taylor and Francis; 2001. pp. 1001–38. [Google Scholar]


