Abstract
In the grim scenario where presently about 70% of pathogenic bacteria are resistant to at least one of the drugs for the treatment, cue is to be taken from traditional/indigenous medicine to tackle it urgently. The Indian traditional knowledge emanates from ayurveda, where Bos indicus is placed at a high pedestal for numerous uses of its various products. Urine is one of the products of a cow with many benefits and without toxicity. Various studies have found good antimicrobial activity of cow’s urine (CU) comparable with standard drugs such as ofloxacin, cefpodoxime, and gentamycin, against a vast number of pathogenic bacteria, more so against Gram-positive than negative bacteria. Interestingly antimicrobial activity has also been found against some resistant strains such as multidrug-resistant (MDR) Escherichia coli and Klebsiella pneumoniae. Antimicrobial action is enhanced still further by it being an immune-enhancer and bioenhancer of some antibiotic drugs. Antifungal activity was comparable to amphotericin B. CU also has anthelmintic and antineoplastic action. CU has, in addition, antioxidant properties, and it can prevent the damage to DNA caused by the environmental stress. In the management of infectious diseases, CU can be used alone or as an adjunctive to prevent the development of resistance and enhance the effect of standard antibiotics.
KEY WORDS: Antibiotic, antifungal, antineoplastic, bioenhancer, Bos indicus, immune-enhancer
INTRODUCTION
Infectious diseases remain a major threat to the public health despite tremendous progress in human medicine. Emergence of widespread drug resistance to the currently available antimicrobials is a matter of deep concern. A high percentage of nosocomial infections are caused by highly resistant bacteria such as methicillin-resistant Staphylococcus aureus or multidrug-resistant (MDR) Gram-negative bacteria. Each year in the United States, about 2 million people become infected with antibiotic resistant bacteria and at least 23,000 people die every year as a consequence of these infections. Many more people die from other conditions that are complicated by an antibiotic-resistant infection [1]. In 2012, there were about 450000 new cases of MDR tuberculosis. Extensively drug-resistant tuberculosis has been identified in 92 countries. Development of resistance to oral drug of choice fluoroquinolones, for urinary tract infections caused by Escherichia coli is very widespread, often sensitivity remains only for injectables [2]. Infections caused by resistant microorganisms often fail to respond to the standard treatment, resulting in prolonged illness, higher health care expenditures, and a greater risk of death. There is a dire need for the development of new antimicrobial agents with sensitivity intact against microorganisms [3,4]. The rational designing of novel drugs from traditional medicines to treat these difficult to treat infections offers a new prospect for the modern health-care system.
Ayurvedic texts (Sushruta Samhita, Ashtanga Sangrah and Bhav Prakash Nighantu) describe cow urine (CU) (gomutra) as an effective medicinal substance/secretion of animal origin with innumerable therapeutic uses. Cow (Kamadhenu) has been considered as a sacred animal in India. In Rigveda (10/15), CU is compared to nectar. In Susruta (45/221) and in Charak (sloka-100) several medicinal properties of CU have been mentioned such as weight loss, reversal of certain cardiac and renal diseases, indigestion, stomach ache, diarrhea, edema, jaundice, anemia, hemorrhoids and skin diseases including vitiligo. Gomutra is capable of removing all the imbalances in the body, thus maintaining the general health [5]. CU contains 95% water, 2.5% urea, minerals, 24 types of salts, hormones, and 2.5% enzymes. It also contains iron, calcium, phosphorus, carbonic acid, potash, nitrogen, ammonia, manganese, iron, sulfur, phosphates, potassium, urea, uric acid, amino acids, enzymes, cytokine and lactose [6].
CU is an effective antibacterial agent against a broad spectrum of Gram-negative and Gram-positive bacteria and also against some drug-resistant bacteria. It acts as a bio-enhancer of some antimicrobial drugs. It has antifungal, anthelmintic, antineoplastic action, is useful in hypersensitivity reactions and in numerous other diseases including increasing the life-span of a person. Recent researches have shown that CU is an immune-enhancer also [7-9]. Therapeutic properties of CU have been validated by modern science also.
MECHANISM OF ACTION OF CU
Different fractions of CU possess antimicrobial activity due to the presence of certain components like volatile and nonvolatile ones [10-13]. Presence of urea, creatinine, swarn kshar (aurum hydroxide), carbolic acid, phenols, calcium, and manganese has strongly explained the antimicrobial and germicidal properties of CU [14-16]. Presence of amino acids and urinary peptides may enhance the bactericidal effect [17] by increasing the bacterial cell surface hydrophobicity. CU enhances the phagocytic activity of macrophages. Higher amounts of phenols in fresh CU than CU distillate (CUD) makes it more effective against microbes.
After photo-activation, few biogenic volatile inorganic and organic compounds such as CO2, NH3, CH4, methanol, propanol and acetone, and some metabolic secondary nitrogenous products are also formed [18]. Photo-activated CU (PhCU) becomes highly acidic in comparison to fresh CU. An increase in bactericidal action may be due to a significant decrease in pH [12], presence of inorganic phosphorus, chloride and dimethylamine may also play an important role [19], along with increased formation of some reactive compounds like formaldehyde, sulfinol, ketones and some amines during photo-activation and long term storage [20]. CU prevents the development of antibacterial resistance by blocking the R-factor, a part of plasmid genome of bacteria [21].
CU contains phenolic acids (gallic, caffeic, ferulic, o-coumaric, cinnamic, and salicylic acids) which have antifungal characteristics [22].
Antioxidant property of uric acid and allantoin present in CU correlates with its anticancer effect. CU reduces apoptosis in lymphocytes and helps them to survive better [5]. This action may be due to the free radical scavenging activity of the urine components, and these components may prevent the process of aging [10]. It efficiently repairs the damaged DNA [5].
Daily consumption of CU improves immunity due to the presence of swarn kshar and fastens the wound healing process, which is due to allantoin [8]. CU enhances the immunocompetence by facilitating the synthesis of interleukin-1 and -2 [23,24], augments B - and T- lymphocyte blastogenesis, and IgA, IgM and IgG antibody titers [25].
Early morning first voided CU is more sterile and have more macro and micronutrients along with other enzyme/urea content could be more effective [26].
AS ANTIMICROBIAL AGENT
Antimicrobial activity of CU from both indigenous and hybrid breeds against E. coli, Salmonella typhi, Proteus vulgaris, S. aureus, Bacillus cereus, Staphylococcus epidermidis, Klebsiella pneumonia, Pseudomonas aeruginosa, Pseudomonas fragi, Streptococcus agalactiae, Enterobacter aerogenes, Aeromonas hydrophila, Micrococcus luteus, Streptococcus pyogenes, Streptomyces aureofaciens, Lactobacillus acidophilus and Bacillus subtilis, and Leishmania donovani has been observed in various studies. In these studies the antimicrobial activity of CU was found to be comparable with ofloxacin, ciprofloxacin, ampicillin, chloramphenicol, nalidixic acid, rifampicin, tetracycline, streptomycin, cefpodoxime and gentamycin in different studies [27-36].
Studies with Indigenous Bos indicus Breeds of Cow
Fresh CU (FCU), Sterile, PhCU and CUD from a healthy Geer cow, was used to assess the antibacterial effect against different strains of bacteria. Against E. coli, FCU had the bigger mean of inhibition zone (15 mm) than Sterile, PhCU, and CUD (~10 mm). FCU had good activity of 15, 16 and 20 mm of inhibition against E. coli, B. subtilis, and S. typhi, respectively. Other forms of CU showed activity against E. coli, S. typhi, P. vulgaris, S. aureus and B. subtilis [27].
Rana and De [28] observed a greater activity against Gram-positive than Gram-negative bacteria with CU obtained from Geer cow. The minimum inhibitory concentration (MIC) in all the four tested Gram-positive bacteria was 134 mg/ml. Among Gram-negative organisms, P. aeruginosa was more sensitive (MIC 134 mg/ml) than P. vulgaris (MIC 268 mg/ml). Mean zone of inhibition (mm)± standard error of the mean against B. subtilis was found to be 18.67±1.15, which was less than 27 for Gentamycin 10 mcg and cefpodoxime 10 mcg. Activity (18.67±1.15) against B. cereus and was similar to that of cefpodoxime (19) but less than with gentamycin (26). Activity (16) against S. aureus and S. epidermidis was <25 for Gentamycin and ~23 with Cefpodoxime. No inhibition against P. aeruginosa was observed with Cefpodoxime while CU had an inhibition of 19.33 ± 1.15 mm and Gentamycin 35 mm. Against P. vulgaris inhibition was comparable between CU (16 ± 1.73), gentamycin (21) and cefpodoxime (20). There was comparable inhibition of P. vulagris by CU (16 ± 1.73), gentamycin (21) and cefpodoxime (20). Against K. pneumoniae, inhibition observed with CU (15.67 ± 0.57) was less than gentamycin (34) and cefpodoxime (20).
Comparatively FCU obtained from Gujarati Geer cow was found to have more antimicrobial activity than its distillate (Table 1).
Table 1.
Antimicrobial activity of CU, CUD (Gujrati Geer cow) in comparison to standard drug Ofloxacin [10]
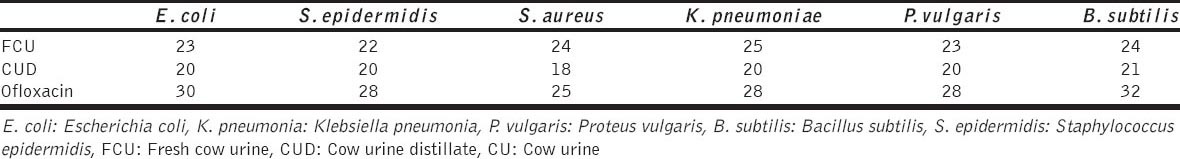
Similar findings were reported by Jarald et al. [29]. Mean zone of inhibition (mm), using Sahiwal CUD, was found to be 19.2 for S. aureus, 20.2 for P. fragi, 18.8 for E. coli, 23 for B. subtilis, 19 for S. agalactiae and 17 for P. vulgaris. There was a progressive decrease in optical density (indicator of antimicrobial activity and was measured by spectrophotometer at 600 nm) over 5 h when FCU was added to the respective inoculums [29].
The antibacterial efficacy (as mean zone of inhibition in mm) of CU Concentrate (CUC) obtained from Karnataka breed, Amrit mahal was comparable with Streptomycin on B subtilis (16:18), S. aureus (16:19), E. coli (14:18) and E. aerogenes (15:18) using Disc diffusion method [30].
In an in vitro study, 30 µL of PhCU of Hariana breed was found to be comparable in efficacy to Tetracycline (30 µg mL). Antimicrobial activity (mean zone of inhibition in mm) of PhCU and Tetracycline, respectively against B. cereus was 17 and 22, S. aureus was 18 and 21, S. typhimurium was 21 and 22, A. hydrophila was 22 and 24, E. aerugenes was 13 and 18 and M. luteus was 15 and 17 [31]. Similar results were found in another study with PhCU of Hariana breed against these bacteria [32].
Studies where breed of cow is not mentioned
In an in-vitro test, activity of FCU was comparable to Streptomycin. Similar mean zone of inhibition (mm) was seen against gram positive organisms E. coli (16:16:13), K. pneumonia (15:17:12) and P. aeruginosa (17:19:15) with FCU and Streptomycin and lesser with PhCU (by keeping urine in sunlight in sealed glass bottles for 72 h), respectively. Comparatively lesser antibacterial activity against gram negative organisms S. aureus (18:26:17), coagulase negative Staphylococci (18:29:15), B. subtilis (20:29:15), and S. pyogenes (20:26:14) was seen for FCU than streptomycin, and still lesser than with PhCU [33]. No antibacterial activity was seen for CUD, which is contradictory to some previous reports [34].
Vats et al. [35] studied the synergistic antimicrobial effect of PhCU and herbs against bacterial and fungal strains. PhCU and Azadirachta indica combination showed a remarkable synergistic antimicrobial activity against Candida tropicalis, Candida glabrata, P. aeruginosa, and S. aureofaciens. PhCU and Terminalia chebula showed maximum activity against S. auereofaciens (45 mm), and P. aeruginosa (zone of inhibition of 40 mm). Piper nigrum, T. chebula and PhCU in combination were most effective against C. glabrata (35 mm) and C. tropicalis (45) mm.
Upadhyay et al. [18] found in in-vitro tests that PhCU has better bactericidal activity against S. aureus, B. cereus, L. acidophilus, M. luteus, K. pneumonia, S. pneumonia and E. coli, when compared with Tetracycline, Ampicillin and Ciprofloxacin. PhCU showed MIC value of 0.25 µl/ml against S. aureus, B. cereus, L. acidophilus and M. luteus, while it was found to be 0.125 µl/ml against E. coli, which was less than that for antibiotics. A combination of CU with Neem (A. indica) oil and Bavchi (Psoralea coryfolia) oil showed a synergistic effect (MIC 0.125-0.25 µl/ml), which was less than that for antibiotics. Neem oil and CU showed 33-35 mm inhibition zones against B. cereus, L. acidophilus, M. luteus, K. pneumoniae and S. pneumonia.
Sathasivam et al. reported the antibacterial activity of CUD (5, 10 and 15 µl) against the B. subtilis, P. aeruginosa, K. pneumoniae and S. typhi. Antibacterial activity (mean zone of inhibition in mm) was observed against B. subtilis (7.6 ± 0.04, 8.6 ± 0.17, 8.8 ± 0.17, respectively) P. aeruginosa (12.6 ± 0.04, 13.6 ± 0.17, 15.4 ± 1.23, respectively) and S. typhi (12 ± 1.23, 13.6 ± 0.17, 15.4 ± 1.23, respectively). This antibacterial activity was more than the positive control of ampicillin (30 mg/disc), which was 7.1 ± 0.01 mm against B. subtilis, 11.2 ± 0.01 mm against P. aeruginosa and 9.6 ± 0.02 mm of inhibition zone against S. typhi. Antibacterial activity against K. pneumonia was 11 ± 0.14 mm with 15 µl of CUD, which was more than the activity (9.5 ± 0.05 mm) with Ampicillin [34].
Yadav et al. reported the antimicrobial property of a herbal formulation containing CU, Dalbergia sissoo, and Datura stramonium. The antimicrobial activity of CU alone was also found to be significant (P > 0.001). It was found that CU extract showed the highest inhibition in gram-positive S. aureus (CI, 213%) and comparable activity in S. pneumoiae (95%) compared to chloramphenicol (30 µg), nalidixic acid (10 µg), rifampicin (30 µg), and ampicillin (10 µg). In gram-negative bacteria all antibiotics were inactive, except chloramphenicol (30 µg), while CU extract showed significant (P < 0.05) activity (35% and 37%, respectively) against E. coli and K. pneumonia as compared to Chloramphenicol [36].
CU has anti-Leishmania donovani effect (Kala-azar) in an in-vitro study [37]. This fact can be further validated by more intensive studies.
PREVENTION OF ANTIBIOTIC RESISTANCE
Pathogenic bacteria are remarkably resilient and have developed several ways to resist antimicrobial drugs. Due to increasing use and rampant misuse of existing antibiotics in human and veterinary medicine, and also in agriculture, threat from antimicrobial resistance is increasing. Resistant strains like Penicillin- and Methicillin- resistant S. aureus, vancomycin resistant Enterococcus, and ciprofloxacin resistance P. aeruginosa are an ever increasing global threat. After photoactivation and purification, CU has been found to be effective against certain drug resistant bacterial strains [38]. CU extract of A. indica showed better MIC values than the organic fractions for MDR E. coli (12.68 mm) and K. pneumonia (9 mm). CU extracts of A. indica showed >8.66 mm zone of inhibition for MDR S. aureus, P. aeruginosa and P. vulgaris [39].
FUNGICIDE AND BIOFUNGICIDE
Fungicidal effect against Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Aspergillus, Malassezia, C. tropicalis and C. glabrata has been observed in various studies. CU was highly stable and capable in inhibiting the growth of Malassezia fungi (90-95%) responsible for causing dandruff for a longer time (4-5 days), than rice water (due to B. cereus growth in rice water) which was stably capable of inhibiting 85-90% of the growth for 3-4 days. Neem leaves extract and Lemon Juice extract were comparatively less effective in this study [40].
15% CU was most active against Aspergillus, Rhizophus and the percentage of inhibition obtained with it was 85% [41]. 5% CUC showed maximum antifungal activity against A. niger (93%), followed by A. oryzae (92.67%) and A. flavus (83%)[30]. CUD showed better antifungal activity against A. fumigatus and C. albicans with mean zone of inhibition of 13 and 11 mm than PhCU [27]. More fungal growth suppression (as mm in diameter) was observed with CUD in A. niger (8 ± 0.14, 11.3 ± 1.2 and 12.6 ± 0.04, respectively) than A. flavus (7.3 ± 0.25, 10 ± 0.26 and 11 ± 1.2, respectively) with the use of 5, 10 and 15 µl of CUD [34].
In vitro antifungal activity (in mm) of Geer CU against A. flavus (17.33 ± 0.57) was in between 50 µg of amphotericin B (15) and 10 µg of clotrimazole (24) and against C. albicans, activity was similar with CU (18.67 ± 1.15) and amphotericin B (19), but less than clotrimazole (30) [28]. Antifungal activity of Geer CU is better than the others where source of CU is not mentioned.
In an in vitro study, it was found that the urine samples of outdoor feeding cow (OCU) was more effective and inhibited growth of fungi more strongly as compared to indoor feeding CU (ICU). This inhibition was concentration dependent. No growth of Penicillium notatum, Trichoderma viridae, and Alternaria solanii was observed with 10% OCU and with 20% ICU and that of Claviceps purpurea, Rhizopus oligosporius, C. albicans and A. candidus, no growth was observed with 20% of OCU only [42].
ANTISEPTIC
Sanganal et al. observed the enhanced wound healing activity of CU in Wistar albino rats [43]. On 4th day, the external application of CU showed significant and progressive increase in wound healing in rats compared to different concentrations of CU and 1% w/w nitrofurazone ointment locally. Similar findings were also observed by Maheshwary et al. [44].
ANTHELMINTIC ACTIVITY
CUC was found to be more effective than piperazine citrate as anthelmintic agent at both 1% and 5% concentrations. For anthelmintic activity, adult Indian earthworm Pheretima posthuma was studied due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. Paralysis of earthworm occurred in 53 and 48 min with 1% piperazine and CUC, respectively and 16 and 13 min with 5% piperazine and CUC, respectively. Time taken for the death of earthworms decreased from 72 min with 1% piperazine to 60 min with 1% CUC, respectively. It further decreased from 28 min with 5% piperazine to 18 min with 5% CUC, respectively [30].
Different compositions of Panchgavya (five products of cow namely milk, curd, ghee, urine and dung) alone and combination of Panchgavya and ethanolic extract of Bauhinia variegata Linn (10%, 50%, 75% in Panchgavya) were found to have excellent anthelmintic activity against adult Indian earthworm (P. posthuma) when we compared to control Piperazine (50 and 100 mg/ml). In combination, the anthelmintic activity was synergistic and with increasing doses, time (in minutes) of onset of paralysis and death in earthworm decreased [45].
BIOENHANCER
A ‘bioenhancer’/‘biopotentiator’ is an agent capable of enhancing the bioavailability and efficacy of a drug with which it is co-administered, without any pharmacological activity of its own at the therapeutic dose used. In ayurveda, this concept is known as ‘yogvahi’ and is used to increase the effect of medicines by increasing the oral bioavailability (especially of medicines with poor oral bioavailability), decreasing their dose and adverse effects, and were used to circumvent the parentral routes of drug administration. We can develop more such useful and economically viable drug combinations, by integrating the knowledge of time tested ayurveda with modern methods of research [8]. CU is the only agent of animal origin which acts as bioenhancer of antimicrobial, antifungal, and anticancer agents [30]. The indigenous CU contains ‘Rasayana’ tatva, which is responsible for modulation of the immune system and also act as a bioenhancer [21].
CUD is more effective bioenhancer than CU [30,46]. CUD enhances the transport of antibiotics like rifampicin, tetracycline and ampicillin across the gut wall by 2-7 folds [47]. It also enhances the potency of taxol against MCF-7 cell lines [48]. It enhances the bioavailability of rifampicin by 80 fold in 0.05 microgm/ml concentrations, ampicillin by 11.6 fold in 0.05 µ g/ml concentrations and clotrimazole by 5 fold in 0.88 µ g/ml concentration [49]. The activity of rifampicin increases by about 5-7 folds against E. coli and 3-11 folds against Gram-positive bacteria, when used along with CU [50]. Potency of paclitaxel has been observed to increase against MCF-7, a human breast cancer cell line in in-vitro assays [49]. The bioenhancing ability is by facilitating the absorption of drugs across the cell membrane. The CU has been granted US Patents for its medicinal properties, particularly as a bioenhancer along with antibiotics, antifungal and anticancer drugs (6896907, 6410059).
CUD alone caused more inhibition of Gram-positive bacteria. Inhibition caused by streptomycin (1 mg/ml) alone was higher (31-34 mm) than that of CUD alone (19-22 mm). With the combination of Pinguicula longifolia, CUD and streptomycin together, S. aureus was inhibited to a more extent (38 mm) followed by P. aeruginosa (37 mm) and an equal inhibition was exhibited by B. subtilis and E. coli (36 mm) [51]. S. aureus and P. aeuroginosa have been recognized as most common bacteria, which have developed resistance against several antibiotics and is a major hospital borne pathogen, which is particularly dangerous to immunocompromised patients. This study is of importance in this scenario.
This bioenhancing activity of CU has been aptly and widely used in various ayurvedic formulations. It is one of the constituents of Hingwadh ghrita, Lashunadh ghrita, Sidhartak ghrita for psychiatric illness used in abdominal tumors and in other formulations like Mandurvatak, Darvi ghrita, and Punnarvamandur. CU is used as yogvahi along with Hareetakyadi yog, Swarnkshiryad yog, Swarnmakshik bhasma and Gvakshyadi churana. These preparations are commercially available in the Indian market. Ghritas are available as semisolid preparations while bhasms, yogs, and churans are in the powder form.
ANTICANCER PROPERTIES
CU has antioxidant properties and is a free radical scavenger, and thus it neutralizes the oxidative stress. Scientists proved that the pesticides even at very low doses cause apoptosis of lymphocytes and tissues through fragmentation of DNA while CU helps the lymphocytes to survive by inhibiting their apoptosis and by repairing the damaged DNA and is, therefore, effective as anti-cancer therapy [52,53].
Chemopreventive potential of CU was observed in a study, which was conducted on 70 Swiss albino mice for 16 weeks. Papillomas were induced by 7, 12 dimethyl benzanthracene and later promoted by repeated application of croton oil. In mice treated with CU, the incidence of tumor (papillomas), tumor yield, and its burden was statistically less than the untreated group [54].
Jain et al. studied the effect of CU therapy on various types of cancers in Mandsaur area. The severity of symptoms (pain, inflammation, burning sensation, difficulty in swallowing, and irritation) decreased from day 1 to day 8 with CU therapy. Percent of patients with severe symptoms decreased from 82.16 to 7.9 on day 8, patients with moderate symptoms increased from 15.8 to 55.3 and with mild symptoms, patients increased from 1.58 to 36.34. The severity of symptoms decreased further with continued CU therapy [15].
Dutta et al. reported the anti-clastogenic and anti-genotoxic effect of redistilled CUD (RCUD) in human peripheral lymphocytes, which have been challenged with manganese dioxide (MnO2) and hexavalent chromium (Cr+6). Protection in number of chromosomal aberrations and frequency of micronuclei were more prominent when these cells were pretreated with CU than simultaneous treatment with CU [55].
IMMUNOSTIMULANT
The use of herbs and minerals (like chavanprash and panchgavya) for improving the overall resistance of the body against common infections and pathogens has been a guiding principal of Ayurveda. Ancient books on Ayurveda state that consuming CU daily increases the resistance to diseases by up to 104%. CU enhances the humoral, and cell-mediated immune response in mice [5]. CUD was found to augment B- and T-lymphocyte blastogenesis; IgG, IgA and IgM antibody titers in mice. It has been observed that CU also increases the phagocytic activity of macrophages and is thus helpful in the prevention and control of bacterial infections. The level of both interleukins -1 and - 2 in mice was increased by 30.9% and 11.0%, respectively, and in rats these levels were increased significantly by 14.75% and 33.6%, respectively [52]. CUD has been reported to be a potent and safe immunomodulator, which increases both humoral, and cell-mediated immunity in mice.
Cell-mediated immune response was evaluated on various parameters in a study by Verma et al. using CU for 30 days. There was a 55% increase in phagocytic index, and a significant increase (16%) in neutrophil adhesion on regular use of whole freeze dried CU. CU has the potential to boost the immune functions by increasing the white blood cells counts and subsequently reducing the red blood cells count to a certain extent [56].
Traditional uses of CU
Some of the traditional uses of CU are in fever, where CU along with pepper, curd and ghee is used; in leprosy, CU is used along with dhruhardi and in deformities associated with leprosy, it is used with Nimbuchal, whereas in chronic leprosy, CU is used along with leaves of Vasaka and kanar, and bark of kuraila and neem [11]. Local traditional healers in Mandsaur prescribe CU for worm infestations, to develop immunity and to avoid aging. They suggest 10-25 ml of CU to be taken on an empty stomach for the same [15].
CONCLUSIONS
On analyzing the effect of different preparations of CU, FCU had better activity than CUD [27-32]. Activity of FCU and CUD from indigenous cows was similar to streptomycin and tetracycline. Ayurveda also mentions that FCU of indigenous cows’ is the best.
More well-planned studies in human subjects are required to fully assess its potential as an effective antimicrobial agent as most of the studies quoted are in vitro studies. Comparative studies between CU obtained from indigenous breeds and of inbred strains may be undertaken, as ayurveda was written when inbred strains of cows were not present. Future development of newer drugs can involve CU in its repository.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1. [Last accessed on 2015 Jan 15]. Available from: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf#page=11 .
- 2. [Last accessed on 2015 Jan 15]. Available from: http://www.who.int/mediacentre/factsheets/fs194/en/
- 3.Murray CK. Infectious disease complications of combat-related injuries. Crit Care Med. 2008;36:S358–64. doi: 10.1097/CCM.0b013e31817e2ffc. [DOI] [PubMed] [Google Scholar]
- 4.Shafer RW, Rhee SY, Bennett DE. Consensus drug resistance mutations for epidemiological surveillance: Basic principles and potential controversies. Antivir Ther. 2008;13(Suppl 2):59–68. [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan RS, Singh BP, Singhal LK. Immunomodulation with Kamdhenu ark in mice. J Immunol Immunopathol. 2001;3:74–7. [Google Scholar]
- 6.Bhadauria H. Cow urine- A magical therapy. Int J Cow Sci. 2002;1:32–6. [Google Scholar]
- 7.Chauhan RS, Garg N. Banglore, Karnataka: Indian Science Congress; 2003. Cow Therapy as an Alternative to Antibiotic. [Google Scholar]
- 8.Randhawa GK. Cow urine distillate as bioenhancer. J Ayurveda Integr Med. 2010;1:240–1. doi: 10.4103/0975-9476.74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randhawa GK, Kullar JS, Rajkumar Bioenhancers from mother nature and their applicability in modern medicine. Int J Appl Basic Med Res. 2011;1:5–10. doi: 10.4103/2229-516X.81972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarald E, Edwin S, Tiwari V, Garg R, Toppo E. Antioxidant and antimicrobial activities of cow urine. Glob J Pharmacol. 2008;2:20–2. [Google Scholar]
- 11.Mohanty I, Senapati MR, Jena D, Pallai S. Diversified uses of cow urine. Int J Pharm Pharm Sci. 2014;6:20–2. [Google Scholar]
- 12.Hu W, Murphy MR, Constable PD, Block E. Dietary cation-anion difference effects on performance and acid-base status of dairy cows postpartum. J Dairy Sci. 2007;90:3367–75. doi: 10.3168/jds.2006-515. [DOI] [PubMed] [Google Scholar]
- 13.Shaw SL, Mitloehner FM, Jackson W, Depeters EJ, Fadel JG, Robinson PH, et al. Volatile organic compound emissions from dairy cows and their waste as measured by proton-transfer-reaction mass spectrometry. Environ Sci Technol. 2007;41:1310–6. doi: 10.1021/es061475e. [DOI] [PubMed] [Google Scholar]
- 14.Achliya GS, Meghre VS, Wadodkar SG, Dorle AK. Antimicrobial activity of different fractions of cow urine. Indian J Nat Prod. 2004;20:14–6. [Google Scholar]
- 15.Jain NK, Gupta VB, Garg R, Silawat N. Efficacy of cow urine therapy on various cancer patients in Mandsaur District, India -A survey. Int J Green Pharm. 2010;4:29–35. [Google Scholar]
- 16.Kumar AA. Kanpur (U.P.): Thesis Submitted to the C.S.A. Univ Agr Techn; 2001. Study on Various Biochemical Constituents in the Urine of Cow, Buffalo and Goat; p. 13. [Google Scholar]
- 17.Badadani M, SureshBabu SV, Shetty KT. Optimum conditions of autoclaving for hydrolysis of proteins and urinary peptides of prolyl and hydroxyprolyl residues and HPLC analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:267–74. doi: 10.1016/j.jchromb.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Upadhyay RK, Dwivedi P, Ahmad S. Antimicrobial activity of photo-activated cow urine against certain pathogenic bacterial strains. Afr J Biotechnol. 2010;9:518–22. [Google Scholar]
- 19.Naotoshi K, Osamu Y, Yoshihiko S, Fuminobu M, Masahiro Y, Yoshimitsu M. Clinico-pathological findings in peripartum dairy cows fed anion salts lowering the dietary cation-anion difference: Involvement of serum inorganic phosphorus, chloride and plasma estrogen concentrations in milk fever. Jpn J Vet Res. 2007;55:3–12. [PubMed] [Google Scholar]
- 20.Türi M, Türi E, Kõljalg S, Mikelsaar M. Influence of aqueous extracts of medicinal plants on surface hydrophobicity of Escherichia coli strains of different origin. APMIS. 1997;105:956–62. [PubMed] [Google Scholar]
- 21.Chauhan RS, Singhal L. Harmful effects of Pesticides and their control through cowpathy. Int J Cow Sci. 2006;2:61–70. [Google Scholar]
- 22.Singh UP, Maurya S, Singh A, Nath G, Singh M. Antimicrobial efficacy, disease inhibition and phenolic acid-inducing potential of chloroform fraction of cow urine. Arch Phytopathol Plant Protect. 2012;45:1546–57. [Google Scholar]
- 23.Chauhan RS. Panchagavya therapy (cow pathy)- Current status and future directions. Indian Cow. 2004;1:3–7. [Google Scholar]
- 24.Singla S, Garg R. Cow urine: An elixir. Innov J Ayur Sci. 2013;1:31–5. [Google Scholar]
- 25.Kumar S. Analysis of cow's urine for detection of lipase activity and anti-microbial properties. J Pharm Biol Sci. 2013;7:1–8. [Google Scholar]
- 26.Pescheck-Böhmer F, Schreiber G. Urine Therapy: Nature's Elixir for Good Health. Rochester: Inner Traditions, Bear & Company; 1999. Healing yourself using urine; p. 152. [Google Scholar]
- 27.Minocheherhomji FP, Vyas BM. Study of the antimicrobial activity of cow urine and medicinal plant extracts on pathogenic human microbial strains. Int J Adv Pharm Biol Chem. 2014;3:836–40. [Google Scholar]
- 28.Rana R, De S. In vitro antimicrobial screening of cow urine – A potential natural anti-microbial agent. Int J Bioassays. 2013;2:436–9. [Google Scholar]
- 29.Ahuja A, Kumar P, Verma A, Tanwar R. Antimicrobial activities of cow urine against various bacterial strains. Int J Recent Adv Pharm Res. 2012;2:84–7. [Google Scholar]
- 30.Kekuda PT, Nishanth BC, Kumar PS, Kamal D, Sandeep M, Megharaj HK. Cow urine concentration: A potent agent with antimicrobial and anthelmintic activity. J Pharm Res. 2010;3:1025–7. [Google Scholar]
- 31.Tyagi PK, Tyagi S, Sarsar V, Pannu R. Cow urine: An antimicrobial activity against pathogens and their possible uses. Int J Pharm Res Sch. 2013;2:427–33. [Google Scholar]
- 32.Sarsar V, Selwal KK, Selwal MK, Pannu R, Tyagi PK. Evaluation of antibacterial activity of photoactivated cow urine against human pathogenic strains. Environ Exp Biol. 2013;11:201–3. [Google Scholar]
- 33.Shah CP, Patel DM, Dhami PD, Kakadia J, Bhavsar D, Vachhani UD, et al. In vitro screening of antibacterial activity of cow urine against pathogenic human bacterial strains. Int J Curr Pharm Res. 2011;3:91–2. [Google Scholar]
- 34.Sathasivam A, Muthuselvam M, Rajendran R. Antimicrobial activities of cow urine distillate against some clinical pathogens. Glob J Pharmacol. 2010;4:41–4. [Google Scholar]
- 35.Vats S, Kumar R, Negi S. Natural food that meet antibiotics resistance challenge: In vitro synergistic antimicrobial activity of Azadirachta indica, Terminalia chebula, Piper nigrum and photoactivated cow urine. Asian J Pharm Biol Res. 2012;2:122–6. [Google Scholar]
- 36.Yadav H, Yadav M, Jain S, Bhardwaj A, Singh V, Parkash O, et al. Antimicrobial property of a herbal preparation containing Dalbergia sissoo and Datura tramonium with cow urine against pathogenic bacteria. Int J Immunopathol Pharmacol. 2008;21:1013–20. doi: 10.1177/039463200802100427. [DOI] [PubMed] [Google Scholar]
- 37.Singh S. Cow urine has anti-leishmania donovani effect in vitro. Int J Cow Sci. 2005;1(2):72–3. [Google Scholar]
- 38.Biddle S, Teale P, Robinson A, Bowman J, Houghton E. Gas chromatography-mass spectrometry/mass spectrometry analysis to determine natural and post-administration levels of oestrogens in bovine serum and urine. Anal Chim Acta. 2007;586:115–21. doi: 10.1016/j.aca.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 39.Rajapandiyan K, Shanthi S, Murugan AM, Muthu GA, Singh AJ. Azadirachta indica - Cow urine extract, a novel controlling agent towards clinically significant multi drug resistant pathogens. J Appl Pharm Sci. 2011;1:107–13. [Google Scholar]
- 40.Kumar S. Analysis on the natural remedies to cure dandruff/skin disease-causing fungus - Malassezia furfur. Adv Bio Tech. 2013;12:1–5. [Google Scholar]
- 41.Vijayalakshmi R, Saranya VT. Effect of “Go-Mutra” on plant growth and its antifungal and antibacterial activity. J Herb Sci Technol. 2010;6:6–11. [Google Scholar]
- 42.Deshmukh SS, Rajgure SS, Ingole SP. Antifungal activity of cow urine. IOSR J Pharm. 2012;2(5):27–30. [Google Scholar]
- 43.Sanganal JS, Jayakumar K, Jayaramu GM, Tikare VP, Paniraj K, Swetha R. Effect of cow urine on wound healing property in wister albino rats. Vet World. 2011;4:317–21. [Google Scholar]
- 44.Maheshwari AK, Gupta AK, Das AK. Effect of cow urine on wounds. Indian Cow. 2004;1:19–24. [Google Scholar]
- 45.Kumar R, Kumar A, Kumar K, Gupta V, Shrivas T, Tripathi K. Synergistic anthelmintic activity of different compositions of panchagavya and Bauhinia variegate Linn. Int J Phytopharmacol. 2014;5:120–2. [Google Scholar]
- 46.Tambekar DH, Kerhalkar SA. Cow urine: A bioenhancer for antibiotic. Asian J Microbiol Biotechnol Environ Sci. 2006;8:329–33. [Google Scholar]
- 47.Khanuja SP, Kumar S, Shasany AK, Arya JS, Darokar MP. Use of bioactive fraction from cow urine distillate (‘Go-mutra’) as a bioenhancer of anti-infective, anti-cancer agents and nutrients. US Patent US 7235262. 2007 [Google Scholar]
- 48.Khanuja SPS, Kumar S, Shasany AK, Arya JS, Darokar MP, Singh M, et al. Pharmaceutical composition containing cow urine distillate and an antibiotic. US Patent US 6410059 B1. 2002 [Google Scholar]
- 49.Tatiraju DV, Bagade VB, Karambelkar PJ, Jadhav VM, Kadam V. Natural bioenhancers: An overview. J Pharmacogn Phytochem. 2013;2:55–60. [Google Scholar]
- 50.Chawla PC. Resorine a novel CSIR drug curtails TB treatment. CSIR News. 2010;60:52–4. [Google Scholar]
- 51.Poornima G, Abhipsa V, Rekha C, Manasa M, Kekuda PT. Antibacterial activity of combination of Polyalthia longifolia thw. extract, cow urine distillate and Streptomycin. Res J Pharm Tech. 2012;5:927–30. [Google Scholar]
- 52.Kumar A, Kumar P, Singh LK, Agrawal DK. Pathogenic effects of free radicals and their prevention through cowpathy. Indian Cow. 2004;6:27–34. [Google Scholar]
- 53.Ambwani S. PhD Thesis. Pantnagar, India: Submitted to Department of Biotechnology and Molecular Biology, College of Basic Sciences and Humanities, GBPAUT; 2004. Molecular studies on apoptosis in avian lymphocytes induced by pesticides. [Google Scholar]
- 54.Raja W, Agrawal RC. Chemopreventive potential of cow urine against 7, 12 dimethyl benz(a) anthracene-induced skin papillomasgenesis in mice. Acad J Cancer Res. 2010;3(1):7–10. [Google Scholar]
- 55.Dutta D, Devi SS, Krishnamurthi K, Chakrabarti T. Anticlastogenic effect of redistilled cow's urine distillate in human peripheral lymphocytes challenged with manganese dioxide and hexavalent chromium. Biomed Environ Sci. 2006;19:487–94. [PubMed] [Google Scholar]
- 56.Verma A, Kumar B, Manish KS, Kharya MD. Immunomodulatory potential of cow urine. Der Pharm Lettre. 2011;3:507–13. [Google Scholar]


