Abstract
Objective:
Increased oxidative stress is associated with the progression of diabetic mellitus. In the present study, we investigated the effects of the ethanolic extract of Nigerian propolis (N. propolis) on markers of oxidative stress, histology of the liver and pancreas and glycaemia in alloxan-induced diabetic rats.
Materials and Methods:
Alloxan-induced hyperglycemic Wistar rats were treated with either metformin (150 mg/kg/d) or N. propolis (200 mg/kg/d and 300 mg/kg/d) for 28 days. At the end of the treatment period, the rats were sacrificed; blood was collected for biochemical analysis while their pancreases and liver were excised and processed for histological studies.
Results:
Serum oxidative stress markers and blood glucose concentration were compared between the treated and control rats. In contrast to the non-treated diabetic rats, blood glucose concentration were not significantly different between treated rats and control (P < 0.05) at 28 days of treatment with N. propolis and metformin. Serum malondialdehyde levels was reduced while superoxide dismutase levels were elevated in the N. propolis group; these levels were converse in the diabetic group, these differences are statistically significant (P<0.05) when compared with the control. Histologically, there was improvement in the treated group compared to the untreated group.
Conclusion:
These findings suggest that the N. propolis confers protection against hyperglycemia-induced oxidative stress in both liver and pancreas of adult Wistar rats.
KEY WORDS: Diabetes, liver, metformin, Nigerian propolis, oxidative stress, pancreas
INTRODUCTION
Diabetic mellitus (DM) has long been defined as a heterogeneous group of metabolic disorders characterized by glucose intolerance and fasting hyperglycemia [1]. Type 1 DM accounts for approximately 10% of all cases and it is essentially immune-mediated with Type 2 DM afflicting about 6% of adult population, and it is essentially a result of insulin resistance and impaired β-cell function [2]. Besides fasting hyperglycemia, several organs develop complications in diabetes. Key organs in this respect include the kidney (nephropathy), eye (retinopathy), brain and peripheral nerves (neuropathy), liver (glycogen storage disease and steatohepatitis), blood vessels (atherosclerosis and microangiopathy) [3-5].
In long-standing DM, the morphology and function of the liver are disturbed. Liver biopsy findings in Type 1 diabetics with hepatomegaly are comparable to hepatic findings in Mauriac’s syndrome and include marked glycogen deposition in hepatocytes [6]. Clark and Diehl [7] reported that in Type 2 diabetes, impaired insulin action usually result in non-alcoholic fatty liver disease, including steatosis and steatohepatitis.
There is ample evidence of an important role of oxidative and glycooxidative stress in the pathogenesis of diabetic complications [8]. The exact contribution of antioxidant enzyme to oxidative stress in diabetes is not fully understood. Learning and memory impairment has also been associated with oxidative stress in streptozotocin-induced diabetes rats [9].
Propolis is a natural product derived from plant resins and collected by honeybees (workers) to be used as glue and as draught-extruder for beehives [10]. Honey another product derived from honeybees was almost the only source of sugar available to the ancients and was valued for its medicinal benefits. It has been reported that propolis contain at least 200 compounds with more than 100 being present in any given sample [11]. These include fatty and phenolic acids and esters, substituted phenolic esters, bioflavonoids (flavones, flavanones, flavonols and others), terpenes, β-steroids, aromatic aldehydes and alcohols, and derivatives of sesquiterpenes, naphthalene and stilbenes [12-14].
The main types of flavonoids that have been reported include rutin (an antihypertensive agent), quercetin (a potent anti-diabetic material), galangin [15] and caffeic acid phenethyl ester [16]. These entire constituents give propolis the ability to perform many functions. Propolis is reported to have hepatoprotective [17], antioxidant [18], antimicrobial [19], anti-inflammatory [19] and anticancer [20] properties.
Several factors is responsible for the variability in the propolis chemical components which includes: Unpredictability of growing plant species around the beehive, condition of the climate, beekeeper actions and nature of the soil [21]. Propolis has been reported to be geographically sensitive and each area has its own peculiar constituent [10]. Gómez-Caravaca et al. [22] reported that despite geographical differences, most propolis sample contain 50% resin, 30% wax, 10% essential oils, 5% pollen and 5% of other organic compounds in their chemical composition. Several studies have been carried out to examine the effects of propolis from different geographical regions on experimentally induced diabetes especially from the Arab world, Brazil and Croatia, but little or no information is available on the anti-diabetic properties of Nigerian propolis (N. propolis).
Currently, botanical drugs are being screened for their efficacy, safety and dosage in the management of DM because they are cheap and readily available. In this work, we report the blood glucose responses of hyperglycemic rats to a regimen of N. propolis. Besides this, histology of the liver and pancreas, activities of hepatic alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) and the antioxidant superoxide dismutase (SOD) and malondialdehyde (MDA) were reported.
MATERIALS AND METHODS
Animals
A total of thirty adult male Wistar rats were bred at the animal holdings of the Department. Animals weighed between 190 and 230 g and were 8 weeks old at the start of the experiment. They were exposed to 12 h light, 12-h dark cycle at 22-24°C. All animals were maintained on a pelletized growers feed (Flour Mill Ltd., Ibadan, Nigeria). Rat pellets and water were given freely. Study was performed in accordance with the ethical guidelines stipulated by the Ethical Committee of the College of the institution. These guidelines were in accordance with the internationally accepted principles for laboratory animal use and care.
Collection and Extraction of Propolis
N. propolis was purchased from the Federal University of Agriculture, Abeokuta, Ogun state in Nigeria. Raw propolis was obtained by scraping it off its hive frames. Ethanolic extract was prepared according to the method described by Ivan et al. [23].
2.3 Induction and assessment of DM
To induce hyperglycemia, 25 animals were fasted overnight. Diabetes was induced by single intra-peritoneal (ip) injection of alloxan monohydrate (100 mg/kg) (Sigma, St. Louis, USA) in sterile normal saline (0.9%). Five Control animals were injected intra-peritoneally with citrate buffer alone at a single dose of 1.2 ml/kg. All animals were allowed free access to feed and water after alloxan injection, and they were left undisturbed for a minimum of 72 h for hyperglycemia to develop [24]. Animals were identified as diabetic on the basis of blood glucose levels (higher than 230 mg/dL) in tail blood using a one touch ultra mini Glucometer (LifeScan Inc., Milpitas, USA) 3 days after alloxan treatment. Weekly record of blood glucose level was taken afterwards.
Administration of Drugs
A total of 25 adult Wistar rats (twenty surviving diabetic rats and five normal rats) were randomly assigned into one of the following treatment groups of five animals each: Control, diabetic, diabetic + N. propolis (200 mg/kg, p.o.), diabetic + N. propolis (300 mg/kg, p.o.) and diabetic + metformin. Metformin (Merck, Germany) was given orally at 150 mg/kg. Administration of both drugs lasted for 28 days and was carried out at 09:00-10:00 daily.
Blood Glucose, Feed Intake and Body Weight (BW)
A One Touch Ultra Mini Glucometer (LifeScan Inc., Milpitas, USA) was used to estimate the blood glucose of treated and control animals. Blood was obtained from the dorsal vein of the animals. Blood glucose was estimated at day 0 and at 1, 3, 5, and 7 h after the first dose of the extract. Thereafter, measurement was done twice a week for 4 weeks. Feed intake was monitored on a daily basis during the experimental period (data not show). BW of the animals was also recorded twice a week.
Termination of Treatment
All animals were fasted and killed under diethyl ether anesthesia 24 h after the last treatment day. Blood was collected into heparinized tubes and centrifuged at 3000 r/m for 20 min in a desktop centrifuge model 90-1 (Jiangsu Zhangji Instruments Co., China). Plasma was stored at −20°C until analyzed. Laparotomy was performed on each animal; the liver and pancreas were excised, rinsed in normal saline and fixed in Bouin’s fluid.
Histological Studies
The livers and pancreases fixed in Bouin’s fluid were processed and stained with hematoxylin and eosin for histological studies. Photomicrographs were taken with a JVC color video digital camera (JVC, China) mounted on an Olympus light microscope (Olympus UK Ltd, Essex, UK).
Assessment of Liver Function
Biochemical analysis of the serum enzymes for AST and ALT was by the method of Reitman and Frankel [25].
Markers of Oxidative Stress Assessment
Serum level of MDA and SOD were assayed by the method of Ohkawa et al. [26] and Misra and Fridovich [27] respectively.
Determination of Serum Lipids
Triglycerides (TG) and total cholesterol (CHOL) were determined by enzymatic methods according to Diniz et al. [28] using commercial diagnostic kits (Randox, UK).
Statistical Analysis
Data obtained were presented as mean ± standard error of mean and analyzed for statistical significance using one-way analysis of variance, followed by Waller-Duncan post-hoc test. P < 0.05 was considered statistically significant.
RESULTS
To evaluate the effect of the ethanolic extract of N. Propolis on alloxan-induced diabetic rats, several biochemical estimations were carried out in all experimental animals for the estimation of plasma glucose, serum cholesterol, serum TG, liver function tests and oxidative stress markers. The histology of the liver and pancreas was also compared. The following pharmacological effects were observed:
Effect on Hyperglycemia
The mean blood glucose level of normal control rats fed on a normal diet was almost invariable throughout the experimental study. On the contrary, the blood glucose level of diabetic untreated rats was significantly increased (P < 0.05) when compared with the normal control group. When alloxan-induced diabetic rats were treated with metformin (150 mg/kg) and N. propolis at doses of 200 and 300 mg/kg, lowering in blood glucose was observed from the second week onwards when compared to the diabetic control group [Figure 1].
Figure 1.
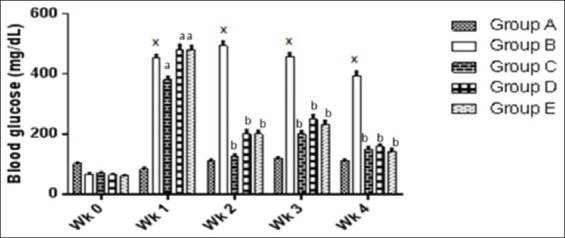
Showing and comparing weekly changes in the blood glucose level of the treated groups compared to the diabetic control group. *P < 0.05 significantly different from non-diabetic control group, aP < 0.05 significantly different from normal control group, bP < 0.05 significantly different from diabetic untreated group. A: Non-diabetic control, B: Diabetic control, C: Diabetic treated (150 mg/kg metformin), D: Diabetic treated (200 mg/kg Nigerian propolis [N. propolis]), E: Diabetic treated (300 mg/kg N. propolis)
Effect on BW
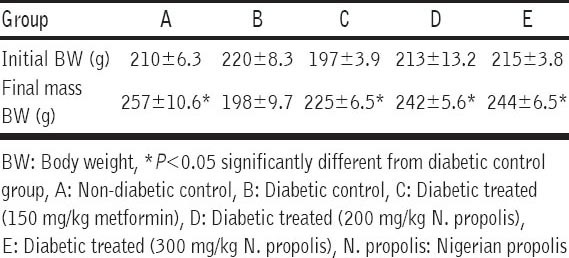
Table 1 shows the initial and final BW of animals in all groups after the 28 day experimental period. BW of animals in the diabetic group decreased after 28 days while there was significant (P < 0.05) increase in the BW of animals in other groups.
Table 1.
Effect of ethanolic extract of N. propolis on BW
Effect on lipid profile
Level of serum TG
Increase in level of serum TG was observed in the diabetic group when compared to the non-diabetic control group. Decrease in serum triglyceride levels was observed between metformin and extract treated groups when compared to the diabetic untreated group [Table 2].
Table 2.
Effect of ethanolic extract of N. propolis on lipid profile
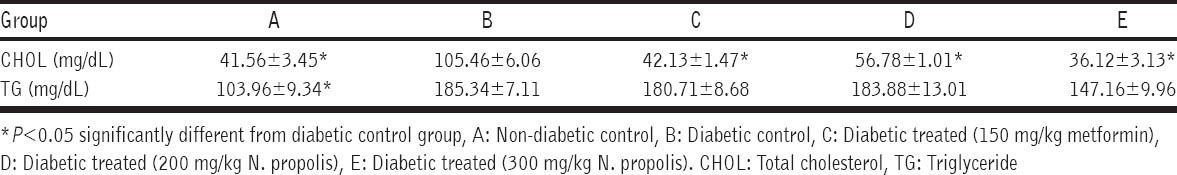
Level of CHOL
Table 2 also shows that serum cholesterol levels of untreated diabetic rats were significantly higher than those in normal control group. Treatment with metformin and N. propolis lowered the level of serum cholesterol with maximum effect seen in the 300 mg/kg N. propolis group.
Effect on Serum ALT and AST
Figure 2a and b shows the serum levels of AST and ALT in all groups. Serum levels of ALT and AST were up regulated significantly (P < 0.05) in the diabetic untreated group when compared to the normal control group. Administration of metformin and N. propolis significantly decreased the elevated level of AST when compared to the normal control group. Metformin had no significant effect on the elevated level of ALT while N. propolis administration significantly brings down the level of ALT when compared to the normal control group.
Figure 2.
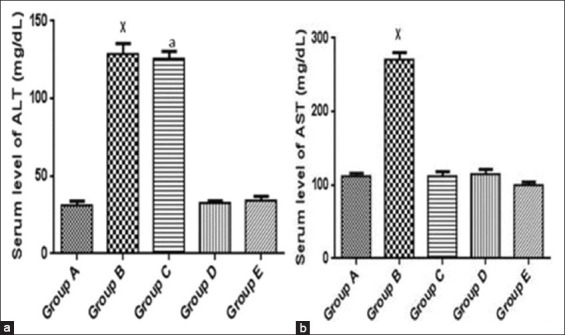
(a and b) Showing and comparing serum level of alanine aminotransferase and aspartate aminotransferase between treated groups and the diabetic untreated group. *P < 0.05 significantly different from normal control group,aP < 0.05 significantly different from normal control group. A: Non-diabetic control, B: Diabetic control, C: Diabetic treated (150 mg/kg metformin), D: Diabetic treated (200 mg/kg Nigerian propolis [N. propolis]), E: Diabetic treated (300 mg/kg N. propolis)
Effect on oxidative stress
Serum MDA levels and SOD activities were assayed for. Alloxan-induced diabetes significantly lowered SOD levels in the diabetic group when compared to normal control group. After administration of metformin and N. propolis, SOD level was not significantly different (P < 0.05) between the normal control and the metformin treated group [Figure 3]; the administration of N. propolis raised the SOD level higher than the control [Figure 3]. Similarly, MDA levels were higher in the untreated diabetic group, which was significantly different (P < 0.05) when compared with the normal control. Administration of N. propolis and metformin significantly (P < 0.05) lowered the MDA levels when compared to the normal control group [Figure 4].
Figure 3.
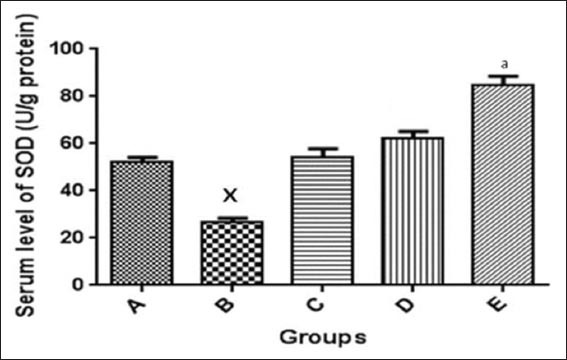
Serum level of superoxide dismutase in the control and experimental groups. *P < 0.05 significantly different from normal control group, aP < 0.05 significantly different from normal control group, A: Non-diabetic control, B: Diabetic control, C: Diabetic treated (150 mg/kg metformin), D: Diabetic treated (200 mg/kg Nigerian propolis [N. propolis]), E: Diabetic treated (300 mg/kg N. propolis)
Figure 4.
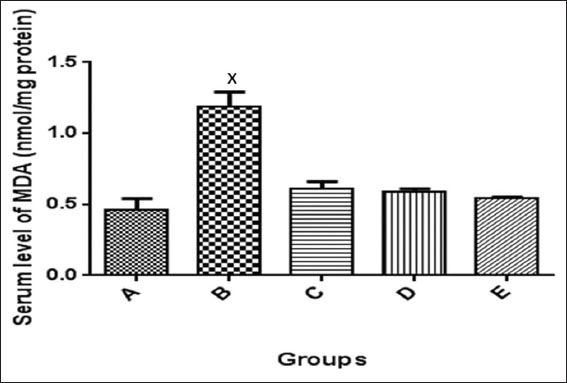
Serum level of malondialdehyde in the control and experimental groups. *P < 0.05 significantly different from the control and treatment groups. A: Non-diabetic control, B: Diabetic control, C: Diabetic treated (150 mg/kg metformin), D: Diabetic treated (200 mg/kg Nigerian propolis [N. propolis]), E: Diabetic treated (300 mg/kg N. propolis)
Histology
Hepatic histology
Figure 5a-e shows histology of the livers in control and treated groups at 28 days. In these groups, hepatic histology was comparable to the control, except in untreated diabetic rats, where hepatic sinusoids had become occluded, central veins were congested and hepatocytes appeared swollen.
Figure 5.
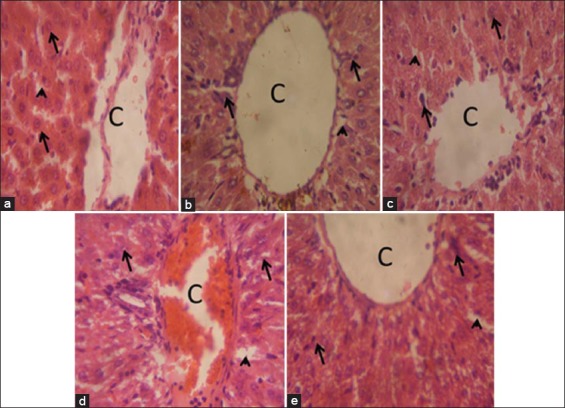
Liver of rats at 28 days of treatment, (a) Control group: The morphology of the liver is normal as indicated by intact central vein (c), sinusoids (arrowhead), and hepatocytes (arrows). (b) Diabetic group; Sinusoids have become largely occluded, perhaps due to swollen hepatocytes (arrows); central vein (c) is also congested. (c) Diabetic + metformin: The liver has normal morphology (c, central vein; arrow, hepatocyte; arrowheads, sinusoids). (d) Diabetic + 200 mg/kg of Nigerian propolis [N. propolis]: Hepatic morphology is comparable to control. (e) Diabetic + 300 mg/kg of N. propolis: The liver show normal morphology. Arrows indicate hepatocytes; arrowheads indicate sinusoids. H and E stain; ×400.
Pancreatic histology
The section of rat pancreas from normal control group reveals normal pancreatic acini and islet cells, a similar result was found in the group treated with N. propolis and metformin. Alloxan-diabetic rats demonstrate degenerative and lytic changes in the islet of Langerhans of the pancreas as seen in Figure 6a-e.
Figure 6.
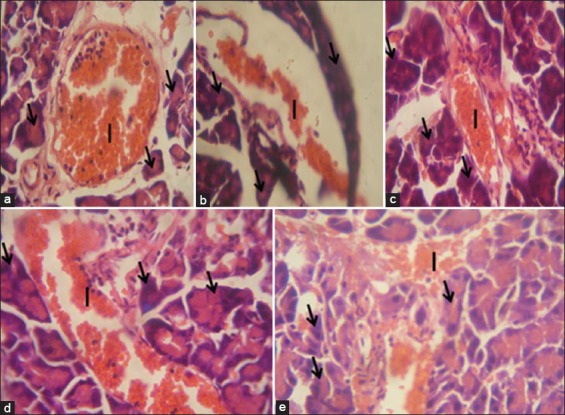
Pancreas of rats at 28 days of treatment. (a) Control group: The morphology of the pancreas is normal as indicated by intact islet of Langerhans cells (I) and acinar cells (arrows). (b) Diabetic group: There are degenerative and lytic changes in the islet of Langerhans of pancreas. (c) Diabetic + metformin: The pancreas has normal morphology (I show islet cells, arrow show acinar cells). (d) Diabetic + 200 mg/kg of Nigerian propolis; Pancreas morphology shows improvement comparable to control. (e) Diabetic + 300 mg/kg of Nigerian propolis. Pancreas morphology is comparable to control (H and E, 400×)
DISCUSSION
In this study, we examined the possible antioxidants effects of N. propolis on diabetic rats. The noted significant decrease in blood glucose level in the N. propolis treated groups [Figure 1] suggests that long-term administration/intake of this extract may have hypoglycemic effect. The reduction in the blood glucose level may be due to the presence of certain bioactive components and the protective effects that the antioxidant components of N. propolis may have on pancreatic β-cells which could enhance their production of insulin and more importantly, the possibility that propolis may enhance cellular response to insulin. In the propolis treated groups, despite the initial hyperglycemia, the antioxidants present in propolis were able to prevent oxidative stress effect of hyperglycemia on the liver and pancreas, this finding is in line with the reports of Al-Hariri et al. [29] who reported the efficacy of Arabian propolis in hyperglycemia. Hypoglycemic agents exact their effects via direct or indirect mechanisms in diabetes rats [30]. N. propolis acted as a direct hypoglycemic agent, by producing hypoglycemic effects when administered to alloxan-treated rats due to the severe destructive effect of alloxan on the β-cells of the pancreas [24]. N. Propolis could also have acted indirectly by stimulating the few surviving β-cells to secrete more insulin rather than aiding the regeneration of necrotic β-cells of the pancreas. Observation from this study shows that N. Propolis exacts its activity by both direct and indirect mechanisms.
Lipid disorders assume a position of utmost importance in patients with diabetes, because of the high risk of microvascular disease in this condition but administration of N. propolis to the rats was able to reverse hyperlipidemia seen in the diabetic rats. Similar reports of Al-Hariri [29] and El-Sayed et al. [31] shows that treatment with propolis can reduce the TG and serum cholesterol level in diabetics. The hypolipidemic effects of N. propolis observed from this study is probably due to the hypoglycaemic potential of N. Propolis, which makes it possible to ameliorate lipid and lipoprotein disorders associated with diabetes.
The ethanolic extract of N. propolis possesses antioxidant components that ameliorated the oxidative stress induced damage associated with alloxan-induced diabetes. The bioflavonoids present in N. Propolis may have confers the antioxidant effect seen in this study as quecretin, a flavonoids present in N. Propolis have been reported to bring down hyperglycemia and oxidative stress in STZ-induced diabetes rats [32].
Increased levels of ALT and AST infiltrates and disturbs functioning of the hepatic cell membranes [33]. Administration of N. propolis ameliorated high levels of ALT and AST following induced-diabetes. The hepatoprotective activity of N. propolis was higher at 300 mg/kg than metformin.
A primary consideration in the assessment of the efficacy of a potential therapeutic agent for hepatic injury is its effect on liver histology. Histological sections of the liver in untreated diabetic rats show extensive occlusion of the sinusoids [Figure 5b]. Reports have revealed swelling of hepatocytes could arise from accumulation of glycogen in these cells - a condition referred to as hepatic glycogenosis of DM [34]. Treatment with the ethanolic extract of N. propolis improved hepatic injuries associated with induced-diabetes.
The pancreas is usually the main organ affected in diabetes with loss in both its exocrine and endocrine functions. This is mainly due to the close anatomical and functional links between the exocrine and endocrine pancreas [35]. Histologically, there was improvement in the islet and the acinar cells in the N. propolis treated group. This is different from what is seen in the untreated diabetic group. Pancreatic cells are usually lost because pancreatic β-cells are highly prone to oxidative stress and damage because they have low activity of antioxidant enzymes, which are the first line of defense against oxidative insult [27].
CONCLUSION
In conclusion, results from this study indicate that the ethanolic extract of N. propolis can ameliorate hyperglycemia, hypercholesterolemia, and hypertriglyceridemia as well as protect the liver and pancreas against alloxan-induced diabetes. This significant protection of N. propolis may be due to the synergistic effect of the constituents of the extract. Further biochemical and pharmacological investigations would be required to know the comprehensive mechanism of action of the N. propolis which our laboratory is currently involve in.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kantarova D, Buc M, Stuchlikova M, Mokan M, Vrlik M. Ethiopathogenesis of autoimmune diabetes morphology of the pancreas in rats with experimental diabetes mellitus. Bull Exp Biol Med. 2007;143:368–71. doi: 10.1007/s10517-007-0114-y. [DOI] [PubMed] [Google Scholar]
- 2.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 3.Guyton WF, Hall EJ. Textbook of Medical Physiology. 10th ed. Vol. 20. New York: Lange Medical Books; 2002. Endocrine functions of the pancreas and regulation of carbohydrate metabolism; p. 322. [Google Scholar]
- 4.Zare K, Tabatabaei SR, Shahriari A, Jafari RA. The effect of butter oil on avoidance memory in normal and diabetic rats. Iran J Basic Med Sci. 2012;15:983–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi I, Farajnia S, Mohammadi M, Sabouri Gannad M. The pattern of brain-derived neurotrophic factor gene expression in the hippocampus of diabetic rats. Iran J Basic Med Sci. 2010;13:146–53. [Google Scholar]
- 6.Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, et al. Glycogenic hepatopathy: An underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30:508–13. doi: 10.1097/00000478-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210–5. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal N, Singh S, Singh N, Kalra S, Srivastava G. Oxidative stress and diabetes. Int J Geriatr Gerontol. 2010;6:1–4. [Google Scholar]
- 9.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Khalil ML. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7:22–31. [PubMed] [Google Scholar]
- 11.Greenaway W, May J, Scaysbrook T, Whatley FR. Identification by gas chromatography-mass spectrometry of 150 compounds in propolis. Z Naturforsch C. 1999;46:111–21. [Google Scholar]
- 12.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima SH. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–6. [Google Scholar]
- 13.Bankova V, Christov R, Kujumgiev A, Marcucci MC, Popov S. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch C. 1995;50:167–72. doi: 10.1515/znc-1995-3-402. [DOI] [PubMed] [Google Scholar]
- 14.Marcucci MC, DeCamargo FA, Lopes CMA. Identification of amino acids in Brazilian propolis. Z Naturforsch C. 1996;51:11–4. [Google Scholar]
- 15.Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001;76:165–70. doi: 10.1016/s0378-8741(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banskota AH, Tezuka Y, Adnyana IK, Ishii E, Midorikawa K, Matsushige K, et al. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine. 2001;8:16–23. doi: 10.1078/0944-7113-00004. [DOI] [PubMed] [Google Scholar]
- 18.Moreira L, Dias LG, Pereira JA, Estevinho L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem Toxicol. 2008;46:3482–5. doi: 10.1016/j.fct.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Silva JC, Rodrigues S, Feás X, Estevinho LM. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem Toxicol. 2012;50:1790–5. doi: 10.1016/j.fct.2012.02.097. [DOI] [PubMed] [Google Scholar]
- 20.Valente MJ, Baltazar AF, Henrique R, Estevinho L, Carvalho M. Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem Toxicol. 2011;49:86–92. doi: 10.1016/j.fct.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Feás X, Pacheco L, Iglesias A, Estevinho LM. Use of propolis in the sanitization of lettuce. Int J Mol Sci. 2014;15:12243–57. doi: 10.3390/ijms150712243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Caravaca AM, Gómez-Romero M, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Advances in the analysis of phenolic compounds in products derived from bees. J Pharm Biomed Anal. 2006;41:1220–34. doi: 10.1016/j.jpba.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Kosalec I, Bakmaz M, Pepeljnjak S, Vladimir-Knezevic S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004;54:65–72. [PubMed] [Google Scholar]
- 24.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–26. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 25.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 28.Diniz YS, Rocha KK, Souza GA, Galhardi CM, Ebaid GM, Rodrigues HG, et al. Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur J Pharmacol. 2006;543(1-3):151–7. doi: 10.1016/j.ejphar.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hariri MT. Propolis and its direct and indirect hypoglycemic effect. J Family Community Med. 2011;18:152–4. doi: 10.4103/2230-8229.90015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Antidiabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol. 1990;42:290–2. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 31.El-Sayed el SM, Abo-Salem OM, Aly HA, Mansour AM. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin-induced diabetic rats. Pak J Pharm Sci. 2009;22:168–74. [PubMed] [Google Scholar]
- 32.Adewole SO, Caxton-Martins EA, Ojewole JA. Protective effect of quercetin on the morphology of pancreatic beta-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2006;4:64–74. doi: 10.4314/ajtcam.v4i1.31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 34.Akinola OS, Akinola OB, Caxton-Martins EA. Vernonia amygdalina upregulates hepatic enzymes and improves liver microanatomy in experimental diabetes mellitus. Pharmacologyonline. 2010;2:1231–42. [Google Scholar]
- 35.Bank S. Chronic pancreatitis: Clinical features and medical management. Am J Gastroenterol. 1986;81:153–67. [PubMed] [Google Scholar]


