Abstract
The incidence of diabetes mellitus continue to rise annually all over the world with India and Nigeria having recorded cases of 65.1 and 3.9 million respectively in 2013 and expected to increase by a large amount in 2035. Hyperglycemia is a pre-condition for the development of diabetic complications and is accompanied by an increase in the production of free radicals. The present available treatment option for diabetes like sulfonylurea, metformin and alpha-glucosidase are restricted by their limited actions, secondary failure rates, and side-effects; and unaffordable to the majority of the population. Hence, the need to screen for more medicinal plants with antidiabetic ability due to the fact that plants are; biodegradable, safe and cheap with fewer side-effects. In this review article, we have presented the current status of diabetes in India and Nigeria and the role of some less commonly used medicinal plants from both countries that have antidiabetic potential.
Keywords: Antidiabetic plants, diabetes, hypoglycaemic activity, medicinal plants, oxidative stress
INTRODUCTION
The World Health Organization (WHO) defines diabetes mellitus (DM) as a degenerative and chronic disease that occurs when the pancreas does not produce enough insulin, or when the body cannot effectively use insulin [1]. It is a disorder of the metabolism of carbohydrates, fats, and lipids, which is characterized by a high fasting blood sugar [2]. It manifests as chronic hyperglycemia and leads to the development of diabetes-specific micro vascular pathology in the retina, glomerulus and peripheral nerve culminating into serious complications affecting the eyes, kidneys and arteries [3,4].
WHO statistics shows that worldwide 347 million people have diabetes and 80% of diabetic deaths occur in low and middle-income countries [1]. According to the International Diabetes Federation, India is ranked second only to China in the list of top ten countries for a number of people with diabetes. [5]. In Africa, it is estimated that about 19.8 million adults have diabetes with Nigeria and South Africa having 3.9 and 2.6 million, respectively. It is estimated that by 2035, the percentage of diabetic patients in Africa would cross an alarming figure of 58% [5].
Type 2 diabetes, is the major form of diabetes accounting for 90-95% of all diabetic cases [6] and nearly half of all patients suffering from the disease are older than 65 years of age [7]. It is a complicated and divergent disease which in addition to blood sugar control requires the management of lipid parameters, blood pressure and thrombotic factors [8].
The treatment for diabetes is both difficult and tedious; it is expensive, costly and not affordable by majority of African and Asian populations [9]. The current treatments for DM include the use of insulin and synthetic drugs such as sulfonylurea, metformin, alpha-glucosidase inhibitors and thiazolidinedione’s in addition to lifestyle adjustments. These synthetic drugs are valuable but restricted by their limited action, pharmacokinetic properties, secondary failure rates and accompanying side-effects like hypoglycemia, damage to liver, lactic acidosis, diarrhea, abdominal pain, weight loss and loss of appetite [7,10-12].
Due to the problems associated with the current treatments, a large percentage of diabetics resort to alternative remedies that are purported to improve glycemic control [8]. The WHO estimated that approximately 80% of the world’s population rely mainly on traditional medicines for their primary health care [13]. The screening of medicinal plants for novel bioactive compounds is, therefore, an important goal for scientists. Importantly, the plant based drugs are biodegradable, safe, and cheap, having fewer side-effects, in India, China and other ancient traditional medicinal systems in the world, medicinal plants have been the major source of treatment for DM since time immemorial [14-16].
The importance of research on medicinal plants is validated by the fact that a plethora of new drugs have been developed from plants; relevant examples include cromolyn used as bronchodilator, developed from Ammi visnaga (L) Lamk; galegine, from Galega officinalis L, which is a model for the synthesis of metformin and other bisguanidine-type antidiabetic drugs, papaverine from Papaver somniferum which forms the basis of cerapramil used in the treatment of hypertension, [17]. Artemisia annua (Quinhaosu) gave rise to artemininin, this compound and it analogs are now used as antimalarial therapy in many countries [18]. Paclitaxel (Taxol®), the most exciting plant-derived anticancer drug discovered in recent years, is derived from several key precursors (the baccatins) in the leaves of various Taxus species; Taxus brevifolia [19].
Although the role of natural product in new drug discovery is encouraging and has frequently resulted in development of new drugs [20], the success of drug discovery depends on evolving stringent criteria to avoid false positive drug candidates. Surfeit of information warrants proper documentation. The evaluation of the scientific efficacy of traditional systems of medicine is an area of great interest especially in developing economies where sometimes the cost of medication may be prohibitive. Excellent reviews [13,21-23] on antidiabetic plants have already been written. This current review aims to bring in focus and document the use of less commonly used antidiabetic plants on which fewer studies have been conducted. Some of these plants, albeit less researched, hold immense potential as antidiabetic therapeutic agents in India and Nigeria.
ANTIDIABETIC PLANTS USED IN NIGERIA AND INDIA
The climatic conditions in Nigeria and India support the growth and thriving of various plant species and hence the use of these plants by the poor population to ameliorate disease conditions. There are about 800 plants that may possess antidiabetic properties according to ethno botanical information [24]. Most of the current drugs available have been directly or indirectly derived from plants. An example is metformin that was derived from the plant G. officinalis L.
The plants with antioxidant and antidiabetic potential included in this review are Azadirachta indica (AI) A. Juss, Mangifera indica (MI) L, Terminalia arjuna Roxb. Ex DC, Terminalia catappa L, Terminalia chebula Retz, Syzygium cumini (L) Skeels, Syzygium aromaticum (L) Merr. and L.M. Perry, Vernonia amygdalina (VA) Delile and Xylopia aethiopica (XA) (Dunal) A. Rich.
The general botanical data, taxonomic data, distribution in the world, experimental design, compounds isolated, mechanism of action, the antidiabetic and antioxidant capability of the plants are presented below:
Mangifera indica L. (Common Name: Mango)
Mango in an important species of the family anacardiaceae and the genus Mangifera, it is native to South East Asia from where it spread all over the world, it is the most popular fruit in the tropical and subtropical regions of the world. It is the national fruit of India, Pakistan, Philippines and the national tree of Bangladesh [25].
The plant is widely grown in Nigeria, where in addition to the fruit consumption it is used for the treatment and management of diabetes [26]. The peel and pulp of the plant contain carotenoids, and polyphenols such as quercetin, kaempferol, gallic acid, caffeic acid, catechins, tannins, mangiferin, leucocyanidin, epiatechin, quercetin and chromogenic acid [27]. Phenolics have scavenging activity on free radicals mainly due to the presence of hydroxyl groups. Recently, Mohan et al. [28] isolated a compound 1, 2, 3, 4, 6-penta-O-gallolyl-β-D-glucose from the methanolic extract fraction of mango that is a potent inhibitor of 11-β-hydroxysteriod hydrogenase enzyme and ameliorates high fat diet (HFD) induced diabetes in C57BL/6 mice.
Mangiferin (1, 3, 6, 7-tetrahydroxy-xanthone-C2-β-D-glucoside) a bioactive compound isolated from MI possesses a wide range of pharmacological actions including being anticancer [29,30], antibacterial [31], anti HIV [32], antioxidants [33], and antidiabetic [34,35].
The administration of mangiferin at a dose of 10 and 20 mg/Kg body weight (i.p.) in type 1 and 2 diabetic rats for 30 days showed significant antidiabetic, hypo-lipidemic, alpha amylase and alpha-glucosidase inhibitory effect [36]. This glucoside has also been shown by Li et al. [37] to improve renal function of diabetic nephropathy in rats and its inhibitory effect on overexpression of transforming growth factor-β1, advanced glycation end and extracellular matrix accumulation, Polyol pathway activation, reactive oxygen species (ROS) generation and mesangial cells proliferation. Miura et al. [38] demonstrated that the mangiferin exerts its antidiabetic activity by decreasing the insulin resistance.
The ethanolic extracts of MI showed significant free radical scavenging activity and have cytoprotective (anti-apoptotic) effect; the leaves and fruits extract reduce the absorption of glucose in type 2 diabetes and stimulate glycogenesis in liver causing reduction in blood glucose level [39].
Vernonia amygdalina Delile (Asteraceae)
VA is a perennial shrub-like plant with green leaves growing up to 1.3-3 m high that is native to Africa, widely grown in Nigeria and West Africa. It is reported to contain phytochemicals useful in the treatment and management of certain diseases. It has been introduced into India and is now being cultivated in parts of central and eastern India [40].
VA is rich in amino acids, minerals and vitamins [41]. The decoction from the leaves is often used in the African traditional treatment for the management of diabetes, malaria, infertility, and sexually transmitted diseases [42-47]. The plant is said to have antimalarial compounds like alkaloids, tannins, and saponins [48] and also anticancer properties [49]. In comparison to other plants, VA accounted for 9.2% of medicinal plants used as an alternative medicine in central Nigeria [50]. In Nigeria, a dosage form of freeze-dried aqueous leaf extract of this plant has been developed and formulated, which is suitable for therapeutic use in the management of DM. Mostly in Nigeria, the decoction from the leaf is often used in combination with that of other plants by traditional healers and medical practitioners to treat diabetes, fever and gastrointestinal problems [51].
The ethanolic extracts of the plant has a strong bioactive compound that has blood sugar lowering action in rats and can serve as an effective antioxidant [52], Ong et al. [53] showed that VA has anti-hyperglycemic effect on streptozotocin (STZ)-induced diabetic rat model and this effect is mediated through the inhibition of key hepatic G6pase, which causes an increase in expression and translocation of GLUT4 in skeletal muscles. The combined leaf extract of A. indica (AI) and VA ameliorates hyperglycemia and hepatic oxidative stress in diabetic rats [54] and the methanolic extract of VA has the ability to mitigate cycasin-induced oxidative damage in colonic tissues [55].
The composite decoctions of VA, Gongronema latifolium (Benth) and Occimum gratissimum (Linn) reduced the postprandial blood glucose concentrations of diabetic subjects [56]. Two flavonoids and terpenoids: Vernolide and edotides have been isolated from the VA plant. Octahydrovernodalin is the most important bitter principle in the plant [57]. Ong et al. [58] also isolated four main polyphenols in the ethanolic extract namely dicaffeoyl-quinic acid, chlorogenic acid, 1,5-dicaffeoyl-quinic acid and luteolin-7-O-glucosidase. Dicaffeoyl-quinic acid is the most abundant in the plant. The administration of 400 mg/Kg body weight of VA extract is found to exert most effective anti-hyperglycemic activity [59].
The two major glucose transporters that regulate glucose uptake into the tissues are GLUT1 (non-insulin responsive) and GLUT4 (insulin-responsive). While G6pase is one of the rate-limiting gluconeogenic enzymes that regulate, the synthesis of glucose and results has shown strong suppression of G6pase activity by extracts of VA [60]. VA extract was found also to protect pancreatic β-cells and the polyphenols present are responsible for this action especially dicaffeoyl-quinic acid.
Most of the traditional uses of the plant have been systematically and scientifically validated and the study of oxidative stress in diabetic rats showed that the aqueous extracts of VA decrease the levels of serum malondialdehyde an indication of the antioxidant property of the plant [60].
Xylopia aethiopica (Dunal) A. Rich
XA, also known as the African pepper or Ethiopian pepper, belongs to the family annonaceae and the genus Xylopia. It is a tropical, slim, tall and aromatic tree that grows up to 15-30 m. It is found in the west, central and southern Africa in humid forest zones, native to Nigeria, Ghana, Kenya, Ethiopia, Senegal and Uganda.
XA is a common ethno medicine in West Africa where it is used in the treatment of rheumatism and arthritis, cough, stomachache, bronchitis, biliousness and dysentery [61]. The fruit and vegetable have many medicinal properties and contains phytochemicals, vitamins and minerals. Phytochemicals like flavonoids are potentially anti-allergic, anti-carcinogenic, anti-viral and antioxidants, the ethanolic extract of XA was found to increase steroid hormone [62], the aqueous extract was also shown to have anti-amylase and anti-lipase activity with antioxidant potentials [63].
A poly-herbal formulation sold in Nigeria containing the following: Stachytarpheta angustifolia, Alstonia congensis, and XA in the ratio 3:2:1 was found to have hypoglycemic and hyper-lipidemic activities [64].
Syzygium aromaticum (Linn.) Merrill and Perry (Myrtaceae) (Common Name: Cloves)
S. aromaticum (clove) belongs to the family myrtaceae and the genus Syzygium. Native to Indonesia, this plant can grow to a height of 8-12 m, it is an aromatic flower bud commonly used in Africa, Asia and other parts of the world for the preparation of different spicy dishes. In Nigeria most traditional medical practitioners use the fruits and cloves by boiling in water and the decoction is administered to patients for the treatment of cough, chest congestion and catarrh and the compound eugenol present in this plant is responsible for the aroma and has antioxidative and antimycotic ability [65].
A triterpenoid compound extracted from the clove plant named oleanolic acid has potent diuretic/saluretic, anti-hyperlipidemic, antioxidant and hypoglycemic effects [66], Ngubane et al. [67] showed that oleonolic acid exhibited anti-hyperglycemic effect in STZ-induced diabetic rats by the attenuation of the activities of glycogenic enzymes and the compound eugenol present in this plant is responsible for the aroma and has antioxidative and antimycotic ability. The oil from the extract of this plant protects experimental animals from hepato-nephrotoxicity and oxidative stress due to aflatoxins [68].
Clove bud powder (CBP) possesses high phenolic content, free radical scavenging activity and metal chelating and reducing properties, the major phenolic compounds found are Kaempferol, isoquercitrin, gallic acid, ellagic acid, and caffeic acid [69]. Dietary supplementation of CBP in type 2 diabetic rats showed anti-hyperglycemic, hepatoprotective, hypolipidemic and antioxidant activities, by suppressing oxidative stress and delaying carbohydrate digestion [70].
Oleanolic acid (3 β-hydroxy-olea-12-en-28-oic acid) and maslinic acid have been reported to modulate the activity of the intestinal glucose transporters and carbohydrate hydrolyzing enzymes thus reducing postprandial hyper-glycaemia and that the ethanolic extract of this plant suppresses elevated blood glucose levels in type 2 diabetic KK-Ay mice [70].
Free and bound phenolic extract of clove bud was found to inhibit carbohydrate hydrolyzing enzymes; alpha-amylase and alpha-glucosidase in a dose-dependent manner (200-800 µg/ml) [71]. Decreasing the postprandial hyperglycemia peak is very crucial in the treatment of diabetes; there is a strong correlation between the phenolic content of clove and the enzyme inhibitory activities and with a strong antioxidant property which is the mechanism and the basis for its anti-diabetic action [71].
Azadirachta indica A. Juss (Common Name: Neem)
AI A. Juss is a member of the Meliacea family and the genus Azadirachta. It is a fast-growing tree that can reach up to 15-20 m and can sometimes reach 40 m. The plant is native to India and adapted to sub-arid and sub-humid tropical climates. It is widely grown in India, Pakistan, Indonesia, Sri Lanka, Caribbean, Nigeria, South and Central America. It is called “Dogonyaro” in Nigeria and grown all over the country, especially in the northern region. The plant has been used in the Indian Ayurveda traditional medicine for over 2000 years for the healing of various diseases and ailments [72].
The composite leaf extract of AI and VA at 500 mg/Kg body weight ameliorates hypoglycemia and hepatic oxidative stress in STZ-induced diabetic rats [54]. AI leaves glucosamine an active component of neem leaves is responsible for immunostimulatory activity in albino mice [73].
The chloroform extract of AI administered on murine diabetic model for 21 days significantly reduced the fasting blood sugar and islet regeneration and protection properties [74]. The administration of 500 mg/kg body weight of AI leaf extract and AI bark extract was effective in improving the antioxidant status in cardiac and skeletal muscles [75]. Khosla et al. [76] showed that azadirachtin and nimbin are the active ingredients in AI and they have the ability to regenerate the pancreatic beta cell. Recently Tiwari et al. [77] showed that the administration of the composite extract of Aegle marmelos, AI, Murraya koengii, Occimum sanctum, and S. cumini at 100 mg/Kg body weight caused a significant reduction in the blood sugar level, total cholesterol, triglyceride, low-density lipoproteins and an increase in the level of high-density lipoproteins.
Syzygium cumini (L.) Skeels
This plant belongs to the family myrtaceae and the genus Syzygium; it is an evergreen tropical plant native to South East Asia and widely grown in Africa. The fruit of the plant is widely used in cooking as spice and condiments to add flavor to foods.
S. cumini is well-known for its antidiabetic properties; gallic acid, rutin and chlorogenic acid are the main phenolic present in this plant and the extracts of all parts of the plant is used in traditional medicine [78]. Aqueous extract is found to improve endothelial dysfunction, antioxidant, anti-inflammatory and anti-thrombic properties of adenosine deamine activity in erythrocytes [78].
A dose of 400 mg/kg body weight of aqueous seed extract of S. cumini has hypoglycemic, insulin sensitizing and hypo-lipidemic activity in HFD-STZ induced rats due to an increase in peroxisome proliferator-activated receptor (PPAR)y and PPARα protein expression [79]. The active fraction of S. cumini was found to regenerate pancreatic islets and insulin secretion in STZ-induced diabetic mice [80].
Sharma et al. [81] demonstrated that the aqueous extract of S. cumini seed when given orally to mice at a dose of 250 mg/kg body weight for 21 days effected and repaired the liver damage associated with alloxan diabetes. The extract of this plant inhibits alpha-glucosidase and alpha-amylase, which are the two enzymes responsible for the metabolism of carbohydrate, and this limits the postprandial glucose and consequently controlling diabetes [82]. The seed extract is found to act as a chemo-protective agent against in vivo oxidative stress and genomic damage [83].
Terminalia catappa. L.
This plant belongs to the family Combretacea and to the genus Terminalia found growing in the warmer parts of India, Asia, Africa and Australia. The tree is primarily used as an ornamental and as a shade tree; the seeds are edible like almonds. The extracts of the bark and leaves are reported to have anticancer and aphrodisiac capability [84], antioxidant and anti-inflammatory [85] and anti-malarial [86]. This may be as a result of high contents of tannins in the plant making them a good source of antioxidants [87]. Kinoshita et al. [88] isolated chebulagic acid and corilagin from the 50% ethanol extract of the plant with a strong free radical scavenging activity and these compounds are found to have hepato-protective and antioxidant actions, by suppressing the generation of ROS followed by the inhibition of apoptosis.
Terminalia arjuna (Roxb) Wight and Arn
This is a plant belonging to the family Combretaceae and genus Terminalia commonly called arjuna. It is a large tree found throughout the South Asia region, and it is an exotic tree in India, it can grow up to a height of 25-30 m. The bark and fruits of this plant is used in traditional Indian medicine as an anti-dysentric, anti-pyretic, astringent, cardiotonic, lithotriptic, anticoagulant, hypolipidemic and anti-microbial, the large amount of flavonoids is responsible for the antioxidant and anti-microbial properties [89]. The bark contains arjunine a lactone, arjunetin, essential oils and reducing sugars. The methanolic extract exhibited analgesic activity and acute anti-inflammatory activity [90]. The extracts of this plant have the presence of alkaloids, triterpenoids, tannins and flavonoids. Gallic acid, apigenin, luteolin, quercetin, epicatechin, ellagic acid and 1-O-galloyl glucose are some of the compounds that have been isolated from this plant [91].
A dose of 250 and 500 mg/kg body weight of T. arjuna extract was found to have reno-protective and antioxidant ability in isolated perfused kidneys [92]. The leaf extracts when administered at a dose of 100 and 200 mg/kg body weight orally to STZ-induced diabetic rats was found to significantly normalize blood glucose level and this is due to its antioxidant role [93]. Due to the presence of tannins, saponin, and flavonoids, the bark extract exhibited antidiabetic activity by enhancing the peripheral utilization of glucose by correcting the impaired liver and kidney glycolysis and by limiting gluconeogenic formation, an action similar to that of insulin [94]. Perveen et al. [95] showed that the antioxidant activity of T. arjuna bark extract is due to the rich concentration of tannins, triterpenoid and saponins like arjunic acid, arjunolic acid, arjungenin, arjunglycosides, gallic acid, ellagic acid, oligomeric proanthocyanidins, and that the antidiabetic activity is due to the stimulation of β-cells of the pancreatic islets. The administration of T. arjuna ethanolic extracts at a dose of 250 mg/kg body weight per oral was found to reverse diabetic condition by inhibiting oxidation and degradation of lipids [96], and due to the fact that T arjuna extract has the ability to reduce postprandial hyperglycemia an important cardiovascular risk factor in type 2 diabetic patients, it has got a promising anti-hyperglycaemic and hypo-lipidemic effects in type 2 diabetics [97].
Terminalia chebula Retz. (Combretaceae)
This plant belongs to the family combretaceae, and the genus Terminalia found growing in the Sub-Himalayan tracts. It is a tall tree plant rising to about 15-25 m. It is a revered plant in India and has been extensively used in the Ayurveda, Unani and Homeopathic medicine. It has a beneficial effect on digestive diseases, urinary diseases, diabetes, skin, heart, irregular fevers, constipation, ulcers, vomiting, colic pain and hemorrhoids [98]. Phyto-constituents present in this plants is hydrolysable tannins like gallic acid, chebulagic acid, punicalagin, chebulamin, corilagin, neochebulini acid, ellagic acid, casuarinas and 2,3,6-tri-O-galloyl-β-D-glucose, 1,6-di-O-galloyl-D-glucose and terchebulin. The methanolic extracts from the plant inhibits lipid peroxide formation and scavenge hydroxyl and superoxide radicals [99]. The methanolic extract of T chebula has antioxidant, anti-inflammatory and anticancer ability and the phenolic derivatives, hydrolysable tannins and oleanane type triterpenoids are the active principles [100]. Chebulagic acid from T. chebula at 100 mg/kg body weight significantly reduced postprandial blood glucose levels of Sprague-Dawley rats when compared to the control group.
CONCLUSION
The present review presents the current scientific literature with respect to the antidiabetic and antioxidant potential of AI, MI, T. arjuna, T. catappa, T. chebula, S. cumini, S. aromaticum, VA and XA; summarize in Table 1. These plants are not the most popular when it comes to their use as antidiabetic plants used in traditional medicine, but yet they are widely used in some traditional medicinal system in India and Nigeria. It is hoped that further studies on these plants will target the isolation, purification and characterization of the bioactive compounds, which may lead to the discovery of potent antidiabetic drugs for the management and treatment of diabetes.
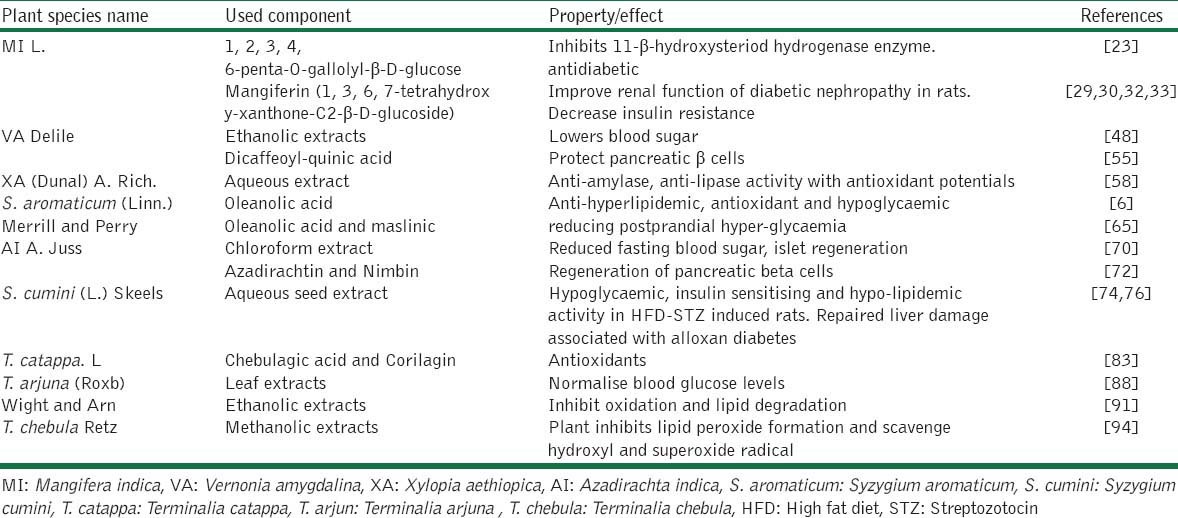
Table 1.
Summary of the selected plant species with their active component and their therapeutic effects
ACKNOWLEDGMENT
Abubakar Mohamed is presently a D. Phil Scholar in the Department of Biochemistry, University of Allahabad, India. His permanent position is at Department of Biochemistry, Bauchi State University, Gadau PMB 065. Bauchi State, Nigeria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geneva: World Health Organization; 2006. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. [Google Scholar]
- 2.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–73. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320–6. doi: 10.2337/dc08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guariguata L, Nola T, Beagley J, Linnenkamp U, Jacqmain O. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. IDF Diabetes Atlas. [Google Scholar]
- 6.Philippe J, Raccah D. Treating type 2 diabetes: How safe are current therapeutic agents? Int J Clin Pract. 2009;63:321–32. doi: 10.1111/j.1742-1241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 7.Virdi J, Sivakami S, Shahani S, Suthar AC, Banavalikar MM, Biyani MK. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol. 2003;88:107–11. doi: 10.1016/s0378-8741(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A, Ibrahim MA, Islam MS. African medicinal plants with antidiabetic potentials: A review. Planta Med. 2014;80:354–77. doi: 10.1055/s-0033-1360335. [DOI] [PubMed] [Google Scholar]
- 9.Bosi E. Metformin – The gold standard in type 2 diabetes: What does the evidence tell us? Diabetes Obes Metab. 2009;11(Suppl 2):3–8. doi: 10.1111/j.1463-1326.2008.01031.x. [DOI] [PubMed] [Google Scholar]
- 10.Asche CV, McAdam-Marx C, Shane-McWhorter L, Sheng X, Plauschinat CA. Association between oral antidiabetic use, adverse events and outcomes in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:638–45. doi: 10.1111/j.1463-1326.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen IB, Henriksen JE, Beck-Nielsen H. The effect of metformin in overweight patients with type 1 diabetes and poor metabolic control. Basic Clin Pharmacol Toxicol. 2009;105:145–9. doi: 10.1111/j.1742-7843.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 12.Jia W, Gao W, Tang L. Antidiabetic herbal drugs officially approved in China. Phytother Res. 2003;17:1127–34. doi: 10.1002/ptr.1398. [DOI] [PubMed] [Google Scholar]
- 13.Demain AL, Sanchez S. Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo) 2009;62:5–16. doi: 10.1038/ja.2008.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi SI, Mishra N. Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res 2013. 2013 doi: 10.1155/2013/712092. 712092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, et al. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci. 2009;10:2367–82. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inayat-ur-Rahman Malik SA, Bashir M, Khan R, Iqbal M. Serum sialic acid changes in non-insulin-dependant diabetes mellitus (NIDDM) patients following bitter melon (Momordica charantia) and rosiglitazone (Avandia) treatment. Phytomedicine. 2009;16:401–5. doi: 10.1016/j.phymed.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller LH, Su X. Artemisinin: Discovery from the Chinese herbal garden. Cell. 2011;146:855–8. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingston DG. Taxol and its analogs. In: Cragg GM, Kingston DG, Newman DJ, editors. Anticancer Agents from Natural Products. 2nd ed. Boca Raton, Fl: Taylor and Francis; 2012. pp. 123–75. [Google Scholar]
- 20.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Therapeutic potential of Indian medicinal plants in diabetic condition. Ann Phytomed. 2013;2:37–43. [Google Scholar]
- 22.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 24.Loh SP, Hadira O. In vitro inhibitory potential of selected Malaysian plants against key enzymes involved in hyperglycemia and hypertension. Malays J Nutr. 2011;17:77–86. [PubMed] [Google Scholar]
- 25.Fasoli E, Righetti PG. The peel and pulp of mango fruit: A proteomic samba. Biochim Biophys Acta. 2013;1834:2539–45. doi: 10.1016/j.bbapap.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Etuk EU, Bello SO, Isezuo SA, Mohammed BJ. Medicinal plants used for the treatment of diabetes mellitus in the North Western region of Nigeria. Asian J Exp Biol Sci. 2010;1:55–9. [Google Scholar]
- 27.Ma X, Wu H, Liu L, Yao Q, Wang S, Zhan R, et al. Polyphenolic compounds and antioxidant properties in mango fruits. Sci Hortic. 2011;129:102–7. [Google Scholar]
- 28.Mohan CG, Viswanatha GL, Savinay G, Rajendra CE, Halemani PD. 1,2,3,4,6 Penta-O-galloyl-β-d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11β-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine. 2013;20:417–26. doi: 10.1016/j.phymed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Shoji K, Tsubaki M, Yamazoe Y, Satou T, Itoh T, Kidera Y, et al. Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-κB in HL-60 cells. Arch Pharm Res. 2011;34:469–75. doi: 10.1007/s12272-011-0316-8. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimi N, Matsunaga K, Katayama M, Yamada Y, Kuno T, Qiao Z, et al. The inhibitory effects of mangiferin, a naturally occurring glucosylxanthone, in bowel carcinogenesis of male F344 rats. Cancer Lett. 2001;163:163–70. doi: 10.1016/s0304-3835(00)00678-9. [DOI] [PubMed] [Google Scholar]
- 31.Subbiya A, Mahalakshmi K, Pushpangadan S, Padmavathy K, Vivekanandan P, Sukumaran VG. Antibacterial efficacy of Mangifera indica L. kernel and Ocimum sanctum L. leaves against Enterococcus faecalis dentinal biofilm. J Conserv Dent. 2013;16:454–7. doi: 10.4103/0972-0707.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang RR, Gao YD, Ma CH, Zhang XJ, Huang CG, Huang JF, et al. Mangiferin, an anti-HIV-1 agent targeting protease and effective against resistant strains. Molecules. 2011;16:4264–77. doi: 10.3390/molecules16054264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das J, Ghosh J, Roy A, Sil PC. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFB pathways. Toxicol Appl Pharmacol. 2012;260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Sellamuthu PS, Muniappan BP, Perumal SM, Kandasamy M. Antihyperglycemic effects of mangiferin in STZ-induced diabetic rats. J Health Sci. 2009;55:206–14. [Google Scholar]
- 35.Ichiki H, Miura T, Kubo M, Ishihara E, Komatsu Y, Tanigawa K, et al. New antidiabetic compounds, mangiferin and its glucoside. Biol Pharm Bull. 1998;21:1389–90. doi: 10.1248/bpb.21.1389. [DOI] [PubMed] [Google Scholar]
- 36.Dineshkumar B, Mitra A, Manjunatha M. Studies on the antidiabetic and hypolipidemic potentials of mangiferin (Xanthone glucoside) in STZ-induced type 1 and type 2 diabetic rat models. Int J Adv Pharm Sci. 2010;1:75–85. [Google Scholar]
- 37.Li X, Cui X, Sun X, Li X, Zhu Q, Li W. Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother Res. 2010;24:893–9. doi: 10.1002/ptr.3045. [DOI] [PubMed] [Google Scholar]
- 38.Miura T, Ichiki H, Hashimoto I, Iwamoto N, Kato M, Kubo M, et al. Antidiabetic activity of a xanthone compound, mangiferin. Phytomedicine. 2001;8:85–7. doi: 10.1078/0944-7113-00009. [DOI] [PubMed] [Google Scholar]
- 39.Ling LT, Yap SA, Radhakrishnan AK, Subramanian T, Cheng HM, Palanisamy UD. Standardised Mangifera Indica extract is an ideal antioxidant. Food Chem. 2009;113:1154–9. [Google Scholar]
- 40.Bhattacharjee B, Lakshminarasimhan P, Bhattacharjee A, Agrawala DK, Pathak MK. Vernonia amygdalina Del (Asteraceae) - An African medicinal plants introduced in India. Zoo's Print. 2013;28:18–20. [Google Scholar]
- 41.Eleyinmi AF, Sporns P, Bressler DC. Nutritional composition of Gongro-nema latifolium and Vernonia amygdalina. Nutr Food Sci. 2008;38:99–109. [Google Scholar]
- 42.Borokini TI, Ighere DA, Clement M, Ajiboye TO, Alowonle AA. Ethno biological survey of traditional medicine practice for the treatment of piles and diabetes mellitus in Oyo State. J Med Plants Stud. 2013;1:30–40. [Google Scholar]
- 43.Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health. 2011;8:2533–55. doi: 10.3390/ijerph8062533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gbolade AA. Inventory of antidiabetic plants in selected districts of Lagos State, Nigeria. J Ethnopharmacol. 2009;121:135–9. doi: 10.1016/j.jep.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Abo KA, Fred-Jaiyesimi AA, Jaiyesimi AE. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J Ethnopharmacol. 2008;115:67–71. doi: 10.1016/j.jep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Erasto P, Van de Venter M, Roux S, Grierson DS, Afolayan AJ. Effect of leaf extract of Vernonia amygdalina on glucose utilization in chang liver C2C12 muscle and 3T3-L1 cells. Pharm Biol. 2009;47:175–81. [Google Scholar]
- 47.Akah PA, Ekekwe RK. Ethno pharmacology of some Asteraceae family used in Nigerian traditional medicine. Fitoterapia. 1995;66:351–5. [Google Scholar]
- 48.Sharma MC, Sharma S. Pharmacognostic and phytochemical screening of Vernonia amygdalina Linn against selected bacterial strains. Middle-East J Sci Res. 2010;6:440–4. [Google Scholar]
- 49.Wong FC, Woo CC, Hsu A, Tan BK. The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One. 2013;8:e78021. doi: 10.1371/journal.pone.0078021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amira OC, Okubadejo NU. Frequency of complementary and alternative medicine utilization in hypertensive patients attending an urban tertiary care centre in Nigeria. BMC Complement Altern Med. 2007;7:30. doi: 10.1186/1472-6882-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atangwho IJ, Egbung GE, Ahmad M, Yam MF, Asmawi MZ. Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina Del. growing in Malaysia. Food Chem. 2013;141:3428–34. doi: 10.1016/j.foodchem.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 52.Atangwho IJ, Ebong PE, Eteng MU, Eyong EU, Obi AU. Effect of Vernonia amygdalina Del. leaf on kidney function of diabetic rats. Int J Pharmacol. 2007;3:143–8. [Google Scholar]
- 53.Ong KW, Hsu A, Song L, Huang D, Tan BK. Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2011;133:598–607. doi: 10.1016/j.jep.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 54.Akinola OB, Omotoso GO, Akinola OS, Dosumu OO, Adewoye ET. Effects of combined leaf extract of Vernonia amygdalina and Azadirachta indica on hepatic morphology and hepatotoxicity markers in streptozotocin-induced diabetic rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:1373–9. doi: 10.3736/jcim20111215. [DOI] [PubMed] [Google Scholar]
- 55.Lolodi O, Eriyamremu GE. Effect of methanolic extract of Vernonia amygdalina (common bitter leaf) on lipid peroxidation and antioxidant enzymes in rats exposed to cycasin. Pak J Biol Sci. 2013;16:642–6. doi: 10.3923/pjbs.2013.642.646. [DOI] [PubMed] [Google Scholar]
- 56.Ejike CE, Awazie SO, Nwangozi PA, Godwin CD. Synergistic postprandial blood glucose modulatory properties of Vernonia amygdalina (Del.) Gongronema latifolium (Benth.) and Occimum gratissimum (Linn.) aqueous decoctions. J Ethnopharmacol. 2013;149:111–6. doi: 10.1016/j.jep.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Yeap SK, Ho WY, Beh BK, Liang WS, Ky H, Yousr AH, et al. Vernonia amygdalina, an ethno veterinary and ethno medical used green vegetable with multiple bioactivities. J Med Plants Res. 2010;4:2787–812. [Google Scholar]
- 58.Ong KW, Hsu A, Tan BK. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem Pharmacol. 2013;85:1341–51. doi: 10.1016/j.bcp.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Ebong PE, Atangwho IJ, Eyong EU, Egbung GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. Juss) (Neem) and Vernonia amygdalina (Del.) (African Bitter Leaf) Am Biochem Biotech2008; 4:239–44. [Google Scholar]
- 60.Eteng MU, Bassey BJ, Atangwho IJ. Biochemical indices of macrovascular complication in diabetic rat model: Compared effects or Vernonia amygdalina, Catharanthus roseus and chlopropamide. Asian J Biochem. 2008;3:228–34. [Google Scholar]
- 61.Fleischer TC, Mensah ML, Mensah AY, Komlaga G, Gbedema SY, Skaltsa H. Antimicrobial activity of essential oils of Xylopia aethiopica. Afr J Tradit Complement Altern Med. 2008;5:391–3. doi: 10.4314/ajtcam.v5i4.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woode E, Alhassan A, Abaidoo CS. Effect of ethanolic fruit extract of Xylopia aethiopica on reproductive function of male rats. Int J Pharm Biomed Res. 2011;2:161–5. [Google Scholar]
- 63.Oben J, Etoundi CB, Kuate D, Ngondi JL. Anti-amylase, anti-lipase and antioxidant effect of aqueous extracts of some Cameroonian spices. J Nat Prod. 2010;3:165–71. [Google Scholar]
- 64.Ogbonna SO, Mbaka GO, Adekunle A, Anyika EN, Gbolade OE, Nwakakwa N. Effect of a poly-herbal formulation, Okudiabet, on alloxan-induced diabetic rats. Agric Biol J North Am. 2010;1:139–45. [Google Scholar]
- 65.Ene AC, Atawodi SE. Ethno- medicinal survey of plants used by the Kanuri's of North-eastern Nigeria. Indian J Tradit Knowl. 2012;11:640–5. [Google Scholar]
- 66.Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 2003;10:115–21. doi: 10.1078/094471103321659807. [DOI] [PubMed] [Google Scholar]
- 67.Ngubane PS, Masola B, Musabayane CT. The effects of Syzygium aromaticum-derived oleanolic acid on glycogenic enzymes in streptozotocin-induced diabetic rats. Ren Fail. 2011;33:434–9. doi: 10.3109/0886022X.2011.568147. [DOI] [PubMed] [Google Scholar]
- 68.Adefegha SA, Oboh G, Adefegha OM, Boligon AA, Athayde ML. Antihyperglycemic, hypolipidemic, hepatoprotective and antioxidative effects of dietary clove (Szyzgium aromaticum) bud powder in a high-fat diet/streptozotocin-induced diabetes rat model. J Sci Food Agric. 2014;94:2726–37. doi: 10.1002/jsfa.6617. [DOI] [PubMed] [Google Scholar]
- 69.Khathi A, Serumula MR, Myburg RB, Van Heerden FR, Musabayane CT. Effects of Syzygium aromaticum-derived triterpenes on postprandial blood glucose in streptozotocin-induced diabetic rats following carbohydrate challenge. PLoS One. 2013;8:e81632. doi: 10.1371/journal.pone.0081632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuroda M, Mimaki Y, Ohtomo T, Yamada J, Nishiyama T, Mae T, et al. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J Nat Med. 2012;66:394–9. doi: 10.1007/s11418-011-0593-z. [DOI] [PubMed] [Google Scholar]
- 71.Adefegha SA, Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.). Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe(2+)-induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed. 2012;2:774–81. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subapriya R, Nagini S. Medicinal properties of neem leaves: A review. Curr Med Chem Anticancer Agents. 2005;5:149–6. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 73.Kumar VS, Navaratnam V, Ramachandran S. Isolation and characterization of glucosamine from Azadirachta indica leaves: An evaluation of immunostimulant activity in mice. Asian Pac J Trop Biomed. 2012;51:561–7. [Google Scholar]
- 74.Bhat M, Kothiwale SK, Tirmale AR, Bhargava SY, Joshi BN. Antidiabetic properties of Azardiracta indica and Bougainvillea spectabilis: In vivo studies in murine diabetes model. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/nep033. 561625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shailey S, Basir SF. Protective role of Azadirachta indica against oxidative damage in skeletal and cardiac muscle of alloxan diabetic rats. Int J Pharm Sci. 2012;4:471–7. [Google Scholar]
- 76.Khosla P, Bhanwra S, Singh J, Seth S, Srivastava RK. A study of hypoglycaemic effects of Azadirachta indica (Neem) in normaland alloxan diabetic rabbits. Indian J Physiol Pharmacol. 2000;44:69–74. [PubMed] [Google Scholar]
- 77.Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacol 2014. 2014 doi: 10.1155/2014/608590. 608590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Bona KS, Bonfanti G, Bitencourt PE, Cargnelutti LO, da Silva PS, da Silva TP, et al. Syzygium cumini is more effective in preventing the increase of erythrocytic ADA activity than phenolic compounds under hyperglycemic conditions in vitro. J Physiol Biochem. 2014;70:321–30. doi: 10.1007/s13105-013-0305-0. [DOI] [PubMed] [Google Scholar]
- 79.Sharma AK, Bharti S, Kumar K, Krishnamurthy B, Bhatia J, Kumari S, et al. Syzygium cumini ameliorates insulin resistance and β-cell dysfunction via modulation of PPARy, dyslipidemia, oxidative stress and TNF-α in type 2 diabetic rats. J Pharmacol Sci. 2012;119:205–13. doi: 10.1254/jphs.11184fp. [DOI] [PubMed] [Google Scholar]
- 80.Dusane MB, Joshi BN. Seeds of Syzygium cumini (L.). Skeels: Potential for islet regeneration in experimental diabetes. Zhong Xi Yi Jie He Xue Bao. 2011;9:1380–7. doi: 10.3736/jcim20111216. [DOI] [PubMed] [Google Scholar]
- 81.Sharma B, Siddiqui MS, Kumar SS, Ram G, Choudhary M. Liver protective effects of aqueous extracts of Syzygium cumini in Swiss albino mice on alloxan-induced diabetes mellitus. J Pharm Res. 2013;6:853–8. [Google Scholar]
- 82.Shinde J, Taldone T, Barletta M, Kunaparaju N, Hu B, Kumar S, et al. Alpha-glucosidase inhibitory activity of Syzygium cumini (Linn.). Skeels seed kernel in vitro and in Goto-Kakizaki (GK) rats. Carbohydr Res. 2008;343:1278–81. doi: 10.1016/j.carres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Arun R, Prakash MV, Abraham SK, Premkumar K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J Ethnopharmacol. 2011;134:329–33. doi: 10.1016/j.jep.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Mininel FJ, Junior CS, Espanha LG, Rosende FA, Varanda EA, Leite CQ, et al. Characterization and quantification of compounds in hydro-alcoholic extract of the leaves from Terminalia catappa Linn. (Combrataceae) and their mutagenic activity. Evid Based Complement Alternat Med 2014. 2014:11. doi: 10.1155/2014/676902. doi: 10.1155/2014/676902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen PS, Li JH. Chemopreventive effect of punicalagin, a novel tannin component isolated from Terminalia catappa, on H-ras-transformed NIH3T3 cells. Toxicol Lett. 2006;163:44–53. doi: 10.1016/j.toxlet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Chanda S, Rakholiya K, Nair CR. Antimicrobial activity of Terminalia catappa L. leaf extracts against some clinically important pathogenic microbial strains. Chin Med. 2011;2:171–7. [Google Scholar]
- 87.Chyau CC, Tsai SY, Ko PT, Mau JL. Antioxidant properties of solvent extracts from Terminalia catappa leaves. Food Chem. 2000;78:483–8. [Google Scholar]
- 88.Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine. 2007;14:755–62. doi: 10.1016/j.phymed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Mandal S, Patra A, Samanta A, Roy S, Mandal A, Mahapatra TD, et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed. 2013;3:960–6. doi: 10.1016/S2221-1691(13)60186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biswas M, Biswas K, Karan TK, Bhattacharya S, Gosh AK, Haldar PK. Evaluation of analgesic and anti-inflammatory activities of Terminalia arjuna leaf. J Phytol. 2011;3:33–8. [Google Scholar]
- 91.Singh PP, Chauhan SM. Activity-guided isolation of antioxidants from the leaves of Terminalia arjuna. Nat Prod Res. 2014;28:760–3. doi: 10.1080/14786419.2013.879304. [DOI] [PubMed] [Google Scholar]
- 92.Raj CD, Shabi MM, Jipnomon J, Dhevi R, Gayathri K, Subashini U, et al. Terminalia arjuna's antioxidant effect in isolated perfused kidney. Res Pharm Sci. 2012;7:181–8. [PMC free article] [PubMed] [Google Scholar]
- 93.Biswas M, Kar B, Bhattacharya S, Kumar RB, Ghosh AK, Haldar PK. Antihyperglycemic activity and antioxidant role of Terminalia arjuna leaf in streptozotocin-induced diabetic rats. Pharm Biol. 2011;49:335–40. doi: 10.3109/13880209.2010.516755. [DOI] [PubMed] [Google Scholar]
- 94.Ragavan B, Krishnakumari S. Antidiabetic effect of T. arjuna bark extract in alloxan induced diabetic rats. Indian J Clin Biochem. 2006;21:123–8. doi: 10.1007/BF02912926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perveen K, Kahn R, Siddiqui WA. Antidiabetic effects afforded by Terminalia arjuna in high fat-feed and STZ-induced type 2 diabetic rats. Int J Diabetes Metab. 2011;19:23–33. [Google Scholar]
- 96.Chander R, Singh K, Khanna AK, Kaul SM, Puri A, Saxena R, et al. Antidyslipidemic and antioxidant activities of different fractions of Terminalia arjuna stem bark. Indian J Clin Biochem. 2004;19:141–8. doi: 10.1007/BF02894274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morshed MA, Haque A, Rokeya B, Ali L. Anti-hyperglycaemic and lipid lowering effect of Terminalia arjuna bark extracts on STZ-induced type 2 diabetic model rats. Int J Pharm Pharm Sci. 2011;3:450–4. [Google Scholar]
- 98.Juang LJ, Sheu SJ, Lin TC. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J Sep Sci. 2004;27:718–24. doi: 10.1002/jssc.200401741. [DOI] [PubMed] [Google Scholar]
- 99.Bag A, Bhattacharyya SK, Chattopadhyay RR. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac J Trop Biomed. 2013;3:244–52. doi: 10.1016/S2221-1691(13)60059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Manosroi A, Jantrawut P, Ogihara E, Yamamoto A, Fukatsu M, Yasukawa K, et al. Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem Biodivers. 2013;10:1448–63. doi: 10.1002/cbdv.201300149. [DOI] [PubMed] [Google Scholar]


