Abstract
Aim:
Methanolic extract of a Fraxinus micrantha (MeFM) was evaluated for antiproliferative activity in vitro using Michigan Cancer Foundation-7 (MCF-7) breast carcinoma cell line. This plant was selected and studied for naturally available bioactive compound as different synthetic drugs available for cancer treatment has certain limitations and side effects.
Materials and Methods:
The anti-proliferative activity of a methanolic extract from the aerial parts of F. micrantha was assessed on MCF-7 breast cancer cell line using 3(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide assay. Furthermore, to understand the mechanism of anti-proliferation, production of nitric oxide (NO) and DNA fragmentation was also determined on MCF-7 cells. Different phytoconstituents of the extract were determined qualitatively based on various biochemical assays.
Results:
The results demonstrated anti-proliferative activity of an MeFM in a concentration and time-dependent manner. The percentage viability determined was 31.24% at 125 µg/ml as compared to 80.46% in negative control group. An MeFM has also shown NO production in a concentration (0.2-125 µg/ml) and time-dependent manner (24-48 h). DNA fragmentation studies showed that a methanolic extract was causing DNA fragmentation thus inducing apoptosis in MCF-7 breast carcinoma cells. Biochemical analysis result showed the presence of flavonoids, polyphenols, and sterols in an MeFM.
Conclusion:
In conclusion, F. micrantha possesses potent anti-proliferative activity on the malignant MCF-7 cell line which is correlated with the production of NO and DNA fragmentation. Further studies are required to identify, isolate, and characterize the phytochemicals present in the methanolic extract that might have antiproliferative potential in the treatment of different cancer conditions.
KEY WORDS: Antiproliferation, DNA fragmentation, Fraxinus micrantha, nitric oxide, Michigan Cancer Foundation-7 breast cancer cell line
INTRODUCTION
Cancer is the disease characterized by uncontrolled and unregulated proliferation of cancer cells. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs. By 2012, about 8.2 million died of cancers [1]. The conventional treatment of cancer includes chemotherapy causes various adverse and toxic side effects. These include damage to blood-forming cells in the bone marrow, hair follicles and cells lining mucous membrane, digestive tract, and reproductive system [2]. In view of this, there is a greater emphasis on search for novel and safer alternative. Plant are the attractive source as number of drugs such as sanguinarine, vinblastine, vincristine, teniposide, taxol, and camptothecin had being derived from these natural products [3]. In this continuing search for new anticancer compounds from plants, the present study evaluated the antiproliferative property of a methanolic extract of Fraxinus micrantha (MeFM) which had been previously reported for a number of medicinal properties.
The genus Fraxinus is in the olive family, Oleaceae. F. micrantha is one of the ashes found in Asia mainly in India and Nepal. In India, it is found in Himachal Pradesh and Uttar Pradesh region [4]. F. micrantha have been studied for its medicinal and economical value worldwide since ancient times. Local inhabitants of Dharchula, Himalayas use the inner bark infusion for the treatment of liver enlargement, jaundice, and other liver diseases [5]. The dried bark of Fraxinus japonica blume is available in the market as an oriental medicine “shinpi” in Japan and has been used since olden times as diuretic, an antifebrile, an analgesic, and an anti-rheumatic [6]. Fraxinus rhyncophylla is traditional Chinese herb and has antidiarrheal properties [7].
Michigan Cancer Foundation-7 (MCF-7) cell line derived from the breast cancer cells was used as an in vitro model to study the anti-proliferative studies because the cell line has retained several ideal characteristics particular to the tumor epithelium [8].
MATERIALS AND METHODS
3(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide (MTT), dimethylsulfoxide, Roswell Park Memorial Institute (RPMI) 1640, fetal bovine serum (FBS), streptomycin, penicillin G (Sigma-Aldrich Chemical Company Germany). Guanidium isothiocyanate reagent (EZ DNA KIT, Biological Industries, Israel, Beit Haemek Ltd.), Gene Ruler 1 kb DNA ladder (Fermentas) were used. Furthermore, Griess reagent was prepared of 1% sulphanilamide and 0.1% N-[naphthyl] ethylenediamine dihydrochloride in o-phosphoric acid (Sigma-Aldrich Chemical Company Germany).
Plant Material, Extraction and Preparation
The dried barks of F. micrantha were purchased from the local market in Delhi around February-March, 2012 and authenticated by a certified botanist. A voucher specimen (USBT#101-19012012FM) was submitted to the herbarium in the laboratory. The dried barks were grinded using an electric grinder and subjected to extraction (1:10) using methanol as solvent system by decoction method for 3-4 days. The solvent thus obtained was subjected to rota-evaporation for 24 h to get an MeFM. The fraction was reconstituted in complete RPMI (cRPMI - with 10% FBS and 1% antibiotic solution) medium and stock concentration of 2 mg/ml was made. The stock of an MeFM was filtered using 0.2 µ syringe filter before making different concentrations (0.2-125 µg/ml).
Anti-proliferative Study on MCF-7 Breast Carcinoma Cell Line
MCF-7 cells were plated at 5 × 105 cells/well in a 96-well plate in cRPMI (100 µl). 24 h after confluency (70-80%) and 48 h after more than 90% confluency, cells were treated with different concentrations of an MeFM (0.2-125 µg/ml). Each concentration was tested in triplicates (100 µl in each well). Untreated cells were used as negative control and taxol was used as a positive control. Morphological and quantitative changes were observed under inverted microscope at ×200 magnification and photographed.
MTT Assay
This assay was based on the cleavage of yellow tetrazolium dye MTT into soluble purple formazan, by succinate dehydrogenase in active mitochondria. Dead cells were unable to perform this reaction. In this assay, the amount of purple formazan generated is spectrophotometrically determined in a multi-well plate reader at 570 nm [9]. Cell viability was calculated by trypan blue dye exclusion method [10], and cells were plated in 96 well tissue culture plates at a final volume of 100 µl (in triplicates). Culture plates were incubated at 37°C and 5% CO2 in a CO2 incubator for 24 h. Afterward, different groups were given incubation with taxol (0.25 µM) and different concentrations (0.2-125 µg/ml) of an MeFM. After incubation, the supernatant was removed for nitric oxide (NO) analysis.
Griess Assay
The amount of NO formed was estimated from the accumulation of the stable NO metabolite, nitrite (NO2-) by Griess assay. In this assay, the culture supernatant (100 µl) from various treatment groups from the previous experimental set up and Griess reagent (100 µl) were mixed, and the absorbance was measured at 550 nm [11]. The amount of nitrite was calculated from a NaNO2 standard curve (0-200 µM).
Detection of DNA Fragmentation
The MCF-7 cells were plated in 24-well plates (2 × 106 cells/well). The cells were treated with taxol and an MeFM at different concentration ranging from 0.2 µg/ml to 125 µg/ml for 24 h (in duplicates). After incubation cells were harvested using phosphate buffered saline (pH 7.4) and trypsin and centrifuged at 10,000 rpm for 10 min at room temperature. The pellet was reconstituted in 1 ml of EZ reagent. 1 ml of ethanol was added, and the contents were mixed thoroughly. After washing twice with ethanol, the pellet was air-dried and was dissolved in 8 mM sodium hydroxide. The samples (20 µl/well) were electrophoresed on 2.5% agarose gel along with molecular marker.
Biochemical Tests on an MeFM
Different biochemical tests such as Mayer’s and Libermann Burchard’s were performed for the qualitative detection of various phytoconstituents (alkaloids, flavonoids, polyphenols, sterols, and saponins) in an MeFM. Extract was treated with Mayer’s reagent (potassium mercuric iodide). Formation of a yellow colored precipitate indicates the presence of alkaloids. Chloroform was added to 1 ml MeFM and then treated with few drops of acetic anhydride, boiled and cooled, followed by addition of concentrated sulfuric acid. Formation of brown ring at the junction indicates the presence of phytosterols. For the detection of saponins, an MeFM was diluted with distilled water to 20 ml, and this was shaken in a graduated cylinder for 15 min. Formation of 1 cm layer of foam indicates the presence of saponins. Flavonoids were detected using alkaline reagent test where an MeFM was treated with few drops of sodium hydroxide solution resulted in formation of intense yellow color, which becomes colorless on addition of dilute acid, indicates the presence of flavonoids. Addition of 3-4 drops of ferric chloride solution to an MeFM forms bluish black color indicating the presence of polyphenols [12].
Statistical Analyses
Statistical tests were performed using Graphpad Prism 4 (GraphPad software, Inc., CA/USA, 2011). Data were presented as mean±standard error. For single comparison, the significance of differences between means was determined by Student’s t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
Morphological Examination of Anti-proliferative Activity of an MeFM against MCF-7 Human Breast Carcinoma Cell Line
The cells were treated with different concentration of an MeFM (0.2, 0.9, 31.25 and 125 µg/ml) for 24 h. After incubation morphological alterations were observed as compared to control groups. Untreated cells were elongated, expanding as monolayers forming interconnections between them on the well surface. Exposure of MCF-7 cells to concentrations of an MeFM as mentioned above at different time intervals (24 h, 48 h) resulted in retraction and rounding of cells. At 24 h incubation, the negative control group [Figure 1a1] showed 70% confluence with normal and healthy growing cells while cells debris were seen in positive control group [Figure 1b1]. At 0.2 µg/ml [Figure 1c1] and 0.9 µg/ml [Figure 1d1] the cells showed slow proliferation rate as they were less in number as compared to the negative control group. At high concentration of 31.25 µg/ml [Figure 1e1] and 125 µg/ml [Figure 1f1] the cells were seen in patches and clumps which is the sign of deteriorated morphology. At 48 h, the negative control cells [Figure 1a2] were fully confluent. The cells treated with taxol [Figure 1b2] showed dead cells and cell debris while cells incubated with an MeFM (0.2 µg/ml and 0.9 µg/ml) showed very less proliferative cells. An MeFM at high concentration (31.25 µg/ml [Figure 1e2] and 125 µg/ml [Figure 1f2]) showed complete inhibition of cell proliferation indicating strong anti-proliferative activity.
Figure 1.

Comparison of Michigan Cancer Foundation-7 breast carcinoma cells at 24 h and 48 h - A1 and A2 only completed Roswell Park Memorial Institute (negative control), B1 and B2 taxol (positive control), C1 and C2 methanolic extract of a Fraxinus micrantha (MeFM) 0.2 µg/ml, D1 and D2 MeFM 0.9 µg/ml, E1 and E2 MeFM 31.25 µg/ml and F1 and F2 MeFM 125 µg/ml (arrow showing cell clumping)
Evaluation of Cytotoxicity of an MeFM against MCF-7 Human Breast Carcinoma Cell Line
The relationship between concentration of an MeFM and its cytotoxic effect on MCF-7 cells was evaluated by MTT assay. The cells were treated with an MeFM at different concentrations (0.2, 0.9, 31.25 and 125 µg/ml) for 24 h [Figure 2a] and 48 h [Figure 2b]. The MeFM was found to be cytotoxic to MCF-7 cells in concentration and time-dependent manner. Similar results were observed when the taxol was used as a positive control. An MeFM at 31.25-125 µg/ml decreased the proliferation of MCF-7 cells by 15-45% at 24 h and by 82-89% at 48 h. Treatment with an MeFM significantly decreased the proliferation (31.24%) of MCF-7 carcinoma cells, P < 0.05 compared to negative control group (80.46%) which was untreated MCF-7 cells. The inhibitory concentration 50 value calculated from the line of best fit was 18.95 µg/ml.
Figure 2.
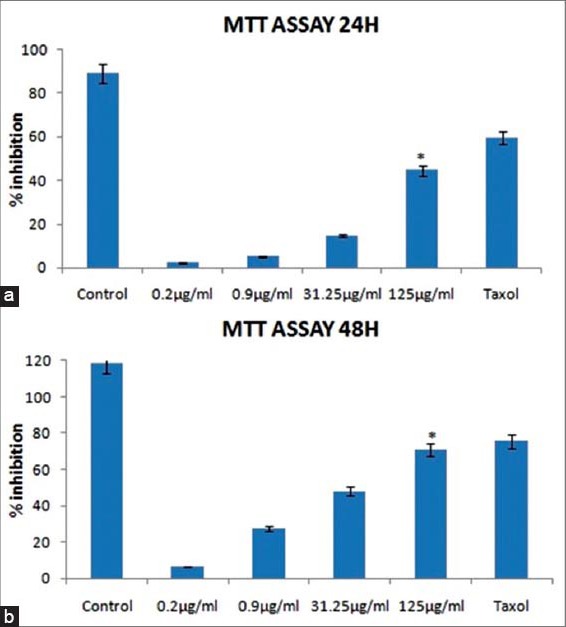
Effect of different concentrations of an methanolic extract of a Fraxinus micrantha (MeFM) (0.2-125 µg/ml) on cytotoxicity in Michigan Cancer Foundation-7 human breast cancer cell line at: (a) 24 h and (b) 48 h; *Antiproliferative potency of an MeFM was found to be significant, p<0.05 compared to untreated negative control group. Data are means±standard error for three independent experiments
NO Production by an MeFM against MCF-7 Human Breast Carcinoma Cell Line
The supernatant collected from different groups was subjected to NO assay by Griess method. The MeFM was found to be inducing NO production in a concentration and time-dependent manner [Figure 3]. With the increase in incubation time with an MeFM, the amount of NO produced was almost doubled (90 µM at 24 h and 187 µM at 48 h). Interestingly, the highest concentration of an MeFM induced NO production at a higher level than the positive control, taxol. The high production in NO after incubation with an MeFM was shown to be significant with P < 0.05 as compared to the positive control group treated with taxol.
Figure 3.
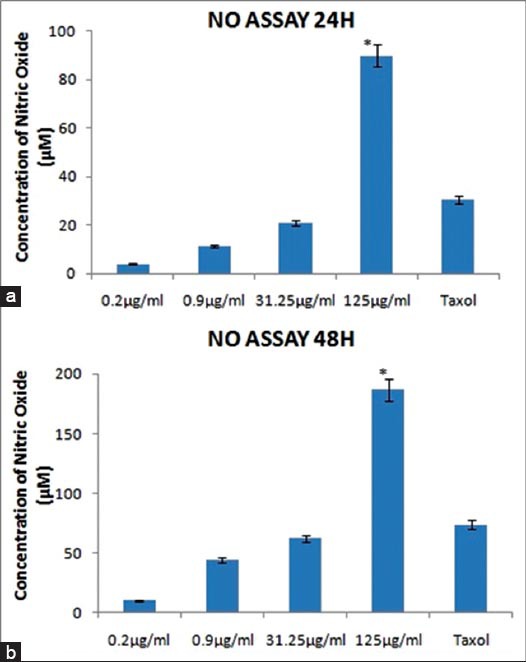
Effect of different concentrations of an methanolic extract of a Fraxinus micrantha (MeFM) (0.2-125 µg/ml) on nitric oxide (NO) production in Michigan Cancer Foundation-7 human breast cancer cell line at: (a) 24 h and (b) 48 h; *Induction of NO production was found to be significant, p<0.05 compared to positive control group. Data are means±standard error for three independent experiments
DNA Fragmentation Analysis by an MeFM
DNA fragmentation of MCF-7 (2 × 106 cells/well) was detected on 2.5% agarose gel after exposing with control groups and concentrations of an MeFM ranging from 0.2 to 125 µg/ml for 24 h. At an MeFM concentration of 125 µg/ml, DNA smear was observed ranging up to 0.2 kb [Figure 4]. This smear (Lane 2) was obtained because of DNA fragmentation induced by an MeFM, which probably causing apoptosis. On the other hand, a sharp clear band was obtained in a negative control (Lane 6). A 1kb DNA ladder was used as a marker.
Figure 4.
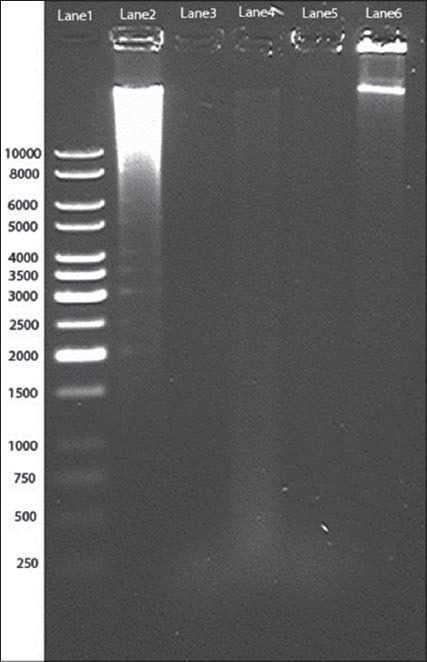
2.5% agarose gel showing DNA fragmentation induced by an methanolic extract of a Fraxinus micrantha (Lane 1-1 kb marker, Lane 2-125 µg/ml, Lane 3-31.25 µg/ml, Lane 4-0.9 µg/ml, Lane 5-0.2 µg/ml, and Lane 6 - Negative control)
Biochemical Assays
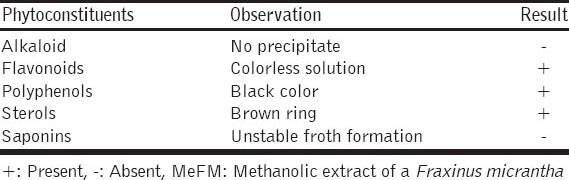
Qualitative analysis showed the presence of flavonoids, polyphenols, and sterols while alkaloids and saponins were absent in an MeFM [Table 1].
Table 1.
Result of phytochemical screening of bark of an MeFM
DISCUSSION
Cancer is one of the invasive diseases caused by the uncontrolled proliferation and growth of otherwise normally growing cells. The drugs used in conventional chemotherapy causes various toxic and side effects such as depression of bone marrow leads to neutropenia, fall of hair follicles, damage to cell lining of mucous membrane, digestive tract, and reproductive system [2]. In view of this, there is an urgent need to search for novel and safer alternative molecules having anti-proliferative property. Natural resources especially plant like F. micrantha found in the Himalayan region of Indian subcontinent have been reported for various medicinal properties [13]. The present study for the first time reporting anti-proliferative property of an MeFM in vitro on MCF-7 cell line.
The MeFM demonstrated significant antiproliferative effect against MCF-7 human breast carcinoma cell line. MTT reduction assay showed concentration and time-dependent cytotoxicity of the MeFM. The morphological changes in MCF-7 breast cancer cells incubated with different concentrations of an MeFM were consistent with MTT assay. At the concentration of 31.25 µg/ml and 125 µg/ml striking morphological changes such as clumping of cells having round morphology, retraction and shrinking of cells were observed in inverted microscope in the same manner as MTT reduction assay showed strong cytotoxic action of an MeFM at these concentrations. The cytotoxicity of an MeFM at high concentration (125 µg/ml) used in the present study was almost comparable to cytotoxicity of taxol. This showed that the phytoconstituents present in the MeFM have strong anti-proliferation potential which might be useful in deriving the novel anticancer molecule in future.
NO is a short-lived free radical, endogenously acts as a signaling molecule in the body. Previous reports showed that an excessive and unregulated NO synthesis has been implicated to abrogation of tumorigenicity and metastasis of tumor cells [14]. NO is also known to inhibit cell proliferation and induces apoptosis in high concentrations [15]. Increased NO production has been reported in breast cancer cells treated with various apoptotic agents, such as tumor necrosis factor-a, phorbol esters, and peptide hormones [14,16]. This study demonstrated that on incubation of MCF-7 cells with different concentrations of an MeFM, NO was produced in concentration- and time-dependent manner. This phenomena might be possible due to enhancing effect of iNOS gene stimulated by an MeFM which may lead to enhanced production of NO [17] which might be responsible for damage of various cellular components (proteins, DNA, other organelles) and finally may result in cell death and apoptosis. This was consistent with our result of DNA damage in DNA fragmentation study after incubation with an MeFM. Similar DNA fragmentation results were reported in a study with positive control taxol [18].
Biochemical tests showed the presence of flavonoids, polyphenols, and sterols present in the MeFM, which might be involved in anti-proliferative activity. Flavonoids have been reported for potent cell growth inhibitory actions over a physiologically relevant concentration range [19].
In conclusion, the results of the present study demonstrated that the MeFM has potent antiproliferative activity which might be inducing the phenomena of programmed cell death or apoptosis. Further studies are required to identify, isolate, and characterize the active phytoconstituents from the extract in order to be used as a potential anticancer agent in the future for cancer chemoprevention.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lyon, France: 2012. [Last accessed on 2014 Sep 01]. International Agency for Research on Cancer. Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from: http://www.globocan.iarc.fr/Pages/fact_sheets_cancer.aspx . [Google Scholar]
- 2.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer treatment side effects. J Natl Cancer Inst. 2001;93:810–23. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhery HJ, Wadhwa BM. Vol. 1. Howrah: Botanical Survey of India, Dept. of Environment; 1984. Flora of Himachal Pradesh: Analysis; pp. 6–670. [Google Scholar]
- 5.Garbyal SS, Aggarwal KK, Babu C. Traditional phytomedicinal knowledge of Bhotias of Dharchula in Pithoragarh. Indian J Tradit Knowl. 2005;4:199–207. [Google Scholar]
- 6.Tsukamoto H, Hisada S, Nishibe S. Coumarins from bark of Fraxinus japonica and Fraxinus mandshurica var japonica. Chem Pharm Bull. 1985;33:4069–73. doi: 10.1248/cpb.32.4482. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JC, Tsai S, Chang WC. Effect of ethanol extracts of three Chinese medicinal plants with anti-diarrheal properties on ion transport of the rat intestinal epithelia. J Pharmacol Sci. 2004;94:60–6. doi: 10.1254/jphs.94.60. [DOI] [PubMed] [Google Scholar]
- 8.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–16. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro HM. 2nd ed. New York: John Wiley & Sons; 1988. Practical Flow Cytometry; p. 129. [Google Scholar]
- 11.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Evans WC. 15th ed. London: Elsevier, WB Saunders Company Ltd; 2002. Trease & Evans Pharmacognosy; pp. 137–9. (230-40). [Google Scholar]
- 13.Thomas SC. 2nd ed. Florida: CRC Press; 2009. Chinese and Related North American Herbs - Phytopharmacology and Therapeutic Values; pp. 201–640. [Google Scholar]
- 14.Tschugguel W, Schneeberger C, Unfried G, Czerwenka K, Weninger W, Mildner M, et al. Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res Treat. 1999;56:145–51. doi: 10.1023/a:1006288526311. [DOI] [PubMed] [Google Scholar]
- 15.Reveneau S, Arnould L, Jolimoy G, Hilpert S, Lejeune P, Saint-Giorgio V, et al. Nitric oxide synthase in human breast cancer is associated with tumor grade, proliferation rate, and expression of progesterone receptors. Lab Invest. 1999;79:1215–25. [PubMed] [Google Scholar]
- 16.Bani D, Masini E, Bello MG, Bigazzi M, Sacchi TB. Relaxin activates the L-arginine-nitric oxide pathway in human breast cancer cells. Cancer Res. 1995;55:5272–5. [PubMed] [Google Scholar]
- 17.Alalami O, Martin JH. ZR-75-1 human breast cancer cells: Expression of inducible nitric oxide synthase and effect of tamoxifen and phorbol ester on nitric oxide production. Cancer Lett. 1998;123:99–105. doi: 10.1016/s0304-3835(97)00404-7. [DOI] [PubMed] [Google Scholar]
- 18.Choi YH, Yoo YH. Taxol-induced growth arrest and apoptosis is associated with the upregulation of the Cdk inhibitor, p21WAF1/CIP1, in human breast cancer cells. Oncol Rep. 2012;28:2163–9. doi: 10.3892/or.2012.2060. [DOI] [PubMed] [Google Scholar]
- 19.Zava DT, Duwe G. Estrogenic and antiproliferative properties of genistein and other flavonoids in human breast cancer cells in vitro. Nutr Cancer. 1997;27:31–40. doi: 10.1080/01635589709514498. [DOI] [PubMed] [Google Scholar]


