Abstract
Objective:
The aim was to determine the chemical constituents and antimicrobial activity of the hexane leaf extract of Anisopus mannii against a wide range of human pathogenic microorganisms.
Methods:
The chemical constituents of the hexane leaf extract was determined using gas chromatography-mass spectrometry (GC-MS) analysis; and the antimicrobial activity was evaluated on “standard strains”, clinical susceptible and resistant bacterial and fungal isolates using the disc diffusion and broth microdilution methods.
Results:
GC-MS analysis of the hexane leaf extract revealed 32 compounds, representing 73.8% of the identified components. The major compounds were hexadecanoic acid, ethyl ester (34%), oxirane, hexadecyl- (11%) and 9, 12, 15-octadecatrienoic acid, ethyl ester, (Z, Z, Z) (9.6%). Results from the antimicrobial activity demonstrated higher inhibition zones against Bacillus cereus (29 mm), followed by Streptococcus pyogenes (28 mm). Other notable inhibitions were observed with Enterococcus faecalis (27 mm), Proteus vulgaris (26 mm) and MRSA (25 mm). The MIC values ranged from 0.625 mg/mL to 1.25 mg/mL while the MBC/MFC values ranged from 2.5 mg/mL to 5.0 mg/mL.
Conclusion:
These results support the traditional use of the plant and demonstrate the huge potential of A. mannii as a source of antimicrobial compounds.
KEY WORDS: Anisopus mannii, antimicrobial activity, hexane extract, gas chromatography-mass spectrometry
INTRODUCTION
Infectious diseases are the primary cause of death, representing about 50% of all the human deaths in tropical countries [1]. The is due to decreasing efficacy of the antimicrobial chemotherapy because of the emergence of drug-resistant pathogens especially in developing countries where poverty and ignorance are high among the populace and basic health facilities are inadequate [2]. The range of these emerging infectious diseases such as methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin resistant enterococci (VRE) and the prevalence of drugs resistant Pseudomonas aeruginosa are threats to public health around the world [1]. This situation calls for an urgent need to identify new or complementary antimicrobial agents from natural sources to combat the resistant pathogens [3]. This screening of plant extracts or plant products for bioactive agents have shown potentials for new compounds or templates for new antibiotics [4,5]. It is desirable to evaluate our indigenous herbal plants as sources of bioactive compounds for drug discovery.
Anisopus mannii N.E. Br. (family Asclepiadaceae), is a perennial herb currently used in the traditional medicinal preparations of Northern Nigeria. Different parts of the plant are used as a remedy for diabetes [6], pile, diarrhea and other infectious diseases (Personal communication). The plant is locally known as “kashe zaki” in Hausa language. Previously, the proximate composition, mineral elements and anti-nutritional factors of A. mannii were reported [7]. The phytochemical and antimicrobial screening of the stem aqueous extract [8], the analgesic and anti-inflammatory studies of methanol leaf extract [9] as well as the isolation of chemical constituents such as 1, 7-naphthyridine alkaloid- named anisopusin, 5-hydroxy-lup-20(29)-en-3-yl eicosanoate, 6-gingerdione, 6-dehydrogingerdione and ferulic acid from acetone extract of the stem bark have been reported [10]. However, no previous phytochemical or biological studies on the non-polar constituents of A. mannii have been reported. Thus, the need to evaluate the lipophilic components is imperative to validate the use of the plant as traditional phytomedicine. It is unarguable that bioactive constituents of medicinal plants are widely distributed between polar and non-polar regions [11,12]. Because A. mannii leaf is the commonly used recipe for infectious disease treatment in Northern Nigeria; we investigated the chemical composition using gas chromatography-mass spectrometry (GC-MS) and antimicrobial effects of hexane extract on some standard, clinical susceptible and resistant strains of human pathogenic microorganisms with had not been reported hitherto.
MATERIALS AND METHODS
Plant Material
The leaves of A. mannii were collected in February, 2011 in Zaria, Kaduna State, Nigeria. It was taxonomically authenticated by Umar Shehu Galla of the Herbarium Unit, Department of Biological Sciences Ahmadu Bello University Zaria, Nigeria. A specimen (Voucher No. 217) was deposited there. The leaves were air-dried for 2 weeks and pulverized to powder using pestle and mortar.
Extraction
The pulverized plant sample (120 g) was extracted with hexane (750 ml) by simple percolation on a shaker (Labcon, South Africa) for 24 h. The extract was filtered using Whatman filter paper No. 2, and concentrated on a rotary evaporator (Büchi Rota vapor R-124) at 40°C to give 12.5 g of the crude hexane extract.
GC-MS
The GC-MS analyses was carried out on an Agilent Technologies (6890 Series) GC coupled with a (5973 Series) Mass Selective Detector. It was equipped with an Agilent HP-5MS capillary column (0.25 µm film thickness) with dimensions 30 m (length) × 0.25 µ I.D). The sample ionization energy of 70eV for GC-MS detection was used. Helium was used as the carrier gas at a pressure of 60 kPa, with the oven temperature programming at 100°C (for 2 min) to 280°C (for 30 min) at a ramping rate of 4°C per min. A 2.0 µl diluted sample was manually injected while the injection temperature was 280°C with a split ratio of 1:50. The system software was driven by Agilent Chemstation software. The relative percentage of each component was calculated by comparing its average peak to the total areas. The identification of the various compounds was carried out by comparison of their mass spectra with those of authentic samples or those obtained from isolated pure compounds in our laboratory. The NIST/NBS 2005 mass spectral database of the GC-MS system was also used to identify some compounds whose structures were confirmed by published data [13].
Test Organisms
The standard strains of organisms such as: Staphylococcus aureus NCTC 6571, Escherichia coli NCTC 10418, Salmonella typhi ATCC 9184, Pseudomonas aeruginosa NCTC 6750 and clinical isolates including MRSA, VRE, Bacillus cereus, Streptococcus pyogenes, Enterococcus faecalis, Candida tropicalis, Candida stellatoidea, Candida krusei and Candida albicans used in this study were obtained from the Department of Microbiology, Ahmadu Bello University Teaching Hospital (ABUTH), Shika. The isolates were purified on nutrient agar (OXOID) plates and characterized using standard microbiological and biochemical procedures as previously described [14,15].
Determination of Antibacterial and Antifungal Activities
The disc diffusion method was used [16]. Stock solution (100 mg/mL) of the hexane extract was prepared using methanol. Disc (6 mm diameter) were prepared using Whatman filter paper and sterilized by autoclaving. The blank sterile discs were placed on the inoculated Mueller Hinton Agar (OXOID) surface and impregnated with 15 µL of stock solutions (300 µg/dics). The plates were incubated at 37°C for 24 h. Standard antibiotic discs were used as positive control: Sparfloxacin (Himedica Laboratories, India) (100 µg/dics) for bacteria and Fluconazole (Saga Laboratories, India) (30 µg/dics) for fungal species. All tests were performed in duplicate, and the antimicrobial activity was expressed as the mean diameter of inhibition zones (mm) produced by the extracts.
Determination of Minimum Inhibitory Concentration (MIC)
MIC was carried out using micro broth dilution in accordance with National Committee for Clinical Laboratory Standards [17]. Serial dilution of sample extract was prepared between 0.1 mg/ml and 6.50 mg/ml concentration. The tests tubes were inoculated with the suspension of the standardized inocula and incubated at 37°C for 24 h. MICs were recorded as the lowest concentration of the extract showing no visible growth of the broth.
Determination of Minimum Bactericidal/Fungicidal Concentrations (MBC/MFC)
MBC/MFC were determined by aseptically inoculating aliquots of culture from MIC tubes that showed no growth, on sterile nutrient agar plates and incubating at 37°C for 48 h. MBC/MFC were recorded as the lowest concentration of the extract showing no bacterial growth.
RESULTS
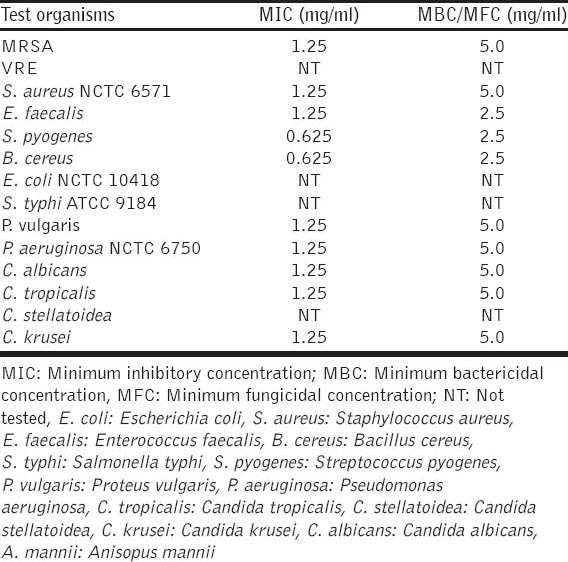
The studies of the active principles in the hexane leaf extract of A. mannii by GC-MS analysis showed the presence of 32 compounds. The active principles with their retention time (RT) and percent relative composition are presented in Table 1. The results of the antimicrobial activity of the hexane leaf extract are presented in Tables 2 and 3
Table 1.
Chemical composition of hexane extract of A. mannii leaf
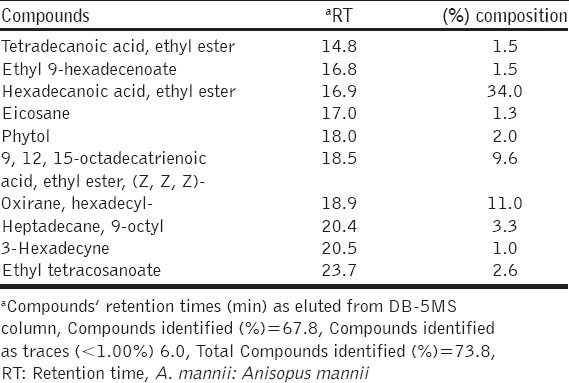
Table 2.
Antimicrobial activity of hexane extract of A. mannii leaf
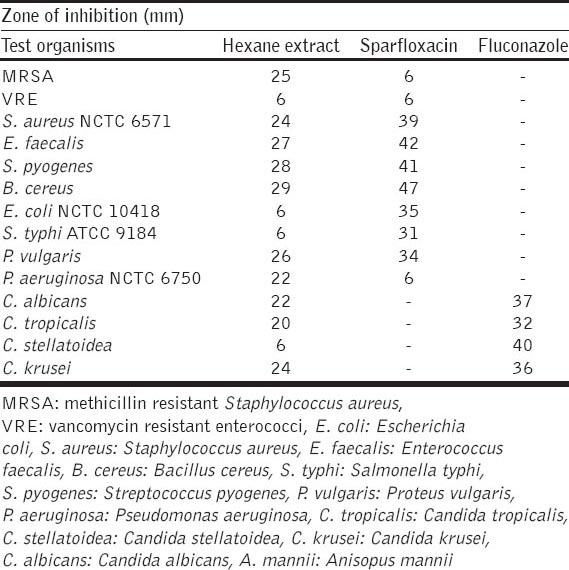
Table 3.
Results of MIC and MBC/MFC of hexane extract of A. mannii leaf
DISCUSSION
The use of A. mannii leaf extracts in the traditional treatment of some infectious diseases had prompted us to investigate the antimicrobial activity and subsequently, the chemical composition of the extract was investigated using GC-MS as a first step towards understanding the nature of bioactive principles. In traditional medicine, A. mannii recipes are prepared as aqueous concoction or taken in powdered form with milk (Personal communication) for different remedies. Plant extracts contain both polar and non-polar components. Thus the phytochemical analysis of non-polar portion is desirable to validate the use as effective phytomedicine against the microbial infections.
Because hexane is non-polar, it was used to extract lipophilic components that have been determined to be largely fatty acid methyl esters (FAME) with hydrocarbons and terpenes as traces. Terpenoids volatile components of essential oil were not detected; they are obtainable from fresh plant samples using a Clevenger hydro distillation technique. Thus, the chemical composition of A. mannii hexane leaf extract is an aggregate mixture of fat soluble compounds of different structural motifs driven by lipophilicity of the solvent. These compounds vary in content or composition from plant to plant due to environmental or genetic factors [18].
The hexane extract demonstrated good in vitro antimicrobial profile especially against MRSA, S. faecalis, S. pyogenes, B. cereus and P. aeruginosa which are known to cause infections that are extremely difficult to treat due to multiple drugs resistance. Furthermore, the extract showed potent activity against both Gram-positive and Gram-negative bacteria, as well as different species of fungi. These findings could indicate that the extract had broad-spectrum antimicrobial effects against a wide range of infectious agents that could be the basis for the folkloric use of the plant. However, it is noteworthy that the test extract showed better antimicrobial activity on B. cereus (29 mm), S. pyogenes (28 mm), E. faecalis (27 mm) and MRSA (25 mm). Previous antibacterial studies on the stem-bark aqueous extract of A. mannii showed higher MIC values of 50 mg/ml (indicating low activity) for S. aureus, S. pyogenes and P. aeruginosa [8] when compared to our findings in which the MIC of the hexane extract for S. aureus and P. aeruginosa was 1.25 mg/ml with S. pyogenes having 0.625 mg/ml [Table 3]. This indicates the potency of the lipophilic hexane extract of A. mannii due to the compounds identified.
FAME have been studied in relation to antibacterial activity and the structural properties, including carbon chain length, unsaturation, esterification and functional groups have enormous influences on the activity [19]. Hexadecanoic acid, ethyl ester (34%), oxirane, hexadecyl (11%) and 9, 12, 15-octadecatrienoic acid, ethyl ester, (Z, Z, Z) (9.6%) are the major constituents identified in the hexane leaf extract. Some of the minor constituents identified are heptadecane, 9-octyl (3.3%), ethyl tetracosanoate (2.6%) and phytol (2.0%). The 32 compounds identified represent 73.8% of the extract. Among the detected major phytochemicals, hexadecanoic acid ethyl ester (palmitic acid ethyl ester) is a neutral lipid soluble form of palmitic acid. Interestingly, the antimicrobial activity of this compound has been reported and subsequently proposed to be the main bioactive antimicrobial agent identified in the ethyl acetate extract of marine Burkholderia cepacia [20]. Furthermore, the other major compound detected in the extract, oxirane tetradecyl has been identified as one of the bioactive antimicrobial compounds of the red algae Laurencia brandenii and Senecio pedunculatus [21,22]. In another study, the leaf extract of Andrographis peniculata demonstrated strong antimicrobial, antioxidant and anticancer activities, which were attributed, in part, to 9, 12, 15-octadecatrienoic acid, ethyl ester, (Z, Z, Z) [23]. However, the antimicrobial activity could probably be accentuated by synergy effects of the different chemical components of the extracts [24].
CONCLUSION
This is the first report on the chemical composition and antimicrobial activity of hexane extract of A. mannii leaf. The findings validate the folkloric use of the plant against infectious agents and identified some chemical entities such as hexadecanoic acid ethyl ester, oxirane, tetradecyl and 9, 12, 15-octadecatrienoic acid, ethyl ester, (Z, Z, Z), which have acted perhaps synergistically for the enhanced antimicrobial activity.
ACKNOWLEDGMENTS
To Ahmadu Bello University, Zaria, Nigeria for travel grant through the ETF/AST & D 2010/13 given to A.B. Aliyu. We are grateful to Mr Neal Broomhead of the Analytical Instrument Laboratory, School of Chemistry & Physics, University of KwaZulu-Natal, Westville Campus, Durban, South Africa, for running the GC-MS analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Iwu MM, Duncan AR, Okunji CO. New antimicrobials of plant origin. In: Janick J, editor. Perspectives on New Crops and Uses. Alexandria, V.A: ASHS Press; 1999. pp. 457–62. [Google Scholar]
- 2.Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agent. 2004;24:105–10. doi: 10.1016/j.ijantimicag.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med. 2013;13:164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer-Grimes B, Macbeth DL, Hallihan B, Delph S. Antimicrobial activity in medicinal plants of the Scrophulariaceae and Acanthaceae. Int J Pharmacogn. 1996;34:243–8. [Google Scholar]
- 5.Afolayan AJ. Extracts from the shoots of Arctotisar totoides inhibit the growth of bacteria and fungi. Pharm Biol. 2003;41:22–5. [Google Scholar]
- 6.Mohammed A, Ibrahim MA, Islam MS. African medicinal plants with antidiabetic potentials: A review. Planta Med. 2014;80:354–77. doi: 10.1055/s-0033-1360335. [DOI] [PubMed] [Google Scholar]
- 7.Aliyu AB, Musa AM, Sallau MS, Oyewale AO. Proximate composition, mineral elements and anti-nutritional factors of Anisopus mannii N.E. Br (Asclepiadaceae) Trends Appl Sci Res. 2009;4:68–72. [Google Scholar]
- 8.Sani D, Sanni S, Ngulde SI. Phytochemical and antimicrobial screening of the stem aqueous extract of Anisopus mannii. J Med Plants Res. 2009;3:112–5. [Google Scholar]
- 9.Musa AM, Aliyu AB, Yaro AH, Magaji MG, Hassan HS, Abdullahi MI. Preliminary phytochemical, analgesic and anti- Inflammatory studies of the methanol extract of Anisopus mannii (N. E. Br) (Asclepiadaceae) in rodents. Afr J Pharm Pharmacol. 2009;3:374–8. [Google Scholar]
- 10.Tsopmo A, Kamnaing P, Watchueng J, Gao J, Konishi Y, Sterner O. Chemical constituents from the bark of Anisopus mannii. Can J Chem. 2009;87:397–400. [Google Scholar]
- 11.Aliyu AB, Musa AM, Abdullahi MS, Ibrahim MA, Tijjani MB, Aliyu MS, et al. Activity of saponin fraction of Anisopus mannii against some pathogenic microorganisms. J Med Plants Res. 2011;5:6709–13. [Google Scholar]
- 12.Ibrahim S, Ibrahim MA, Musa AM, Aliyu AB, Haruna SN, Okafor AI. Indigofera pulchra leaves extract contain anti- Plasmodium berghei agents. Bangladesh J Pharmacol. 2011;6:69–73. [Google Scholar]
- 13.Masada Y. New York: John Wiley & Sons; 1976. Analysis of essential oil by gas chromatography and spectrometry. [Google Scholar]
- 14.Cowan ST, Steel KF. 2nd ed. London: Cambridge University Press; 1974. Manual for Identification of Medical Bacteria; pp. 97–115. [Google Scholar]
- 15.Mac Faddin JF. Baltimore: The William and Wilkins Company; 1977. Biochemical Tests for Identification of Medical Bacteria; pp. 392–452. [Google Scholar]
- 16.Nostro A, Germanò MP, D’angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 2000;30:379–84. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 17.7th ed. Wayne: NCCLS; 2002. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial disc Susceptibility Tests. Approved Standard, NCCLS Document M2-A8. [Google Scholar]
- 18.Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJ. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J. 2008;23:213–26. [Google Scholar]
- 19.Zhang H, Zhang L, Peng LJ, Dong X, Wu D, Wu VC, et al. Quantitative structure activity relationships of antibacterial fatty acids and derivatives against Staphylococcus aureus. J Zhejiang Univ-Sci-B. (Biomed & Biotech) 2012;13:83–93. doi: 10.1631/jzus.B1100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousry MG, Manal MA, Magdy KS, Khouloud MB. Characterization of marine Burkholderia cepacia antibacterial agents. J Nat Prod. 2010;3:86–94. [Google Scholar]
- 21.Manilal A, Sujith S, Sabarathnam B, Kiran GS, Selvin J, Shakir C, et al. Biological activity of the red alga Laurencia brandenii. Acta Bot Croat. 2011;70:81–90. [Google Scholar]
- 22.Joshi N, Sah GC, Mishra D. GC-MS analysis and antimicrobial activity of essential oil of Senecio pedunculatus. IOSR J Appl Chem. 2013;6:61. [Google Scholar]
- 23.Wei LS, Wee W, Siong JY, Syamsumir DF. Characterization of antimicrobial, antioxidant, anticancer properties and chemical composition of Malaysian Andrographis paniculata leaf extract. Pharmacol Online. 2011;2:996–1002. [Google Scholar]
- 24.Eloff JN, McGaw LY. Plant extract used to manage bacterial, fungal and parasitic infections in South Africa. In: Ahmad I, Aqil F, Owais M, editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. Weinheim: Wiley-VCH, Verlag GmbH & Co. KGaA; 2006. p. 104. [Google Scholar]


