Abstract
Aim:
Since there is an increasing need for gastric ulcer therapies with optimum benefit-risk profile. This study was conducted to investigate gastro-protective effects of N-acetylcysteine (NAC) against ethanol-induced gastric ulcer models in mice.
Materials and Methods:
A total of 41 mice were allocated into six groups consisted of 7 mice each. Groups 1 (normal control) and 2 (ulcer control) received distilled water at a dose of 10 ml/kg, groups 3, 4 and 5 were given NAC at doses 100, 300 and 500 mg/kg, respectively, and the 6th group received ranitidine (50 mg/kg). All drugs administered orally once daily for 7 days, on the 8th day absolute ethanol (7 ml/kg) was administrated orally to all mice to induce the acute ulcer except normal control group. Then 3 h after, all animals were sacrificed then consequently the stomachs were excised for examination.
Results:
NAC administration at the tested doses showed a dose-related potent gastro-protective effect with significant increase in curative ratio, PH of gastric juice and mucus content viscosity seen with the highest dose of NAC and it is comparable with that observed in ranitidine group.
Conclusion:
The present findings demonstrate that, oral NAC shows significant gastro-protective effects comparable to ranitidine confirmed by anti-secretory, cytoprotective, histological and biochemical data, but the molecular mechanisms behind such protection are complex.
KEY WORDS: Ethanol, gastric ulcer, gastroprotection, N-acetyle cystein
INTRODUCTION
N-acetylcysteine (NAC) is a derivative of thiol-containing amino acid that is a precursor for the intracellular antioxidant glutathione. It has potent antioxidant effects, NAC detoxifies reactive neutrophils and enhances the eradication of free radicals through either conjugation or reduction reactions [1], as well as suppression of cytokine expression and inhibition of nuclear factor kappa B [2]. Recently, there has been a growing interest in the therapeutic potential of NAC in a wide range of diseases where oxidative stress, cellular degeneration and inflammation are critical drivers in disease progression [3]. It is well-known that imbalance between antioxidants and oxidizing agents can create oxidative damages to cellular components; however, it has been hypothesized that high concentrations of antioxidants, such as glutathione and vitamins E and C could generate pro-oxidant effects, mainly through the reduction of transition metal ions and the involvement of Fenton reaction [4,5]. Several studies demonstrated that lipid peroxidation and protein oxidation are the major outcome of reactive oxygen species (ROS) generation [6], which plays a significant role in gastric ulceration. In accordance, thiol containing compounds like NAC reduce gastric mucosal injury that is caused by various ulcerogenic factors [7]. Gastric ulcer disease is characterized by the presence of sores in the gastric linings, which may occur due to imbalance between aggressive and gastro-protective factors [8]. Oxidative stress and lipid peroxidation play a crucial role in the etiology and development of gastric ulceration [9]. On the other hand, inflammatory cytokines (tumor necrosis factor-α [TNF-α], interleukin [IL-6] and IL-10) and over expression and translocation of the nuclear factor-κB (NF-KB) subunits play additive roles in the acute phase of inflammation as well as in maintenance of gastric ulcers [10]. Globally, gastrointestinal problems have now become a common disorder, affecting 8-10% of the population, and increasingly many research resources are dedicated to address these problems [11]. Acid suppression therapy and eradication of Helicobacter pylori infection contributed to a reduction in the prevalence of gastric ulcer disease [12]; however, there is an increasing need for gastric ulcer therapies with optimum benefit-risk profile. Although the antioxidant anti-inflammatory properties of NAC have been established in other conditions, the gastro-protective effects of NAC as a potential treatment for gastric ulcer have not been previously studied [13]. This study aims to investigate gastro-protective effects of NAC against ethanol-induced gastric ulcer models in mice.
MATERIALS AND METHODS
Materials
NAC was purchased from America Medic and Science - AMS, USA. Absolute ethanol was purchased from BDH chemicals, Ltd., Poole, England. Ranitidine was obtained from SDI Company, Samarra, Iraq. Diethyl Ether was purchased from May and Baker, England. 42 male albino mice (27-32 g) were included throughout the study. The mice were purchased from College of science, University of Basra, housed in the animal house of College of Pharmacy, University of Basra, Iraq. This study was conducted in College of Pharmacy, University of Basra from May to August 2014. The animals were housed in polypropylene cages with three mice per cage under a 12-h light-dark cycle (7 a.m.-7 p.m., lights on), and maintained conventionally during the study with regulated air temperature (15-21°C), relative humidity (40-70%) and ventilation (air volume change 20 times/h). The animals were allowed to acclimatize for at least 1 week prior to the experiment. They had free access to standard diet and water ad libitum. Animal experiments were in accordance with National Institute of Health guidelines and approved by the Animal Ethics Committee of College of Pharmacy, University of Basra.
Experimental Design and Animal Treatment Protocol
The animals were allocated into six groups consisted of 7 mice each: Groups 1 (normal control) and 2 (ulcer control) received distilled water at a dose of 10 ml/kg, groups 3, 4 and 5 were given NAC at doses 100, 300 and 500 mg/kg, respectively, and the 6th group received ranitidine (50 mg/kg), as a reference drug for treatment of gastric ulcer. The doses of NAC were selected based on previously published reports suggesting that NAC was not toxic at these doses [14]. In addition, NAC does not show any signs of toxicity at doses even greater than those administered in this study [15]. All drugs dissolved in distilled water and administered orally once daily for 7 days using gastric gavage needle before induction of gastric ulceration. On the 8th day and after fasting for 22 h (but had free access to water), all mice received daily regular doses of drugs and vehicle 1 h before the induction of gastric ulcer. Absolute ethanol induced ulcer was performed according to the method described by Palacios-Espinosa et al. with slight modification [16], where absolute ethanol (7 ml/kg) was administrated orally to all mice to induce the acute ulcer except normal control group. Then, 3 h after ethanol administration, all animals were sacrificed by inhalation of high dose of diethyl ether, the abdomen opened and ligatures placed around the esophagus and pylorus then consequently the stomach was excised for examination.
Evaluation of Gastric Ulcer Area (UA) and Gastro -protection
The UA was measured using digital pictures under a dissection microscope, with the aid of the software UTHSCSA Image Tool 3.0 program to measure damage area [17,18]. The sum of total UA (mm2) was divided by the number of animals to obtain the mean UA for each group. Meanwhile, the percent of gastro-protection was calculated according to the following equation [19]:
Curative ratio = ([UA control−UA treated] × 100)/UA control
Histological Assessment
For histopathological examination, stomach was fixed in 10% formalin solution for 4 days, sectioned, and then embedded in paraffin. Histological sections were cut at 4-5 µm and then stained with routine hematoxylin and eosin [20], for examination under a light dissection microscope by a histopathologist for analysis of necrotic lesions, congestion, edema, hemorrhage, surface epithelium disruption and infiltration of epithelium.
Determination of Stomach Acidity and Mucus Content
The stomach of each mouse was cut along the greater curvature. Hydrogen ion concentrations of stomach content were measured using digital pH meter [16]. The gastric mucosa of all mice were gently scraped using a flat piece of glass slide and the mucus obtained was weighed using electronic weighing balance [21].
Statistical Analysis
Data were expressed as mean ± standard deviation; the values were first analyzed using one-way analysis of variance (ANOVA). Bonferroni’s post hoc analysis was then performed to compare treated groups. Values with P <0.05 were considered significantly different. Analysis was performed using GraphPad Prism software for Windows (version 5.0, GraphPad Software, Inc., San Diego, CA).
RESULTS
Photographic Investigation under Dissection Microscope
Gastric mucosa shows that absolute ethanol induced significant oxidative injury on the surface epithelial cells; the lesions produced were deep, inflamed with massive hemorrhage and edema in ulcer control group while in normal group no macroscopic or microscopic lesions were observed, as shown in Figure 1a and b. The mice pre-treated with different doses of NAC [Figure 1c-e] and ranitidine [Figure 1f] had considerably reduced areas of gastric lesions compared with ulcer control group depend on dose used with greatest gastro-protection observed in groups treated with the highest dose of NAC (500 mg/kg) and ranitidine.
Figure 1.
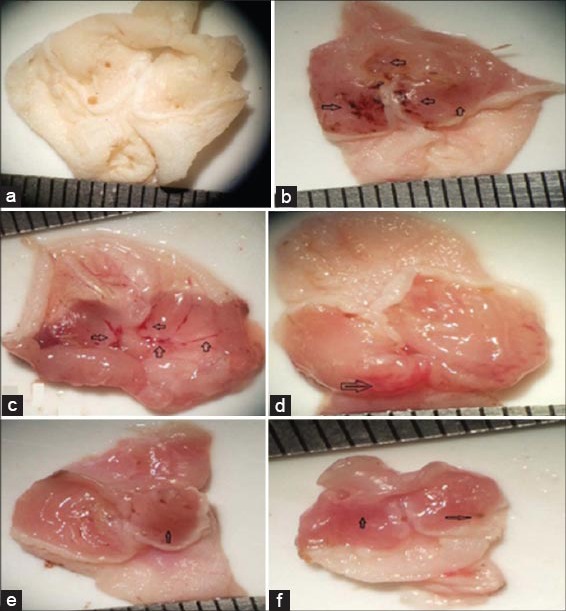
Photographs of gastric mucosa in controls and treated groups. Black arrows indicate ulcer areas. a Normal control group; b ulcer control group; (c) N-acetylcysteine (NAC) 100 mg/kg + absolute ethanol 7 mL/kg; (d) NAC 300 mg/kg + absolute ethanol 7 mL/kg; (e) NAC 500 mg/kg + absolute ethanol 7 mL/kg; (f) Ranitidine 50 mg/kg +absolute ethanol 7 mL/kg
Histopathological Investigation
Histopathological studies further confirmed that pretreatment with NAC inhibits the absolute ethanol induced gastric ulcer, necrotic lesions, congestion, edema, hemorrhage and extensive disruption of the surface epithelium in gastric mucosa as summarized in Figure 2a-f
Figure 2.
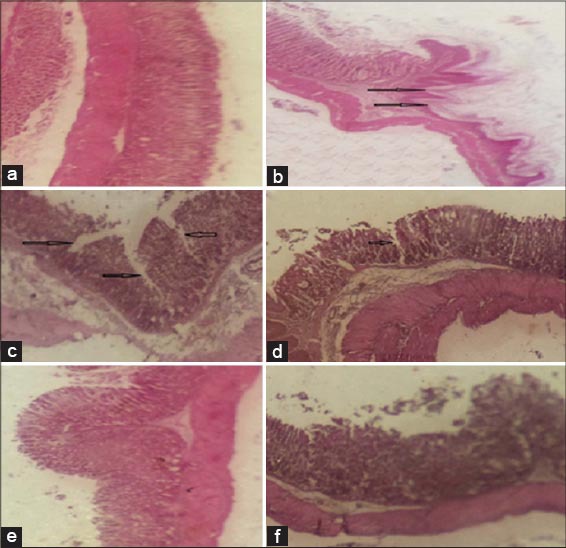
Photomicrographs of mice gastric mucosa stained with hematoxylin and eosin (×100). Ethanol induced damage to the gastric mucosa is indicated by the black arrows. (a) Normal control; (b) ulcer control; (c) NAC 100 mg/kg + absolute ethanol 7 mL/kg; (d) NAC 300 mg/kg + absolute ethanol 7 mL/kg; (e) NAC 500 mg/kg + absolute ethanol 7 mL/kg; (f) Ranitidine 50 mg/kg + absolute ethanol 7 mL/kg
Ulcer Index and Curative Ratio
Orally administered absolute ethanol induced severe gastric mucosal lesion (lesion area = 24.5 ± 0.85); NAC at the tested doses (100, 300 and 500 mg/kg), showed a dose-related significant protective effect against gastric lesions, with significant reduction in the ulcer size achieved with the highest dose of NAC, and it is comparable with that observed in ranitidine group [Figure 3]. Figure 4 demonstrates both doses of NAC (300 and 500 mg/kg) exhibited marked increase in curative ratio, compared with that observed with ulcer control group; such increment were relatively comparable to that observed in ranitidine (as reference drug) treated group.
Figure 3.
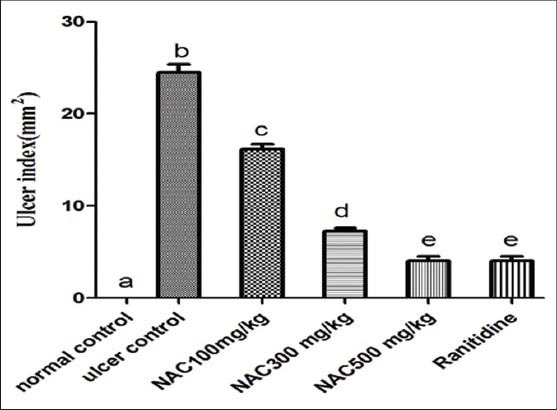
Gastric ulcer index of absolute ethanol induced acute gastric lesions in mice (n = 7), ANOVA: Values with different letters a, b, c, d and e, P < 0.05 versus normal control, ulcer control, N-acetylcysteine (NAC) 100 mg/kg, NAC 300 mg/kg and NAC 500 mg/kg respectively
Figure 4.
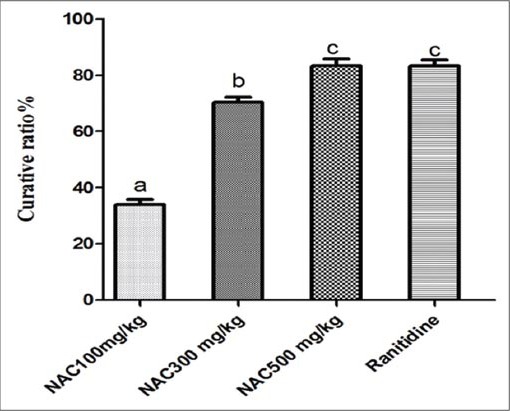
Curative ratio of different doses of NAC and ranitidine on gastric ulcer in mice (n = 7), ANOVA: Values with different letters a, b, c, P < 0.05 versus normal control, ulcer control, N-acetylcysteine (NAC) 100 mg/kg, NAC 300 mg/kg and NAC 500 mg/kg respectively
Biochemical Investigation
In comparison with ulcer control group as shown in Figures 5 and 6, there was a significant increase in pH of gastric juice and mucus content viscosity of the gastric layer in NAC pretreated group and ranitidine treated group, where NAC administration antagonizes mucus depletion by ethanol and raised the pH of gastric juice significantly. Nevertheless, NAC, administered in low dose (100 mg/kg), was found to be ineffective in preventing the depletion of mucus content, compared with other groups and comparable with that measured in ulcer control group.
Figure 5.
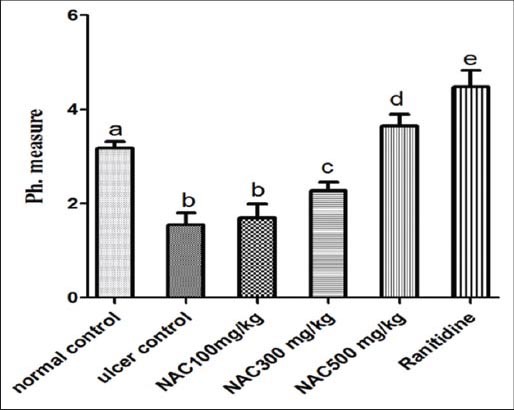
pH values in mice with acute gastric ulcer induced by absolute ethanol (n = 7), ANOVA: values with different letters a, b, c, d and e, P < 0.05 versus normal control, ulcer control, N-acetylcysteine (NAC) 100 mg/kg, NAC300 mg/kg and NAC500 mg/kg respectively
Figure 6.
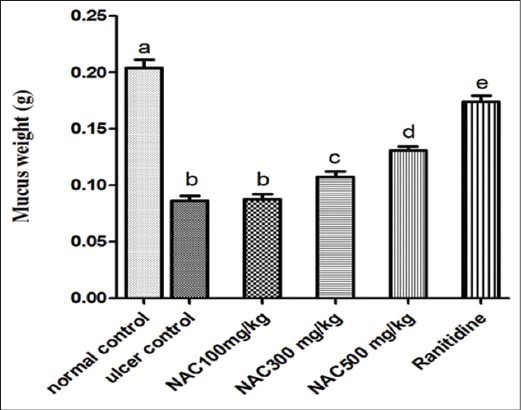
Mucus content in stomach of absolute ethanol induced acute gastric lesions in treated groups in mice (n = 7), ANOVA: values with different letters a, b, c, d and e, P < 0.05 versus normal control, ulcer control, N-acetylcysteine (NAC) 100 mg/kg, NAC300 mg/kg and NAC500 mg/kg respectively
DISCUSSION
The present study shows significant gastro-protective effects of oral NAC administration in ulcer induced by absolute ethanol. Aggressive factors such as acid-pepsin secretion, oxygen free radical production, lipid peroxidation, and matrix metalloproteinases play a crucial role in the pathophysiology of gastric ulceration [9,22]. Therapeutic management of peptic ulcer depends on modulation of such factors, and on this basis, several classes of drugs had been introduced, like proton pump inhibitors and histamine receptor blockers as antiulcer agent [23]. Aggressive factors involved in the pathogenesis of gastric ulceration are induced by several kinds of stress, including ethanol, NSAIDs or H. pylori [24]. In this study, we used a model of absolute ethanol-induced gastric ulceration for the evaluation of the activity of NAC as an antiulcer compound. Absolute ethanol affects the gastric mucosa within a few minutes of contact by different mechanisms, including gastric mucus depletion, decrease in mucosal blood flow and direct mucosal damage [25]. NAC (500 mg/kg) produces a significant gastro-protection, similar to that observed in ranitidine (reference drug), indicating the role of NAC protective mechanisms in providing reduced glutathione (GSH) as an endogenous antioxidant to protect the gastric mucosa through its function as a scavenger of free radical, which protect thiol groups from oxidation [3,26]. In the presence of NAC, the ROS production and consequently oxidative stress were significantly decreased, as reported in many in vitro and in vivo studies. This occurs directly or indirectly by restoring glutathione content [27]. Moreover, the significant inhibitory effect of NAC on ethanol-induced gastric injury can be explained by different mechanisms. NAC is a -SH containing compound with multiple regulatory and protective roles. They are involved in the defense against different chemicals that induce tissue injury, since both non protein and protein thiols can scavenge free radicals and affect enzyme activity, cell viability and membrane stability; such protective mechanism was reported in the stomach and liver injury [28]. Evidences from a variety of pathological and toxicological studies, such as ischemia-reperfusion injury, chemically induced oxidative injury, radiation damage, aging and degenerative disease, indicate that GSH is a major component of physiological systems protecting against oxidative and free radical mediated cell injury [29]. Many in vitro and in vivo studies support this mechanism. In an in vitro model by Mann et al. to assess gastro-protective effect of NAC, they found the healing of mucosal lesions that induced by HCl was promoted and hastened by low-dose of NAC [30]. Furthermore, in an in vivo study of gastric ulcer induced by HCl in the rat, depletion of glutathione with subcutaneous administration of diethylmaleate impaired gastric mucosal healing [31]. These results are supported by the findings of various previous studies. Intra peritoneal administration of GSH protected gastric mucosa against stress induced mucosal ulcerations [32]. In addition, oral administration of NAC has been found to protect the gastric mucosa against ethanol-induced gastric injury [33]. While oral NAC administration protected against ulcer [34], other study showed that intra-peritoneal administration of NAC increased the amount of gastric mucosal lesions after ethanol application secondary to reduced gastric mucosal blood flow [35]. Furthermore, NAC given intra-peritoneally in very high dose (1200 mg/kg) increased gastric injury [33]. The gastro-protective effect of oral NAC administration in the present study, which lowers mean ulcer size, may be due to the action against myeloperoxidase (MPO)/H2O2/Cl− system. This fact was supported by a study done by Antwerpen et al.; they indicated that NAC and its lysinate salt were the most efficient thiol-containing molecules in scavenging of hypochlorous acid, which was produced by MPO, and appeared as efficient inhibitors of MPO/H2O2/Cl− system, probably because of their relatively small size [36]. In addition, oxidative stress plays a critical role in enhancing inflammatory response. This leads to progression and deterioration of inflammatory conditions, where free radical may be implicated in the activation or modulation of a number of signaling pathways like NF-KB, and cytokines release, which promotes serious cellular injuries, and contributes to several inflammatory processes and human diseases [37,38]. NAC attenuates and reduces the inflammation through inhibiting variety of cytokines such as TNF-α, interferon-y, IL-8, and IL-6, and/or to restore the cellular redox-status, and regulates the activity of redox sensitive cell signaling pathways, such as NF-KB that control pro-inflammatory genes, indicating its protective action against cytokines-induced organ damage [13,39]. In light of these issues, studies gave evidence that both anti-oxidant and anti-inflammatory activities of NAC are the major properties that actively participate in gastro-protective activity. Since ulcer formation is directly related to factors such as gastric juice acidity and mucus production, assessment of such factors gave an expression of ulcer status. Interestingly, our study showed that oral NAC administration significantly increased gastric juice pH level with concomitant increase in mucus content viscosity specifically in high dose of NAC. These results are contradictory to the assumption that NAC have mucolytic properties and should reduce mucus viscosity by breaking disulfide bonds between mucin polymers [40]. However, our finding came in tune with study done in estrous mares by Witte et al., they conclude that NAC treatment seems to induce mucus production and viscosity in endometrium of mares [41]. While most of the previous literature data focus on therapeutic potential of NAC on acute lung injury, acute kidney injury, hemorrhagic shock, hepatic damage after acetaminophen overdose, cardiovascular disease, diabetes, carcinogenesis and neuropsychiatric disorders [3,39], effect of NAC on gastric mucosa is an important matter. In conclusion, oral NAC administration shows significant gastro-protective effects comparable to ranitidine in absolute ethanol-induced gastric ulcer models confirmed by anti-secretory, cytoprotective, histological and biochemical data but the molecular mechanisms behind such protection are complex and still unclear. Indeed, further pharmacological and toxicological studies are required to delineate such mechanism(s).
ACKNOWLEDGMENT
The authors gratefully thank Dr. Saosan haroon, a specialist in pathology department (medicine collage- Basra University), for providing pathological consultation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stec M, Kurzeja E, Gaweł S, Wardas P, Maciejewska-Paszek I, Pawłowska-Goral K. In vitro studies of the effect of theophylline and/or N-acetylcysteine on redox homeostasis and ketogenesis in rat hepatocytes. Bull Vet Inst Pulawy. 2011;55:519–24. [Google Scholar]
- 2.Zhu J, Yin R, Shao H, Dong G, Luo L, Jing H. N-acetylcysteine to ameliorate acute renal injury in a rat cardiopulmonary bypass model. J Thorac Cardiovasc Surg. 2007;133:696–703. doi: 10.1016/j.jtcvs.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–9. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–55. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Vitamin C. Antioxidant or pro-oxidant in vivo. Free Radic Res. 1996;25:439–54. doi: 10.3109/10715769609149066. [DOI] [PubMed] [Google Scholar]
- 6.Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523–34. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- 7.Barreto JC, Smith GS, Tornwall MS, Miller TA. Protective action of oral N-acetylcysteine against gastric injury: role of hypertonic sodium. Am J Physiol. 1993;264:G422–6. doi: 10.1152/ajpgi.1993.264.3.G422. [DOI] [PubMed] [Google Scholar]
- 8.Mynatt RP, Davis GA, Romanelli F. Peptic ulcer disease: clinically relevant causes and treatments. Orthopedics. 2009;32:104. [PubMed] [Google Scholar]
- 9.da Silva LM, Allemand A, Mendes DA, Dos Santos AC, André E, de Souza LM, et al. Ethanolic extract of roots from Arctium lappa L . accelerates the healing of acetic acid-induced gastric ulcer in rats: Involvement of the antioxidant system. Food Chem Toxicol. 2013;51:179–87. doi: 10.1016/j.fct.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 11.Calam J, Baron JH. ABC of the upper gastrointestinal tract: Pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ. 2001;323:980–2. doi: 10.1136/bmj.323.7319.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin JM, Vagin O, Munson K, Kidd M, Modlin IM, Sachs G. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci. 2008;65:264–81. doi: 10.1007/s00018-007-7249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Penugonda S, Ercal N. Comparative evaluation of N-acetylcysteine (NAC) and N-acetylcysteine amide (NACA) on glutamate and lead-induced toxicity in CD-1 mice. Toxicol Lett. 2011;201:1–7. doi: 10.1016/j.toxlet.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Midaoui A, Ismael MA, Lu H, Fantus IG, de Champlain J, Couture R. Comparative effects of N-acetyl-L-cysteine and ramipril on arterial hypertension, insulin resistance, and oxidative stress in chronically glucose-fed rats. Can J Physiol Pharmacol. 2008;86:752–60. doi: 10.1139/Y08-090. [DOI] [PubMed] [Google Scholar]
- 16.Li WF, Hao DJ, Fan T, Huang HM, Yao H, Niu XF. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem Biol Interact. 2014;208:18–27. doi: 10.1016/j.cbi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Sousa AT, Vasconcelos JM, Soares MJ. Software image tool 3.0 as an instrument for measuring wounds. J Nurs UFPE. 2012;6:2563–9. [Google Scholar]
- 18.Palacios-Espinosa JF, Arroyo-García O, García-Valencia G, Linares E, Bye R, Romero I. Evidence of the anti-Helicobacter pylori, gastroprotective and anti-inflammatory activities of Cuphea aequipetala infusion. J Ethnopharmacol. 2014;151:990–8. doi: 10.1016/j.jep.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Navarrete A, Trejo-Miranda JL, Reyes-Trejo L. Principles of root bark of Hippocratea excelsa (Hippocrataceae) with gastroprotective activity. J Ethnopharmacol. 2002;79:383–8. doi: 10.1016/s0378-8741(01)00414-7. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, Weinstein WM. Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation. Gastroenterology. 1988;94:1254–62. doi: 10.1016/0016-5085(88)90661-0. [DOI] [PubMed] [Google Scholar]
- 21.Salga MS, Ali HM, Abdulla MA, Abdelwahab SI. Gastroprotective activity and mechanism of novel dichlorido-zinc(II)-4-(2-(5-methoxybenzylideneamino)ethyl)piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem Biol Interact. 2012;195:144–53. doi: 10.1016/j.cbi.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang YC, Wei Su, Zhang CY, Xue CH, Chang YG, Wu XL, et al. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012;133:1414–9. [Google Scholar]
- 23.Mard SA, Bahari Z, Eshaghi N, Farbood Y. Antiulcerogenic effect of Securigera securidaca L. seed extract on various experimental gastric ulcer models in rats. Pak J Biol Sci. 2008;11:2619–23. doi: 10.3923/pjbs.2008.2619.2623. [DOI] [PubMed] [Google Scholar]
- 24.Parra-Cid T, Calvino-Fernández M, Gisbert JP. Helicobacter pylori and peptic ulcer – role of reactive oxygen species and apoptosis. In: Chai J, editor. Peptic Ulcer Disease. Ch. 9. InTech; 2011. pp. 167–72. DOI: 10.5772/21968. [Google Scholar]
- 25.Konturek JW, Hengst K, Konturek SJ, Sito E, Stachura J, Domschke W. Physiological role of cholecystokinin in gastroprotection in humans. Am J Gastroenterol. 1998;93:2385–90. doi: 10.1111/j.1572-0241.1998.00692.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka J, Yuda Y. Lipid peroxidation in gastric mucosal lesions induced by indomethacin in rat. Biol Pharm Bull. 1996;19:716–20. doi: 10.1248/bpb.19.716. [DOI] [PubMed] [Google Scholar]
- 27.Gillissen A, Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92:609–23. doi: 10.1016/s0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- 28.Szabó S. Role of sulfhydryls and early vascular lesions in gastric mucosal injury. Acta Physiol Hung. 1984;64:203–14. [PubMed] [Google Scholar]
- 29.Shan XQ, Aw TY, Jones DP. Glutathione-dependent protection against oxidative injury. Pharmacol Ther. 1990;47:61–71. doi: 10.1016/0163-7258(90)90045-4. [DOI] [PubMed] [Google Scholar]
- 30.Mann NS, Mann SK, Brawn PN, Weaver B. Effect of zinc sulfate and acetylcysteine on experimental gastric ulcer in vitro: study. Digestion. 1992;53:108–13. doi: 10.1159/000200978. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi K, Okada M, Ueshima K, Ohuchi T, Okabe S. Endogenous sulfhydryls in healing gastric mucosal injury induced by HCl in the rat. Digestion. 1993;54:91–7. doi: 10.1159/000201019. [DOI] [PubMed] [Google Scholar]
- 32.Hirota M, Inoue M, Ando Y, Hirayama K, Morino Y, Sakamoto K, et al. Inhibition of stress-induced gastric injury in the rat by glutathione. Gastroenterology. 1989;97:853–9. doi: 10.1016/0016-5085(89)91488-1. [DOI] [PubMed] [Google Scholar]
- 33.Lopez RA, Tornwall MS, Henagan JM, Smith GS, Miller TA. N-acetyl-cysteine: protective agent or promoter of gastric damage? Proc Soc Exp Biol Med. 1991;197:273–8. doi: 10.3181/00379727-197-43255. [DOI] [PubMed] [Google Scholar]
- 34.Henagan JM, Smith GS, Schmidt KL, Miller TA. N-acetyl-cysteine and prostaglandin. Comparable protection against experimental ethanol injury in the stomach independent of mucus thickness. Ann Surg. 1986;204:698–704. doi: 10.1097/00000658-198612000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ovrebø KK, Sørbye H, Svardal A, Grong K, Svanes K. Glutathione and N-acetylcysteine reduce gastric mucosal blood flow in rats. Dig Dis Sci. 1997;42:1765–74. doi: 10.1023/a:1018882019802. [DOI] [PubMed] [Google Scholar]
- 36.Van Antwerpen P, Boudjeltia KZ, Babar S, Legssyer I, Moreau P, Moguilevsky N, et al. Thiol-containing molecules interact with the myeloperoxidase/H2O2/chloride system to inhibit LDL oxidation. Biochem Biophys Res Commun. 2005;337:82–8. doi: 10.1016/j.bbrc.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Nam JH, Moon JH, Kim IK, Lee MR, Hong SJ, Ahn JH, et al. Free radicals enzymatically triggered by Clonorchissinensis excretory-secretory products cause NF-KB-mediated inflammation in human cholangiocarcinoma cells. Int J Parasitol. 2012;42:103–13. doi: 10.1016/j.ijpara.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Rada B, Gardina P, Myers TG, Leto TL. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011;4:158–71. doi: 10.1038/mi.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Jo YH, Kim K, Lee JH, Rim KP, Kwon WY, et al. Effect of N-acetylcysteine (NAC) on acute lung injury and acute kidney injury in hemorrhagic shock. Resuscitation. 2013;84:121–7. doi: 10.1016/j.resuscitation.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 40.Matsuyama T, Morita T, Horikiri Y, Yamahara H, Yoshino H. Improved nasal absorption of salmon calcitonin by powdery formulation with N-acetyl-L-cysteine as a mucolytic agent. J Control Release. 2006;115:183–8. doi: 10.1016/j.jconrel.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Witte TS, Melkus E, Walter I, Senge B, Schwab S, Aurich C, et al. Effects of oral treatment with N-acetylcysteine on the viscosity of intrauterine mucus and endometrial function in estrous mares. Theriogenology. 2012;78:1199–208. doi: 10.1016/j.theriogenology.2012.05.013. [DOI] [PubMed] [Google Scholar]


