Abstract
Aim:
This study assessed within 4 days of suppressive test in vivo antimalarial activity of Ethanolic extract of root and stem bark of Cassia sieberiana DC against chloroquine sensitive strain of Plasmodium berghei NK65 in mice.
Methodology:
Two sets, each of five groups of four mice per each group were used. The groups of animals were administered with 100, 200, and 300 mg extract/kg body weight respectively, while positive control group were administered with 5 mg chloroquine/kg body weight and the negative control, were administered with 5 m1 distilled water/kg body weight. Oral acute toxicity was evaluated using up and down procedure.
Result:
Both the root and stem bark extract of C. sieberiana showed antimalarial property for suppressive tests. Chemo suppression of the root extract exerted significant (P < 0.05) dose-dependent reduction in the level of parasiteamia of 30.7%, 52.7%, and 55.8%. And from stem extract 17.6%, 38.0%, and 63.9% were recorded on mice when compared with 96.0% suppressive rate obtained from weight of chloroquine. The phytochemical screening of the plants root and stem bark extract revealed the presence of alkaloids, anthraquinones, flavonoids, triterpenoids, tannins, cardiac glycosides, saponins, reducing sugars and carbohydrates. The oral median lethal dose was determined to be >3000 mg/kg body weight.
Conclusion:
The acute toxicity results of this study showed that the plant parts used are assumed to be safe and has anti-plasmodial activity that can be explored for the management of malaria.
KEY WORDS: Anti-malaria, phytochemicals, suppressive test
INTRODUCTION
Cassia sieberiana (D.C.) belongs to the Family; Caesalpiniaceae Leguminosae, common name; African laburnum also called “Malga” in Hausa language, is a common plant in Saharan and sub-Saharan Africa. It is a savannah tree found in the dry areas of the forest and thickets [1]. It is often part of remedies in African veterinary and human therapies [2-4] particularly in north eastern and north western Nigeria where it is used for treatment of malaria, inflammatory conditions, rheumatism, jaundice, diarrhea, deworming, and aphrodisiacs [5]. The herb is usually available, affordable and acceptable to most of the consumers [6].
One of the major public health problems and greatest health challenges faced by 40% of the world’s population is the malaria disease, which is caused by Plasmodium species, and transmitted by the bite of female Anopheles mosquito. The disease has caused much suffering and premature death in the poorer region of tropics and subtropics [7]. It has been estimated that 1.2 billion population are at high risk of transmission (≥1 case per 1000 population), and half of these population live in the African region; of which 80% cases are strenuous in 13 countries, and over half in Nigeria, Congo, Tanzania, Kenya, and Ethiopia. A quarter of all malaria cases in Africa, is presented in Nigeria where disease transmission occurs all year round in the southern part of the country and seasonal in the north [8]. At present, the still leading Africa’s health problem is malaria, this occurs due to drug resistance to most anti-malarial drugs, insecticide resistance, war and civil disturbances, climatic changes, environmental changes, population increase and travel [8].
Therefore, the search for new anti-malarial compounds, either synthetic or natural is important for the killing of either the vector or parasite [9]. The use of plant-derived drugs for the treatment of malaria has a long and successful tradition [10,11]. For example, in the 1950’s quinine was isolated from Cinchona and artemisinin from quinghaosu [12]. This illustrates the potential value of investigating traditionally used anti-malarial plants for developing pharmaceutical anti-malarial drugs [13]. Unfortunately, chloroquine resistance now occurs throughout the whole world [14].
Certainly, native plants play an important role in the treatment of many diseases and 80% of people worldwide have been estimated to use herbal remedies [15]. However, information obtained on efficiency and safety of herbal use is few, despite the fact that motives of traditional practices could lead to novel strategies in malaria control. Different types of African plants used by traditional healers to treat and cure malaria symptoms have already been tested [16]. The increased number of drug-resistant malaria parasites, such as chloroquine-resistant malaria parasite has made the development of novel antimalarials urgent, and the high cost of malaria treatment has left the poor masses of Nigeria heavily reliant on traditional practitioners and medicinal plants for the treatment of the disease. For this study, the investigations on rodent malaria models for the antimalarial activity of C. sieberiana was carried out to verify the use experientially of the root and stem plant parts as ingredients in the traditional remedies for the treatment of malaria in aboriginal Northern Nigeria. With this view, the present study was executed to provide scientific evidence of its efficacy and continuous use in ethno therapeutic management of malaria.
METHODOLOGY
This study evaluates the in vivo antiplasmodial effect of ethanolic extract of C. sieberiana on mice. Stem bark and root of C. sieberiana were collected from Dundaye village of Usmanu Danfodiyo University, Sokoto State, Nigeria. The plant was identified and botanically authenticated at the herbarium unit, Department of Pharmacognosy Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria. A voucher specimen (pcg/udus/caes/0010) was prepared and deposited for future reference at the Pharmacognosy herbarium unit, Usmanu Danfodiyo University Sokoto. The root and stem samples were cleaned, air-dried and pounded into a fine powder using mortar and pestle. The powder was then stored in a dry air-tight container. Extraction was carried using soxhlet apparatus. The soxhlet method of extraction [17] was employed in extracting the plant material using ethanol. The extraction was carried out by placing 400 g of each of the powdered plant part in the upper chamber in a trimble and 700 ml of the solvent in the bottom of the flask. After the plant was extracted, the vapor was condensed to extract the sample to dryness using steam evaporator to obtain the crude extract of the plant. The dried ethanol crude extracts were weighed and stored in the refrigerator at 4°C in airtight plastic container until use for this study. The extracts were weighed and dissolved in distilled water for preparation of appropriate doses on each day of the experiment.
Phytochemicals Screening
A combination of several methods was used to identify the phytochemicals of the root and stem part. Standard screening tests using conventional protocol, procedure and reagents were used for detecting the major secondary metabolites present in the plant [18].
Experimental Animal
Sixty two mice of both sexes of Swiss Albino mice having body weight of 16-33 g, were obtained from Laboratory Animal Housing Unit of National Veterinary Research Institute (NVRI), VOM, Jos. They were kept at the Animal Housing Unit of the Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto, Nigeria. The consent of the ethical committee was obtained from the veterinary research and ethical committee on the use of Laboratory animal for research purposes of Usmanu Danfodiyo University Sokoto, Nigeria. The animals were housed in standard cages for 5 days prior to dosing to allow for acclimatization to the laboratory conditions in accordance with the National Institute of Health guideline for the care and use of Laboratory animals [18].
Rodent Parasite Used
The rodent parasite Plasmodium berghei NK 65 inoculated in four donor mice was sourced from the Department of Pharmacology and Toxicology, National Institute of Pharmaceutical Research and Development (NIPRD), Idu Abuja, Nigeria, and were kept at the Animal housing unit of the Department of Phamarcology and Toxicology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto, Nigeria. Prior to commencing the study, four of the infected mice (donor mice) were kept and observed to reproduce disease symptoms similar to malarial human infection [19]. The donor mice (parasitaemia of about 20-30%) were anesthetized with chloroform, and their infected blood were collected via cardiac puncture with a pre-heparinized sterile syringe and needle. To avoid variability in parasitemia, the blood collected was pooled together. The collected blood was then diluted with 0.9% physiological saline, and the dilution was made based on the parasitemia of the donor mice and the red blood cell (RBC) count of averagely healthy mice (4.5×109 RBC/ml) [20] in such a way that 0.2 ml of blood contains 1×107 infected erythrocytes. Each mouse was administered intra-peritoneally with 0.2 ml of this diluted blood, which contains 1×107 P. berghei infected erythrocytes. The parasites were kept alive by continuous intra-peritoneal passage in mice every four days, and the re-infected mice were used for the study [21].
Acute Toxicity Studies
Acute toxicity of C. sieberiana ethanolic root and stem bark extract was carried out using up and down procedure to test the oral acute toxicity on two groups of five female mice which were selected at random and marked to permit individual identification. To conduct the limit test, food (excluding water) was withheld from the mice for 3 h. The fasted body weight of each animal was determined, and the dose was calculated according to the body weight. Water extract of crude extract of the leaves and fruits was administered orally in concentrations of 2000 mg/kg, body weights dose to five groups of animals (each consisting of one animal) using a stomach tube one after the other at the grace observation period of 24 h, 48 h and up to 14 days in a single oral dose while the control group received distilled water only. After the substance has been administered, food was withheld for a further 3-4 h and observation of toxic symptoms was made and recorded systematically at 1, 2, 4 and 6 h after administration. Finally, the number of death and survivors was noted after 24 h, 48 h and up to 14 days for each animal. The dosed mice were observed for the first 1, 2, 4 and 6 h after administration and intermittently for the next 24 h for signs of toxicity. The second mouse was dosed at 24 h interval until all five mice were dosed. The control group received distilled water only. And all five mice were observed thereafter for the next fourteen days for any delayed toxic effect. Observation of toxic symptoms was made and recorded systematically at 1, 2, 4 and 6 h after administration. Finally, the number of death and survivors was noted after 24 h, 48 h and up to 14 days for each animal. The toxicological effect was assessed on the basis of toxicity sign: paw licking, salivation, stretching of the entire body, weakness, sleep, respiratory distress, tremor, off-feed, depression coma and mortality, which were expressed as lethal doze 50 (LD50) [22].
Anti-plasmodial Analysis
The Peter’s 4-day suppressive test against Chloroquine sensitive P. berghei (NK 65) infection in mice was employed [23]. Twenty albino mice of both sexes were inoculated. They were randomly grouped with the same number of male and female and four mice in each group. The animal were then administered extract daily for 4 consecutive days. On day 1 (D0); heparinized blood was prepared from the donor mouse as explained earlier, treatment started 2 h after the mice were infected with the parasite. Group 1 that served as control was orally administered with 1 ml/kg body weight of normal saline, Groups 2, 3 and 4 were orally administered with 100, 200 and 300 mg extract/kg body weight daily respectively, while group 5 was administered with 5 mg chloroquine/kg body weight orally [23]. On Day 2-4 (D1-D3); the mice were treated again with the same doses and through the same route as in D0 at interval of 24 h, 48 h and 72 h post-infection [24]. On the 5th day (D4), blood was collected from the tail of each mouse and spread on a microscope slide to make a thin film. The blood films were fixed with methanol for 1 min stained with 3% Giemsa solution for 30 min and examined microscopically [25]. Eight fields containing 250 erythrocyte and the number of parasitized erythrocytes were recorded while the suppression of parasitemia was expressed as percent for each dose, by comparing the parasitemia in the control group with the treated one. The mice were observed for signs of toxicity after treatment for the first 4 (critical) h, then over a period of 24 h, thereafter daily for 7 days. Mortality occurring at a particular dose will indicate either to continue administration of subsequent higher dose or to estimate the LD50 by comparing the mortality to a fixed LD50 cut-off values provided in the guideline [26].
Data Analysis
Results were expressed as mean ± standard error of the mean. The data obtained were entered and analyzed using Analyze-it version 2.22 Excel 12+ statistical package. Chi-square test at a 95% confidence level was used to compare the result and values of P < 0.05 were considered as statistically significance.
RESULTS
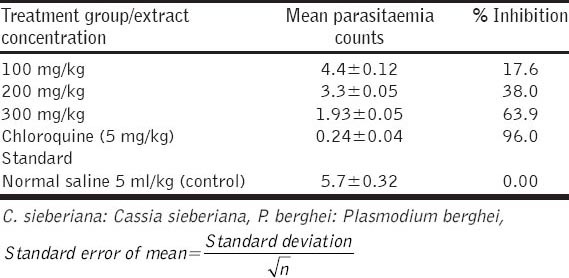
The results obtained showed significant decrease in parasitaemia of P. berghei infected mice treated with the ethanolic root and bark extract of C. sieberiana [Tables 1 and 2]. This significant suppression of parasitaemia observed was dose dependent (P < 0.05). The crude root extract (300 mg/kg) caused 55.8% suppression and the crude stem extract (300 mg/kg) caused 63.9% suppression in parasitaemia of P. berghei infected mice while chloroquine a standard antimalarial drug (5 mg/kg) exerted 96% suppression.
Table 1.
Antiplasmodial suppressive test of ethanolic root extract of C. sieberiana (suppressive test on P. berghei)
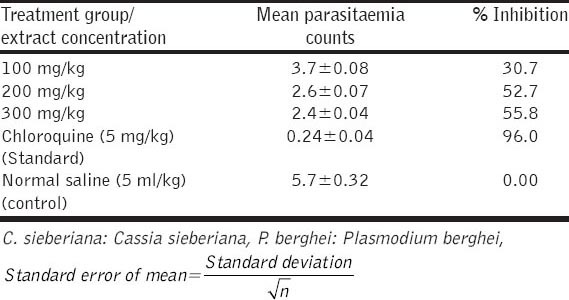
Table 2.
Antiplasmodial suppressive test of ethanolic stem extract of C. sieberiana (suppressive test on P. berghei)
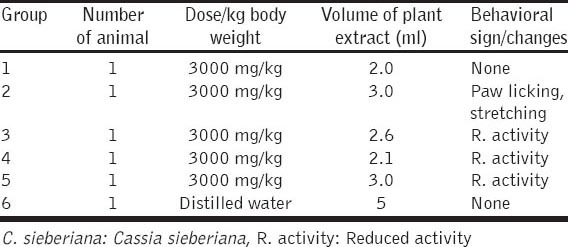
The oral median LD50 of the extract was estimated to be ≥2000 mg/kg in mice. There were no remarkable behavioral changes in the extract administered mice (reaction to food supply, contact and noise), though activity was reduced in some of the extract administered groups within the first 4 h. And no mortality occurred within the observation period of 14 days. However, behavioral signs of toxicity were observed in mice given 2000 mg stem extract/kg body weight which include; paw licking, salivation, stretching and reduced activity. There was however no mortality at all the dose levels used. And the oral median LD50 was estimated to be ≥2000 mg extract/kg body weight [Tables 3 and 4].
Table 3.
Acute toxicity study of ethanolic root extract of C. sieberiana
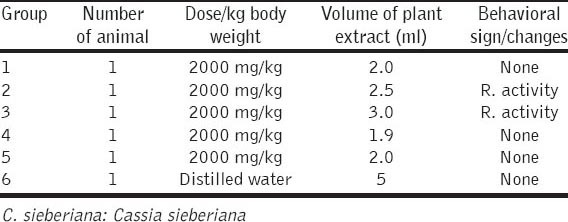
Table 4.
Acute toxicity study of ethanolic root extract of C. sieberiana
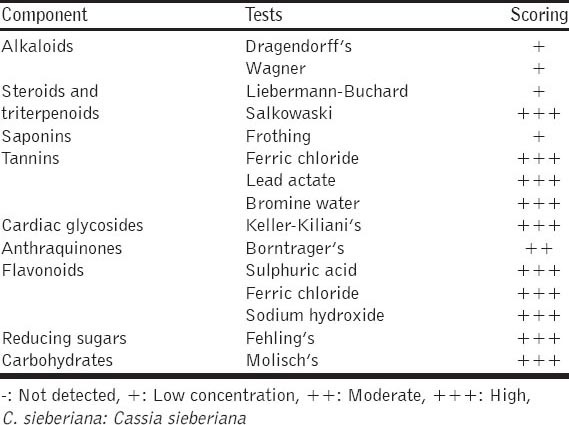
The result from the phytochemical analysis of ethanolic root bark extract of C. sieberiana showed; high amount of Carbohydrates using Molisch’s test, high amount of Reducing Sugars using Fehling’s test, high amount of Tanins using Ferric chloride test, Lead Actate test and Bromine water test, high amount of Flavonoids using Sulfuric acid test and Ferric chloride test, high amount of Saponins using Frothing test, high amount of Steroids and Triterpenoid using Liebermann–Buchard test and Salkowaski test, and also high amount of Cardiac Glycosides using Keller-Kiliani’s test, using Borntrager’s test Anthraquinones was in moderate amount while using Dragendorff’s test and Wagner’s test Alkaloids scored low [Table 5]. The ethanolic stem bark extract of C. sieberiana indicated; high amount of flavonoids in Sulfuric acid test and Ferric chloride test, high amount high amount of reducing sugars in Fehling’s test, high amount of carbohydrates in Molisch’s test, high amount of cardiac glycosides in Keller-Kiliani’s test, high amount of tannins in Ferric chloride test, Lead Actate test and Bromine water test, in Salkowaski’s test, Steroids and Triterpenoids were high but in Liebermann–Buchards test they were both low, there was moderate amount of Antraquinones in Borntrager’s test and Saponins and Alkaloids were low using Frothing’s test, Dragendorff’s test and Wagner’s, respectively [Table 6].
Table 5.
Phytochemical analysis of ethanolic root extract of C. sieberiana
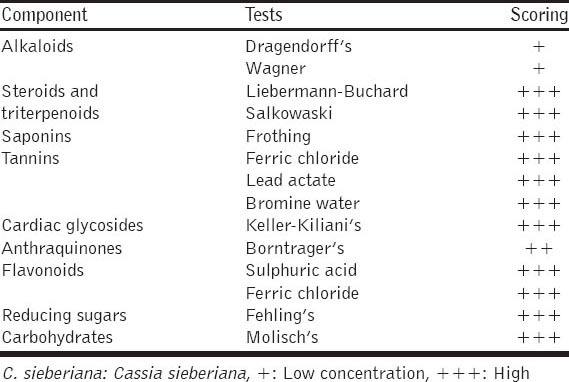
Table 6.
Phytochemical analysis of ethanolic stem extract of C. sieberiana
DISCUSSION
The four-day suppressive test which is a standard test commonly used for antimalarial screening and determination of percentage inhibition of parasitaemia in laboratory animal as used in this study indicated a mean group parasitaemia level of less than or equal to 96% of the mock-treated control animals given a suggestion that the test material is active in standard screening studies [23]. It has been reported that when a standard antimalarial drug is used on mice infected with P. berghei, it suppresses parasitaemia to non-detectable levels [27]. The observed antimalarial activity is consistent with the traditional use of the plant as an herbal medication against the disease in Nigeria. The extracts exerted significantly repository effect in mice treated with 100, 200 and 300 mg/kg body weight respectively [Tables 3 and 4]. This effect was however lower in groups that received low dose. This effect may be due to short duration of action of the extract occasioned by rapid metabolism, and so parasite clearance could not be total. It may also be explained by the fact that not all anti-malarials are completely active against P. berghei model [28]. The rodent model of malaria that was employed in this study for prediction of efficacy of anti-malarial effect of C. sieberiana root and stem bark extract, was also used for conventional antimalarial agents such as chloroquine, halofantrine, mefloquine and more recently artemisinin derivatives [29]. P. berghei are used in the prediction of treatment outcomes. Hence it was an appropriate parasite for the study. Since this parasite was sensitive to chloroquine, this drug was used as the standard drug in this study. The choice of 4 weeks old male mice for the study was done to avoid the effect of anemia in the old mice and the effect of possible physiological changes associated with ageing and gender may induce on the treatment outcome [30]. An in vivo model was employed for this study because it takes into account possible prodrugs effect and possible involvement of the immune system in eradication of infection [20]. Certain compounds form the basis of the pharmacologic effects of such plants [31,32]. Presence of alkaloids ranks among the most efficient and therapeutically significant plant compounds, moreover pure alkaloids and their synthetic derivatives are used as basic medicinal agents e.g. morphine is an analgesic, quinine is antiplasmodial, colchicines is used for gout, reserpine is a tranquilizer, vincristine and vinblastine have antitumor effects [31], terpenes, anthraquinones, and flavonoids screened plants has been implicated in antiplasmodial activity [33,34]. Earlier studies [35], reported the oxidant generation potential of Acacia nilotica extract, based on the ability of the extract to increase conversion of reduced glutathione to oxidized glutathione. Increased oxidation has also been shown to create an intracellular environment that is unfavorable to plasmodial growth [36,37]. The mechanism of action of artemisinin, which depends on oxidant action for its potent antimalarial activity, validates this [35]. However, lack of oxidizing action in some plants does not rule out anti plasmodial activity since they may be active through other biochemical mechanisms. The oral median LD of ≥3000 mg/kg body weight obtained for the Ethanolic root and stem bark extract of C. sieberiana DC is 31 times greater than the minimum effective dose of 100 mg/kg. Earlier reports have shown that if the median LD of a test substance is three times more than the minimum effective dose, the substance is considered a good candidate for further studies. It was also reported that oral administration is about 100 times less toxic than the intra-peritonial [38]. These findings suggested that the extract could be safe, and this partly explain the safe use of the plant by the local people who have been using it in traditional management of malaria in Nigeria.
CONCLUSIONS
It is evident based on these findings that C. sieberiana possess potent anti-plasmodial effect justifying its folkloric usage in the management of anti-malarial. However, the active principle(s) are yet to be identified, and there is a need for the identification. In view of this fact, attempts are being made to carry out anti-plasmodial curative test, prophylactic test as well as guided fractionation of the root and stem ethanolic extract to isolate the active compounds and also to test for the cytotoxicity of the extract.
ACKNOWLEDGMENTS
The authors are thankful to SQN LD Aminu B, for financial support, to Dr. Bulus (NIPRD), Patrick M, for practical support. We are grateful to the Community of Usmanu Danfodiyo University Sokoto, for providing facilities during the course of the study and for their permission to publish the data. We are also thankful to National Institute for Pharmaceutical Resaerch Ndu, Abuja for support and funding.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Geary TG, Divo AA, Bonanni LC, Jensen JB. Nutritional requirements of Plasmodium falciparum in culture. III. Further observations on essential nutrients and antimetabolites. J Protozool. 1985;32:608–13. doi: 10.1111/j.1550-7408.1985.tb03087.x. [DOI] [PubMed] [Google Scholar]
- 2.Kasonia K. Thèse Doct’es Sci. Belgium: Vét. Univ. Liége; 1998. Une reconnaissance des saviors paysans: Plantes medicinales et médicinales vétérinaire traditionnelle d’afrique centrale; p. 279. [Google Scholar]
- 3.Sy GY, Fall AD, Diatta W, Gueye M, Badji K, Bassène E, et al. Analgesic and anti-inflammatory activity of aqueous root extract of Cassia sieberiana D.C. (Caesalpiniaceae) Afr J Pharm Phamarcol. 2009;3:651–3. [Google Scholar]
- 4.Tamboura HH, Bayala B, Lompo M, Guissou IP, Sawadogo L. Ecological distribution, morphological characteristics and acute toxicity of aqueous extract of Holarrhena floribunda, Leptadenia hastata and Cassia sieberiana (D.C.) used by veterinary healers in Burkina Faso. Afr J Trop Complement Alternat Med. 2005;2:13–24. [Google Scholar]
- 5.Obidah W, Sa’ad UA, Wurochekke AU. Toxic effects of aqueous stem bark extract of Cassia sieberiana on some biochemical parameters in rats. Afr J Biochem. 2009;3:229–31. [Google Scholar]
- 6.Sofowora A. Nigeria: Association with Spectrum Books Limited Ibadan; 1985. Medicinal Plants and Traditional Medicine in Africa; pp. 142–5. [Google Scholar]
- 7.Greenwood BM, Fidock DA, Kyle DE, Kappe SH, Alonso PL, Collins FH, et al. Malaria: progress, perils, and prospects for eradication. J Clin Invest. 2008;118:1266–76. doi: 10.1172/JCI33996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. World Malaria Report. Vol. 34. Geneva, Switzerland: World Health Organization; 2008. [Last accessed on 2014 Jun 22]. pp. 22–7. [Google Scholar]
- 9.Asase A, Kokubun T, Grayer RJ, Kite G, Simmonds MS, Oteng-Yeboah AA, et al. Chemical constituents and antimicrobial activity of medicinal plants from Ghana: Cassia sieberiana, Haematostaphis barteri, Mitragyna inermis and Pseudocedrela kotschyi. Phytother Res. 2008;22:1013–6. doi: 10.1002/ptr.2392. [DOI] [PubMed] [Google Scholar]
- 10.Dapper DV, Aziagba BN, Ebong OO. Antiplasmodial effects of the aqueous extract of Phyllantus amarus Schumach and Thonn against Plasmodium berghei in Swiss albino mice. Niger J Physiol Sci. 2007;22:19–25. doi: 10.4314/njps.v22i1-2.54857. [DOI] [PubMed] [Google Scholar]
- 11.Adebayo JO, Krettli AU. Potential antimalarials from Nigerian plants: a review. J Ethnopharmacol. 2011;133:289–302. doi: 10.1016/j.jep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Rieckmann K. A bioassay for derivatives of qinghaosu (artemisinin) Trop Med Parasitol. 1992;43:195–6. [PubMed] [Google Scholar]
- 13.Srisilam K, Veersham C. Nishan I, Khanu A, editors. Antimalarials of plant origin. Role of Biotechnology in Medicinal and Aromatic Plants. 2003;7:17–4. [Google Scholar]
- 14.Phillipson JD, Wright CW. Can ethnopharmacology contribute to the development of antimalarial agents? J Ethnopharmacol. 1991;32:155–65. doi: 10.1016/0378-8741(91)90113-r. [DOI] [PubMed] [Google Scholar]
- 15.Benoit-Vical F, Valentine A, Peletier Y. Differential antimalarial activity in vitro of root and bark extracts of Nauclea latifolia. J Ethanpharmocol. 1998;61:173–8. doi: 10.1016/s0378-8741(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 16.Jensen E, William B. The Origin of the Soxhlet Extractor. J Chem Educ. 2007;84:1913–4. [Google Scholar]
- 17.Trease A, Evans WC. 13th ed. London: Bailiere Tindall; 1989. Trease and Evans Pharmacognosy; pp. 342–83. [Google Scholar]
- 18.National Institute of Health (NIH). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Academic Press; 1985. pp. 155–61. [Google Scholar]
- 19.English MC, Waruiru C, Lightowler C, Murphy SA, Kirigha G, Marsh K. Hyponatraemia and dehydration in severe malaria. Arch Dis Child. 1996;74:201–5. doi: 10.1136/adc.74.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waako PJ, Katuura E, Smith P, Folb P. East African medicinal plants as source of lead ycompounds for the development of new antimicrobial drugs. Afr J Ecol. 2007;45:102–6. [Google Scholar]
- 21.Adzu B, Haruna AK, Salawu OA, Katsayal UA, Njan A. In vivo antiplasmodial activity of ZS-2A: A fraction from chloroform extract of Zizyphus spina-christy root bark against P. berghei berghei in mice. Int J Bio Chem Sci. 2007;1:281–6. [Google Scholar]
- 22.Organization for Economic Cooperation and Development (OECD) Oral toxicity study in rodents. Acute Oral toxicities: Up and Down Procedure. Guideline for the Testing of Chemicals. Vol. 42. OECD; 2001. pp. 1–26. [Google Scholar]
- 23.Peter IT, Anatoli VK. Malaria Parasite Biology, Pathogenesis, and Protection. WDC, USA: ASM Press; 1998. The current global malaria situation; pp. 11–22. [Google Scholar]
- 24.Madara AA, Ajayi JA, Salawu OA, Tijani AY. Anti-malarial activity of ethanolic leaf extract of Piliostigma thonningii Schum. (Caesalpiniacea) in mice infected with Plasmodium berghei berghei. Afr J Biotechnol. 2010;9:3475–80. [Google Scholar]
- 25.Monica C. 2nd ed. New York: Cambridge University Press; 2005. District Laboratory Practice in Tropical Countries Part 1; pp. 249–51. [Google Scholar]
- 26.Ettling M, Bloland PB, Ruebush TK. Vol. 3. Washington, D.C: EHPAR; 1991. Chloroquine Efficacy Study in Zambia; pp. 213–5. [Google Scholar]
- 27.Kamei K, Matsuoka H, Furuhata SI, Fujisaki RI, Kawakami T, Mogi S, et al. Anti-malarial activity of leaf-extract of hydrangea macrophylla, a common Japanese plant. Acta Med Okayama. 2000;54:227–32. doi: 10.18926/AMO/32291. [DOI] [PubMed] [Google Scholar]
- 28.Dow GS, Reynoldson JA, Thompson RC. Plasmodium berghei: in vivo efficacy of albendazole in different rodent models. Exp Parasitol. 1998;88:154–6. doi: 10.1006/expr.1998.4238. [DOI] [PubMed] [Google Scholar]
- 29.Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004;3:509–20. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 30.Pierrot C, Adam E, Lafitte S, Godin C, Dive D, Capron M, et al. Age-related susceptibility and resistance to Plasmodium berghei in mice and rats. Exp Parasitol. 2003;104:81–5. doi: 10.1016/s0014-4894(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 31.Haidet A. The medicinal value of the rainforest. Final Paper on Tropical Field Courses Submitted to the Department of Interdisciplinary Studies. USA: Miami University; 2003. pp. 21–3. [Google Scholar]
- 32.Jigam AA, Akanya HO, Adeyemi JJ. Antimicrobial and antiplasmodial effects of Momordica balsamina. Niger J Nat Med. 2004;8:11–2. [Google Scholar]
- 33.Phillips RE, Warrell DA, White NJ, Looareesuwan S, Karbwang J. Intravenous quinidine for the treatment of severe falciparum malaria. Clinical and pharmacokinetic studies. N Engl J Med. 1985;312:1273–8. doi: 10.1056/NEJM198505163122001. [DOI] [PubMed] [Google Scholar]
- 34.Kremsner PG, Winkler S, Brandts C, Neifer S, Bienzle U, Graninger W. Clindamycin in combination with chloroquine or quinine is an effective therapy for uncomplicated Plasmodium falciparum malaria in children from Gabon. J Infect Dis. 1994;169:467–70. doi: 10.1093/infdis/169.2.467. [DOI] [PubMed] [Google Scholar]
- 35.Etkin NL. Antimalarial plants used by Hausa in northern Nigeria. Trop Doct. 1997;27(Suppl 1):12–6. doi: 10.1177/00494755970270S106. [DOI] [PubMed] [Google Scholar]
- 36.Borris RP, Schaeffer JM. Phytochemical Resources for Medicine and Agriculture. New York: Plenum; 1992. Antiparasitic agents from plants; pp. 117–58. [Google Scholar]
- 37.Levander OA, Ager AL. Malaria parasites and oxidant nutrients. Parasitology. 1993;107:95–106. doi: 10.1017/s0031182000075533. [DOI] [PubMed] [Google Scholar]
- 38.Jutamaad NS, Aimmon S, Yodhtai T. Toxicological and antimalarial activity of the eurycomalactone and Eurycoma longifolia back extract in mice. Thai J Phytopharm. 1998;5:14–27. [Google Scholar]


