Abstract
Ethno Pharmacological Relevance: Traditional medicinal plants are practiced worldwide for treatment of arthritis especially in developing countries where resources are meager. This review presents the plants profiles inhabiting throughout the world regarding their traditional usage by various tribes/ethnic groups for treatment of arthritis.
Materials and Methods:
Bibliographic investigation was carried out by analyzing classical text books and peer reviewed papers, consulting worldwide accepted scientific databases from the last six decades. Plants/their parts/extracts/polyherbal formulations, toxicity studies for arthritis have been included in the review article. The profiles presented also include information about the scientific name, family, dose, methodology along with mechanism of action and toxicity profile. Research status of 20 potential plant species has been discussed. Further, geographical distribution of research, plants distribution according to families has been given in graphical form.
Results:
485 plant species belonging to 100 families, traditionally used in arthritis are used. Among 100 plant families, malvaceae constitute 16, leguminasae 7, fabaceae 13, euphorbiaceae 7, compositae 20, araceae 7, solanaceae 12, liliaceae 9, apocynaceae, lauraceae, and rubiaceae 10, and remaining in lesser proportion. It was observed in our study that majority of researches are carried mainly in developing countries like India, China, Korea and Nigeria.
Conclusion:
This review clearly indicates that list of medicinal plants presented in this review might be useful to researchers as well as practioners. This review can be useful for preliminary screening of potential anti-arthritis plants. Further toxicity profile given in the review can be useful for the researchers for finding the safe dose.
KEY WORDS: Arthritis, plant, polyherbal, traditional uses
INTRODUCTION
Immune system of our body plays a crucial role, as an overactive immune system may lead to certain fatal disease because of various hypersensitive or allergic reactions which may cause numerous derangements; loss of normal capacity to differentiate self from non-self resulting in immune reactions against our own’s cells and tissues called autoimmune diseases. Certain common autoimmune diseases like myasthenia gravis, serum sickness, pernicious anemia, reactive arthritis etc., are the severe issues for medical and pharmaceutical community because of unknown etiology [1]. According to WHO, 0.3-1% of the world population is affected from rheumatoid arthritis (RA) and among them females are three times more prone to the disease as compared to males [2]. RA is a chronic, inflammatory, and systemic autoimmune disease [3]. The primary symptoms of RA include pain, swelling, and destruction of cartilage and bone as a result of which permanent disability occur. Although the exact etiology is unknown but several hypotheses said that it is triggered by the combination of genetic predisposition and exposure to environmental factors like viruses [4]. The exact pathophysiology is still unknown but release of certain free radicals such as nitrous oxide and superoxide radicals generated as by-products of cellular metabolism. The release of such free radicals may induce the production of interleukins (IL) and tumor necrosis factor (TNF-α) from T-cells which ultimately influence the production of growth factors, cytokines and adhesive molecules on immune cells as such factors may cause tissue destruction and inflammation [5]. Pathological changes in RA are hyperplasia of synovial membrane, infiltration of inflammatory cells and neovascularization, which results into cartilage erosion and articular destruction [3].
The goal of treatment for rheumatoid arthitic patients is to eliminate symptoms, slow disease progression, and optimize quality-of-life [6]. Therefore, before starting the treatment of RA certain goals must be kept in mind such as relief of analgesia, reduction of inflammation, protection of articular structure, maintenance of function, and control of systemic involvement [5]. Presently for the treatment of RA, strategies have changed from traditionally used non-steroidal anti-inflammatory drugs (NSAIDs) or disease modifying antirheumatic drugs (DMARDs) to novel biological agents, like TNF monoclonal antibody. Clinically, the treatment of RA includes five strategies. The foremost approach is the use of NSAIDs followed by mild doses of glucocorticoids to minimize the signs of inflammation as well as progression of disease. In chronic patients, the use of DMARDs such as methotrexate, sulfasalazine, gold salts or D-pencillamine can be included in the treatment. In certain cases, TNF-α neutralizing agents like infliximab, etanercept etc; IL-1 neutralizing agents like anakinra; and the drugs which interfere with T-cell activation such as abatacept can also be included in treatment of chronic cases. Finally, immunosuppressive and cytotoxic drugs such as cyclosporine, azathioprine, and cyclophosphamide are used for the treatment of chronic patients [5,7,8]. The above-mentioned therapeutic agents reduce the inflammation and joint destruction but their long-term risks are still unknown. However, long-term risks of drugs includes gastrointestinal ulcers, cardiovascular complications, hematologic toxicity, nephrotoxicity, pulmonary toxicity, myelosuppression, hepatic fibrosis, stomatitis, cirrhosis, diarrhea, immune reactions, and local injection-site reactions. Moreover, higher costs and side effects which include high risks of infections and melagnancies reguires continous monitoring [1].
Herbal Therapy for the Treatment of Arthritis
Herbal medicines are used for the treatment of various ailments from ancient times and it is not an exaggeration to say that the use of the herbal drugs is as old as mankind [9]. Herbal medicines are synthesized from the therapeutic experience of generation of practicing physicians of ancient system of medicine for more than hundreds of years [10]. Nowadays, researcher shows a great interest in those medicinal agents that are derived from plants because the currently available drugs are either have certain side effects or are highly expensive [11]. Nature has blessed us with enormous wealth of herbal plants which are widely distributed all over the world as a source of therapeutic agents for the prevention and cure of various diseases [12]. According to WHO, world’s 80% population uses herbal medicines for their primary health care needs. Herbal medicines will act as parcels of human society to combat disease from the dawn of civilization [13]. The medicinally important parts of these herbal plants are chemical constituents that produce a desired physiological action on the body [14].
Since ancient time India uses herbal medicines in the officially alternative systems of health such as Ayurveda, Unani, Sidha, Homeopathy, and Naturopathy [15]. In India, there are more than 2500 plants species which are currently used as herbal medicaments. For than 3000 years, the herbal medicines are used either directly as folk medication or indirectly in the preparation of recent pharmaceuticals [16]. Thus, from the knowledge of traditional plants, one might be able to discover new effective and cheaper drugs [17]. In this review article, we have tried to cover all the ayurvedic strategies that are followed for the treatment of RA without any possible side effects. The future treatment of RA should provide more effective relief [5].
MATERIALS AND METHODS
In this review, bibliographic investigation was carried out during July 2011-December 2013, by refering various text books and certain review papers and research papers, consulting globally accepted databases from last some decades. The data were gathered from various databases i.e. Science Direct, PubMed, and Google and the information is compiled by reviewing more than 250 research and review articles. The data which are relevant would be considered. The botanical correct names and families were mentioned after verification from published literature and databases.
The method of scrutining the data for this review article includes those plants: (i) Which are native to India and other countries such as America, Africa or Europe, (ii) used in traditional systems and in various polyherbal preparations, (iii) with reported anti-arthritic activity, (iv) appropriate dosage, (v) mechanism of action, (vi) safety profile, and (vii) models used. Plants/their parts/extracts used traditionally in acute rheumatic attacks, chronic analgesia, and chronic rheumatism have been considered as anti-arthritic agents. Further, detailed information on research status of 20 plant species has been explained.
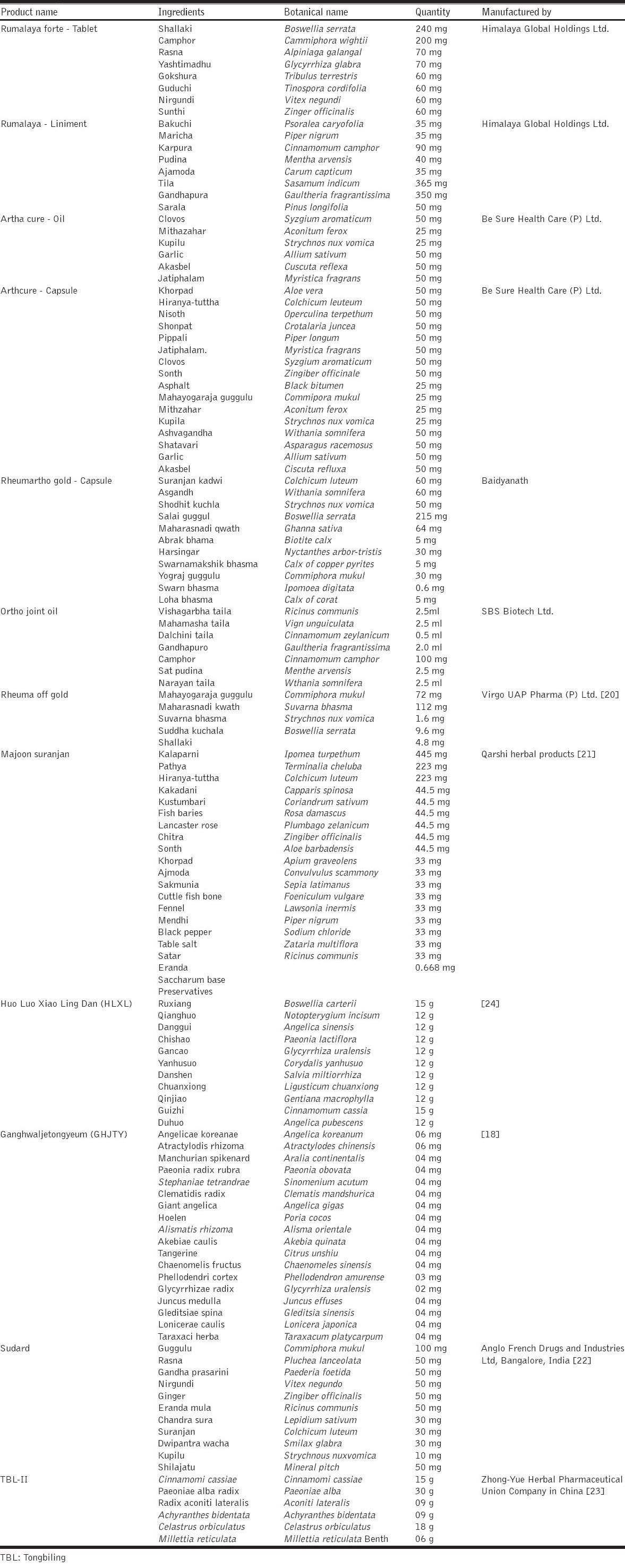
Polyherbal Formulations for Arthritis
Analgesics and NSAIDs are helpful in reducing pain and inflammation in either acute or chronic RA patients [18]. Although the treatment of RA is available but due to potential adverse effects or irreversible organ damage the new approaches are developed for maintaining the balance between these potential risks and acknowledged benefits [19]. Currently for the treatment of RA safer and more potent medicaments are developed from oriental sources. Large number of herbal extracts and products such as polyherbal formulations are prepared to reduce such side effects and increase the benefits [18].
Rheum off Gold is a poyherbal formulation that is commonly recommended by Ayurvedic medical practitioners for the treatment of arthritis. The anti-arthritic activity was confirmed on complete Freund’s adjuvant (CFA) induced arthritis model in wistar rats and it was observed that significant reduction in arthritis index, paw thickness and inflammatory markers such as C-reactive protein, serum rheumatoid factor and erythrocyte sedimentation rate (ESR) when compared with dexamethasone. Thus, the formulation possesses a potential anti-arthritic activity [20].
A Unani polyherbal formulation was evaluated for its anti-arthritic activity in rats. The anti-arthritic efficacy of Manjoon Suranjan was evaluated using formaldehyde and CFA induced arthritis models. The data obtained suggested the anti-arthritic activity of the formulation [21].
Evaluation of Sudard as a potent anti-arthritic polyherbal formulation was studied using formaldehyde and adjuvant induced arthritis models in wistar rats. The formulation at the doses of 150 mg/kg and 300 mg/kg p.o. proves to have an anti-inflammatory and anti-arthrtic activity [22].
Anti-arthritic potential of Tongbiling (TBL-II) which was prepared by some modification in Chinese herbal formulation TBL. The anti-arthritic efficacy of formulation was studied using the collagen induced arthritis model in wistar rats and it was revealed that at the doses of 100 and 300 mg/kg p.o. the levels of IL-1β and TNF-α was significantly reduced. Thus it was concluded that the formulation have an anti-arthritic potential [23].
Chinese herbal formula HLXL was used in the treatment from last hundred years for the treatmnent of inflammation and arthritis. Moreover, after certain modifications in HLXL herbal formulation it was evaluated for its anti-arthritic property using CFA model in rats. It was concluded that the polyherbal formulation shows an anti-arthritic activity through significant inhibition of paw edema and levels of TNF-α and IL-β [24].
The therapeutic effect of Ganghwaljetongyeum on RA in rabbit knee synovial membrane was evaluated. It was observed that there would be significant inhibition of proliferation of HG-82 cells which shows that the polyherbal formulation have an anti-arthritic activity. Moreover, there was significant reduction in TNF-α, IL-10 and NO species [18]. Various polyherbal formulations are described in Table 1.
Table 1.
Polyherbal formulations
RESULTS
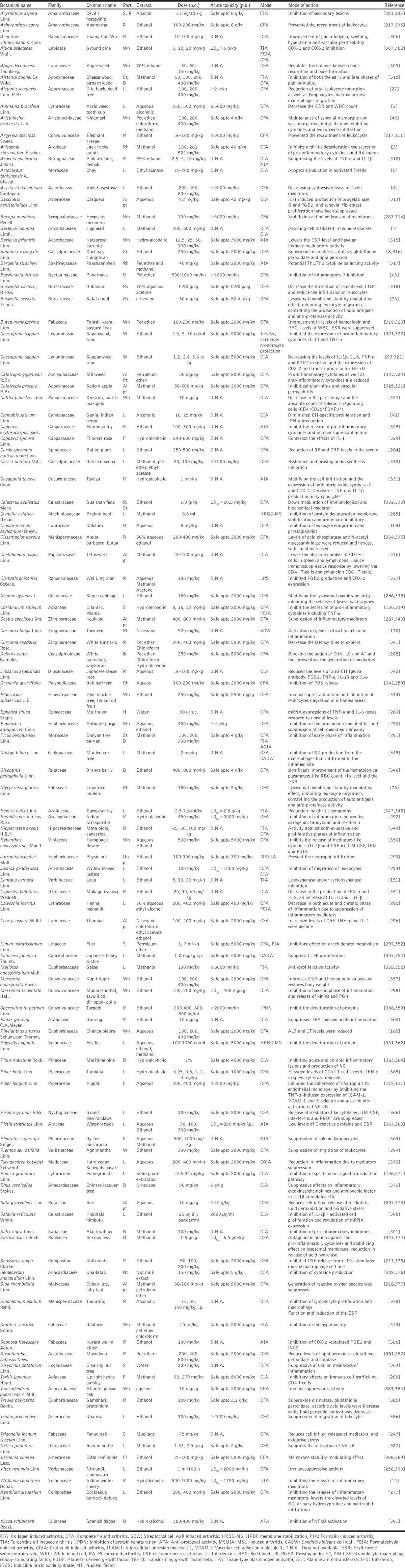
About more than 350 articles were reviewed. More than 20 articles were studied for searching the traditional use of plants in arthritis [Table 2]. Around 108 articles were referred for citing the proved anti-inflammatory and anti-arthritic activities of plants along with mechanism of action, acute toxicity profile, and doses [Table 3].
Table 2.
Traditionally used anti-arthritic plants
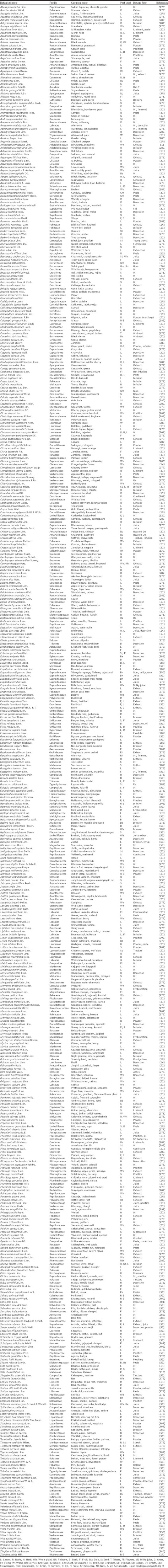
Table 3.
Plants with reported anti-arthritic activity
The detailed information on research status of following 20 plant species was gathered from multiple references.
Alstonia scholaris Linn. (AS)(Family-Apocynaceae)
AS is commonly known as saptaparni or devil’s tree, widely distributed in dried forests of India as Western Himalayas, Western Ghats, and in the Southern region. AS is a medium to large tree about 40 m high with a somewhat tessellated corky grey to grey-white bark [25]. Traditionally, bark of AS is used in the treatment of rheumatism, malarial fevers, abdominal disorders, leprosy, asthma, bronchitis, pruritis, and chronic ulcers [12]. Milky juice is mixed with oil and was applied in rheumatic pains. The chief alkaloids present in AS are echitamine, tubotaiwine, akaummicine, echitamidine, picrinine, and strictamine. AS flowers also contains amino acids, carbohydrates, phenol, tannins, cardiac glycosides, saponins, flavanoids, steroids, fixed oil, and fats [26]. The plant showsimmune-stimulatory, hepato-protective [27], anti-cancer [28], anti-plasmodial [29], and anti-hypertensive [30] activities. Extract of AS possess an anti-diabetic, anti-hyperlipidemic [31], anti-bacterial [32], anti-inflammatory, analgesic [33], antioxidant [27], immunostimulant [34], anti-cancer [35], anti-asthmatic [36], hepatoprotective [37], and anti-anxiety activity [12,25,38]. The ethanolic extract of AS leavesat doses of 100 and 200 mg/kg confirmed anti-arthritic activity in male wistar rats. The anti-arthritic activity was mainly by reducing the total leukocyte migration as well as lymphocytes and monocytes/macrophages migration. It can be concluded that AS shows an anti-arthritic activity on male wistar rats [39].
Aristolochia bractaeta Lam. (AB)(Family-Aristolochiaceae)
AB commonly known as worm killer or kidamari is a shrub found in Deccan Gujarat, western and southern India, Bihar, Sindh, and Bengal [16]. Traditional use of AB was found in gonorrhea, syphilis, inflammation, ulcer, amenorrhea, skin disease, dermatitis, leprosy, jaundice, and helminthiasis [16]. The major chemical constituents of the AB are alkaloids, triterpenoids, steroids, flavonoids, saponins, carbohydrates, proteins, and cardiac glycosides [40,41]. The studies of extract have shown anti-pyretic [42], anti-allergic [43], anti-inflammatory, anti-arthritic [1], anti-ulcer [44], anti-fungal [45], anti-microbial [46], antioxidant [47], wound healing [48], anti-implantation, and abortificient activities [49]. The petroleum ether, methanol, and chloroform extract of whole plant of AB possess comparable anti-arthritic activity at doses of 100, 200, and 400 mg/kg body weight. AB revealed anti-arthritic activity by maintaining the synovial membrane and vascular permeability thus inhibiting cytokines and leukotriene infiltration. In conclusion, AB possesses an anti-arthritic effect on wistar albino rats of either sex [1].
Boerhaavia diffusa Linn. (BD)(Family-Nyctagineae)
BD is found all over India especially during rain. Two varieties of BD are explored, one with white flowers called “shwethpurna” and other flowers called “raktapurna.” The medicinally important part is root (MateriaMedica, 1982). BD is traditionally significant due to their laxative, diuretic, expectorant, diaphoretic, and emetic properties [50]. A paste made up of roots together with Colchicum, Solanum nigrum, Tamarind stone, Stag’s horn and dried ginger, all in equal parts, are used in rheumatic and gouty painful joints. Root is used as powder in drachm doses or decoction or infusion for the treatment of inflammatory disorders like arthritis. Chakradatta used it in the treatment of chronic alcoholism and various other ailments i.e. phthisis, insominia, and rheumatism [51]. The air-dried plant was found to contain large quantities of potassium nitrate and also contains an alkaloid, panarnavine, present in very small quantity of 0.01%. Recent investigations reported that BD possess an antistress, adaptogenic [52], antioxidant [53], immunosuppressive [54], anti-carcinogenic [55], hepatoprotective [56,57], diuretic [58], anti-diabetic [59], anti-viral [60], and anti-inflammatory activities [61,62]. The petroleum ether extract of roots at dose 1000 mg/kg has been evaluated as anti-arthritic using CFA model and showed 81.5% response as compared to indomethacin [63].
Boswellia serrate Roxb. (BS)(Family-Burseraceae)
BS is a deciduous middle-sized tree, grown in tropical parts of Asia and Africa [64]. Boswellic acid is the first terpenoids isolated from oleo gum resins. The oleo gum resin of BS is used in various Unani and Ayurvedic preparations. Folkloric uses of BS are in the treatment of bronchitis, rheumatism, asthma, cough, intestinal problems, syphilitic, jaundice, dysentery, and pulmonary diseases. It acts as both internal and external stimulant, expectorant, diuretic, and stomachic [51,64]. Boswellia is a traditional natural remedy that has been used for thousands of years to treat swelling and inflamation in Ayurvedic medicine and traditional Chinese medicine. In 2003, medical researchers conducted a randomized blind placebo controlled trial of BS on 30 patients suffering from osteoarthritis of the knee. The data showed an increased range of motion and less swelling in their knees from arthritis than before they began the treatment. The essential oil of BS predominantly comprised monoterpenoids, of which β-pineneis the major constituent. Other monoterpenoids includes β-pinene, cis-verbenol, trans-pinocarveol, borneol, myrcene, verbenone, limonene, and p-cymene, while a-copaene was the only sesquiterpene identified [65,66]. BS possess an anti-inflammatory [67], analgesics [68], immunomodulatory [69], anticancer [70,71,72], hepatoprotective, hypolipidemic [73], antiasthmatic [74], osteoarthritis, and hypoglycemic activities [75]. The n-hexane extract of gum resins of BS in combination with methanolic extract of rhizomes of Glycyrrhiza glabra (GY) exhibited anti-arthritic activity at doses of 50 or 100 mg/kg in male wistar rats. The anti-arthritic activity is mainly by decreasing the activity of membrane marker enzymes such as alkaline phosphatase, serum glutamic oxaloacetic transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and by the prevention of leucocytes migration in the inflamed area. In conclusion, BS possesses a significant anti-arthritic activity on male albino wistar rats [76].
Caesalpinia sappan Linn. (CP)(Family-Leguminosae)
CP commonly known as sappanwood, bakam or patang, is a native of South India, Madhya Pradesh, Orissa, West Bengal, Malaya, and Sri Lanka. The tree spreads to a height of 10 m and is cultivated for its large, ornamental penicals of yellow flowers. A very strong barrier is formed by the branches when they are interlaced [11]. The heartwood of the CP is traditionally used for the treatment of ulcers, leprosy, rheumatism, skin disease, diarrhea, dysentery, epilepsy, convulsions, diabetes, odontopathy, stomatopathy, and leucorrhea. The heartwood of the CP is bitter, astringent, sweet, acrid, refrigerant, constipating, sedative, and hemostatic. In Yunani system, the decoction of wood was useful in rheumatism [77,78]. CP is reported to have an anti-anaphylactic [79], anti-coagulant [80], anti-bacterial [81-83], anti-fungal [83], anti-inflammatory [84], anti-tumor [85-87], anti-viral [88,89], immunostimulant [87], and semen coagulating activities [86]. CP also causes the inhibition of phosphodiesterase [90] and stimulation of glutamate pyruvate transaminase [91] and tyrosinase enzymes [92]. The ethanolic extract at doses 1.2, 2.4, and 3.6 g/kg of CP wood showed anti-arthritic activity on wistar rats by declining the levels of IL-1β, IL-6, TNF-α, and prostaglandin E2 (PGE2) in serum. The study concluded that CP possesses an anti-arthritic activity on rats [93].
Cannabis sativum Linn. (CT)(Family-Urticaceae)
CT, a pistillate plant, is a native of Persia, Western and central Asia, and is now largely cultivated all over India. Dried flowering or fruiting tops are medicinally important. CT possesses traditional significance in infections of eye, local inflammation, neuralgia, acute mania, whooping cough, asthma, and to relieve pain in dysmenorrhea and menorrhagia. Oil extracted from seeds is used in rheumatism. The chief chemical constituent is a resin volatile oil composed of canabene, canabene hydride, canabinon, and canabin; which consist of cannabinol, pseudo-cannabinol, cannabinin, and several terpenes [51,94]. Around more than 166 research papers confirm that cannabis and related therapies will be helpful in relieving the pain associated with arthritis. Moreover, cannabinoid component of cannabis shown to possess anti-arthritic activity. It has been claimed to use as anxiolytic, antidepressant [95,96] in schizophrenia [97] and RA. The active moiety of CT i.e. cannabidiol at a dose of 10 and 25 mg/kg, orally, administered in collagen-induced arthritic ratssignificantly decreases the arthritic score and inhibits the release of inflammatory mediators. Thus, it was concluded that the cannabidiol have an anti-arthritic activity by possessing anti-inflammatory and immunosuppressive action [98].
Cinnammomum zeylicanium Blume. (CZ)(Family-Lauraceae)
CZ a topical evergreen tree grows to a height of 7-10 m in its mild state and has deeply veined ovate leaves that are dark green underneath. It is commonly known as cinnamon or Ceylon cinnamon. CZ is cultivated in Sri Lanka, Mayanmar, and Southern Coastal strips of India. Treatment of vaginitis, rheumatism, neuralgia, wounds, toothache, diabetes, inflammation of eyes, impotence, and leucorrhea is its traditional uses. CZ was also used to treat abdominal pain associated with diarrhea, dysmenorrhea, and amenorrhea. The active constituents of the CZ are cinnamaldehyde and eugenol. The other constituents are cmphene, sibinene, myrcene, fenchone, nerol, bornyl acetate, cinnamyl acetate, and geranial [99]. The CZ is reported to have an analgesic, anti-pyretic [100], anti-fungal [101], anti-inflammatory, anti-microbial [102,103], insecticidal [104], anti-diabetic [105,106], and antioxidant activities [107,108]. The polyphenolic extract of the CZ bark at a dose of 8 mg/kg revealed anti-arthriticpotential in male wistar rats in CFA model by improving the body weight and the level of serum C-reactive proteins when compared with control group. Thus, anti-arthritic activity was mediated through inhibition of leukocyte emigration and prostaglandin synthesis [109].
Coriander sativum Linn. (CS)(Family-Umbelliferae)
CS is a herbaceous plant distributed all over India and used for its seeds, fruits and leaves. Traditionally, plant is used as stimulant, carminative, stomachic, diuretic, tonic, and aphrodisiac. Oil is very useful for rheumatism in a dose of 1-4 minim on sugar or in emulsion. Coriander oil which contains linalool/coriandrol, geraniol, and boborneol, extracted from its fruit, is volatile and essential [51,110]. Externally seeda can be used as a lotion or have been bruised and used as a poultice for the treatment of arthritis. Cineole, one of the 11 components of the essential oils, and linoleic acid, present in coriander, possess antirheumatic and anti-arthritic properties [111]. CS possesses an antibacterial [112,113], anti-spasmodic [114], antioxidant [115-117], anticarcinogenic [118], and hypolipidemic activities [119]. The hydroalcoholic extract of seeds at doses of 8, 16, and 32 mg/kg showed reduction in paw swelling induced by formaldehyde and CFA methods in male wistar rats by inhibiting the pro inflammatory cytokines and TNF-α. In conclusion, the extract of CS shows a potent anti-arthritic activity on rats [120].
Curcuma longa Linn. (CL)(Family-Scitaminaceae)
CL is a perennial herb that measures up to 1 m high with a short stem, distributed throughout tropical and subtropical regions of the world, and is widely cultivated in Asian countries, mainly in India and China [121]. There are two varieties of CL one with rich-colored oval rhizomes and other with softer, larger, lighter-colored long rhizomes which are edible. Turmeric paste mixed with lime and saltpeter can be used externally in rheumatism. The major chemical constituents are curcumin, methylcurcumin, demethoxy curcumin, sodium curcuminate, and Ar-turmerone. Traditionally, CL is used in wound healing, helminthic infections, fevers, skin eruption, conjunctivitis, cough, parasitic infections, and liver diseases [51,121]. Later on, it was investigated the effect of herbomineral formulation (comination of turmeric, ashwagandha, sallai guggul, and jasad bhasma based on Ayurveda medicinal system) on 90 patients suffering from arthritis. It was observed that there was significant reduction in disability and pain. The plant is reported to be highly valued as anti-inflammatory [122,123], antiprotozoal [124,125], nematocidal [126], antibacterial [127], anti-tumor [128], and hepatoprotective [129]. The anti-arthritic activity was shown by essential oils of rhizomes of CL with streptococcal cell wall induced arthritis. It can be concluded that the turmeric essential oil possess an anti-inflammatory as well as anti-arthritic activities [130].
GY (Family-Fabaceae)
GY commonly known as mulethi is a herb/shrub of 2 m height mainly found in subtropical or temperate areas. The underground growth of stem is up to 2 m and is highly branched consisting short taproot with number of rhizomes. GY is commercially grown in Spain, Sicily and England. In India, it is mainly cultivated in Punjab and Sub Himalayan tracts [51]. The plant is reported to be traditionally used in anemia, gout, asthma, epilepsy, fever, cough, skin disease, rheumatism, paralysis, and hemorrhagic diseases. Roots in the form of infusion, decoction, extract or lozenge are useful as a demulcent in inflammatory affections [10,51]. The clinical trials reveal that glycyrrhizin has favorable effects on RA, when administered along adrenocorticotropic hormone or cortisone, in comparison, when administered alone. Hence, it was suggested that the main effect of liquorice is to potentiate rather than mimic endogenous steroids. The active chemical constituent is glycyrrhizin present in the form of potassium and calcium salts of glycyrrhizic acid. GY also contains sucrose, glucose, resins, bitter principles, mannites, asparagines, and fat [131]. GY have shown anti-microbial, hypolipidaemic, antiviral, hypotensive, anti-ulcer, anti-diuretic, anti-inflammatory, anti-mutagenic, expectorant, hepatoprotective, antioxidant, and antipyretic activities [132-134]. The methanolic extracts of rhizomes of GY at a dose of 150 mg/kg possess anti-arthritic activity in male wistar rats by inhibiting the leukocyte migration and auto antigens production and exhibit anti-protinase activity. The study concluded that GY possess a significant anti-arthritic activity [76].
Lantana camara Linn. (LC)(Family-Verbinaceae)
LC popular as lava or red sage is a low erect or subscandent vigorous shrub with tetrangular stem, stout recurved pickles and comprises strong odour ofblack currents. LC is native to India and reaches to a height of 1-3 m [135]. Traditionally, LC is used in the treatment of sores, chicken pox, measles, fever, cold, rheumatism, asthma, ulcers, and high blood pressure [135]. In Asian countries like India, the decoction of leaves of the plant LC was used traditionally for the treatment of rheumatism. In Ghana, the infusions of whole plant are used against arthritis. Nyctanthes arbor tristis is used in Bangladesh for treatment of fever, bacterial infections, and rheumatism as well as other ailments [136]. The active constituents are flavones, isoflavones, antocyanins, coumarins, lignins, alkaloids, tannins, saponins, triterpinoids, catechins, and isocatechins [137]. LC is reported to have an antioxidant [138], anti-diabetic [139,140], anti-inflammatory [141], anti-motility [142], anti-fungal [143,144], anti-bacterial [145,146], anti-fertility [147], cytotoxic [148], larvicidal [149], and wound healing activities [17,150]. The ethanolic extract of leaves of LC at doses 5, 10 and 20 mg/kg proved to have anti-arthritic activity by inhibiting the lipoxygenase and cyclooxygenase [151].
Phyllanthus amarus Schum and Thomm. (PA)(Family-Euphorbiaceae)
PA is a 10-60 cm tall herb which grows in tropical and subtropical sandy regions. Its common name is chancapiedra. Traditionally, PA is used in jaundice, dropsy, diarrhea, dysentery, urino-gental disease, scabies, ulcer, and wounds. In addition, it is used as astringent, stomachic, diuretic, antiseptic, bitter, and febrifuge [51,152]. In the Hand Book of African Medicinal Plants it is reported that PA was traditionally use for its anti-inflammatory activity. Moreover, in Amazonia and Brazil, the whole plant was used for the treatment of various inflammatory disorders like arthritis. PA comprised of active constituents found in all parts of the plant aslignans, glycosides, flavonoids, alkaloids, ellagitannins, and phenylpropanoids [152]. Studies have proved that PA have anti-inflammatory [153], anti-microbial [154,155], anti-cancer [156], anti-fertility [157], hepatoprotective [158], anti-diabetic [159], anti-diarrheal [160], antioxidant [161], anti-oedemotgenic [162], diuretic [163] and chmoprotective [164] activity. The aqueous extract of whole plant at a dose of 100, 200, and 400 mg/kg shows anti-arthritic activity in male wistar rats. The extract at various doses reduced the levels of aspartate transaminase and alanine transaminase and thus maintains its anti-arthritic activity [165].
Piper longum Linn. (PL)(Family-Piperaceae)
PL is a slender, climbing, under shrub, creeping, and rooting below. The young shoots are downy, the leaves are 5-10 cm long; 5 cm wide; ovate; cordate with broad rounded lobes at the base; sub-acute and entire. PL is indigenous to North-Eastern and Southern India and Ceylon [51]. PL is used in cold cough, asthma, hoarseness, and snake bite since ancient times. In rheumatism, roasted aments are bitten up with honey and taken in a prescribed dose. In Java and Indonesia, the whole plant was applied topically, as it relieves muscular pains and inflammation [51,166]. Major constituents are piperine, piperlongumine, piperlonguminine, and methyl 3,4,5-trimehoxycinnamate. Others include resin, volatile oil, starch, fatty oil, and inorganic matter [167]. Medicinally, PL finds its importance as an anti-inflammatory [168], anti-amoebic [169], anti-asthmatic [170], hepato-protective, and immune-modulatory activities [171]. The aqueous extract of seeds of PL at two doses (200 and 400 mg/kg) shows a 46.32% inhibition in paw swelling in Freund’s complete adjuvant induced arthritis in rats by inhibiting the adherence of neutrophils to endothelial monolayer by suppressing the TNF-α induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin, and also inhibits the NF-κB. In conclusion, PL possess a significant anti-arthritic activity on male wistar rats [172].
Punica granatum Linn. (PG)(Family-Lythraceae)
PG is popular as pomegranate is a native of India, East Indies, Southern Asia, tropical Africa, California, and Arizona. PG grows tillan height of 12-16 feet with number of spiny branches and has long lifespan. Traditionally, PG is used in diarrhea, ulcers, and diabetes and also useful as antiparasitic agent and blood tonic [51,173]. In Iranian Traditional Medicinal system, the seeds and juice are considered as a tonic for the treatment of rheumatism. Pomegranate fruit consumption reduced composite disease activity index in RA patients, and this effect could be related to the anti-oxidative property of pomegranates. Dietary supplementation with pomegranates may be a useful complementary strategy to attenuate clinical symptoms in RA patients [174]. Some of the major chemical constituents present in the PG aregallic acid, anthocyanins, ellagitannins, flavones, flavonoids, antocyanidins, sterols, quercitin, rutin, and other fatty acids [173]. The plant is of high value due to its anti-inflammatory [175], anti-carcinogenic [176,177], antioxidant [178,179], hypotensive [180], hypolipidaemic [181], anti-artheroseclerotic [182], and anti-diabetic activities [183]. PG is also used in the treatment of myocardial ischemia [184], prostrate cancer [185,186], dental plaques [187], denture stomatitis [188], bacterial infections [189,190], erectile dysfunctions [191], male infertility [192], alzheimer’s disease [193], and ischemic brain injury [194,195]. The fruits of PG show an anti-arthritic activity at doses of 13.6-34 mg/kg by inhibiting the spectrum of signal transduction pathway in male wistar rats. Thus, it can be concluded that PG have potent anti-arthritic activity [196].
Ruta graveolens Linn. (RG)(Family-Rutaceae)
Rue is an herbaceous perennial plant, originally growing in the Mediterranean region [197]. RG is traditionally used as antiseptic, anthelminthic, antispasmodic, stimulant, abortificient, expectorant, and anti-rheumatic [51]. The major chemical constituents isolated from the RG are rutin, quercitin, rutacridone, rutacridone epoxide, graveoline, and gravacridonodiol [197]. RG is reported to have anti-inflammatory [198,199], analgesics [200], antiandrogenic [201,202], antihyperglycemic [203,204], antihyperlipidemic [205], anticancer activity [206], and anti-rheumatic properties. The polyphenolic fraction of aerial parts of RG at a dose of 10 mg/kg, b.w. showed an anti-arthritic activity in male wistar rats induced by CFA model. The polyphenolic fraction revealed its activity by inhibiting the prostaglandins synthesis, decreasing CRP level, ceruloplasmin, lipid peroxidation and release of other inflammatory mediators. In conclusion, RG possess anti-arthritic activity [207].
Saussurea lappa Clarke. (SL)(Family-Compositae)
SL herbs grow abundantly on the Himalayas and Valley of Kashmir. Roots contain odorous principle composed of a solid resin, salt of valeric acid and ash which contains manganese. SL is mainly useful in asthma, helminthiasis, fever, cough, skin disease, rheumatism, malaria, and leprosy. Roots in the form of infusion with little cardamoms are used in chronic rheumatism. Oil of the root composed of camphene, phellandrene, costene, aplotaxene, costol, and costic acid [51]. In the Southern part of Kashmir, Himalaya, and Punjab regions, the roots and root stalk are used for the treatment of rheumatism. In Unani system of medicine, it is useful in rheumatism [208]. The combination of Cyperus rotundus, Tinospora cordifolia and SL clinically proved to have an anti-arthritic activity through significant reduction of pain in double-blinded, comparative, parallel clinical trial design [209]. The SL extracts exhibited other biological activities including anti-diarrheal [210], antiulcerogenic [211,212], antibacterial [213], anticancer [214], anticonvusant [212], hepatoprotective [215], antiviral [216], anti-inflammatory, antioxidant [217], and anti-arthritic activities. The ethanolic extract of SL at dose levels of 50-400 mg/kg showed potent anti-arthritic activity. A sesquiterpene lactone “cynaropicrin” isolated from SL strongly inhibited TNF-α release from lipopolysaccharide (LPS) - stimulated murine macrophage cell line and dose-dependently suppressed the proliferation of lymphocytes stimulated. Another sesquiterpene lactone “dehydrocostus lactone” from SL suppressed LPS-induced nitric oxide production. The investigation concluded that the SL shows a significant anti-inflammatory and anti-arthritic activity [217].
Sida rhombifolia Linn. (SR)(Family-Malvaceae)
SR is a small erect under shrub having rough branches with stellate hairs commonly found in dry countries such as India and Ceylon [218]. Traditionally, the plant is used as nutritive, tonic and for the treatment of gonorrhea, piles, rheumatism, as diuretic, and aphrodisiac [51]. In Indonesia and Johore medicinal system, juice of whole plant pounded with little water is given indoses of ¼ seer for the treatment of rheumatism. β-phenethylamine, N-methyl-β-phenethylamine, S-(þ) N-β-methyl tryptophan methyl ester, vasicinol, vasicinone, vasicine, choline, hypaphorine methyl ester, hypaphorine, and betaine [219] have been isolated from the plant. The reported activities of plant include cytotoxic [220], antimicrobial [221], antibacterial [222], anti-inflammatory, antipyretic [223], and anti-arthritic. The aqueous and ethanol extract of aerial parts of the SR at doses 30 and 100 mg/kg reduced the paw edema induced by CFA method. Thus, it is concluded that the plant possess a potent anti-arthritic activity [218].
Terminalia chebula Retz. (TC)(Family-Combrataceae)
TC is a well-known traditional plant of Indian traditional medicinal system and the most frequently used herb in ayurveda. In tribal of Tamil Nadu in India, the TC is commonly known as Kadukkai and was used for treating various ailments such as fever, cough, diarrhea, gastroenteritis, skin diseases, candidiasis, urinary tract infections, and wound infections [51]. TC is a medium-sized deciduous tree of variable appearance with usually short cylindrical bole of 5-10 m length and 60-80 cm diameter. The phytoconstituents of TC are tannins, flavonoids, resins, fixed oil, fructose, amino acids, and sterols. Moreover, the active constituents of tannins include chebulic acid, ellagic acid, chebulagic acid, chebulinic acid, and gallic acid. TC was used in Thai traditional system as a carminative, expectorant, and antioxidant. A polyherbal formulation “Triphala” of TC, Terminalia bellerica and Emblica officinalis is commonly used in chronic constipation, detoxification, poor digestion and rejuvenator of the body [224]. TC possesses an anti-bacterial [225], anti-viral [226], anthemintic [227], anti-fungal [228], anti-ameobic [229], anti-neoplastic [230], anti-plasmodial [231], antioxidant [232], anti-diabetic [233] and anti-ulcerogenic [234] activity. The TC reported to have an immunomodulatory [229], radioprotective [235], cytoprotective [236], cardioprotective [237], and hepatoprotective [238] activity. Moreover, the hydroalcoholic extract of TC produces a significant inhibition of joint swelling in formaldehyde induced arthritis and CFA induced arthritis models. The anti-arthritic potential of the extract was due to significant reduction in the levels of TNF-α, IL-6, and IL-1β [239].
Trigonella foenum-graecum Linn. (TF)(Family-Papilionaceae)
TF, commonly known as Fenugreek, is an herbaceous plant which has found wide applications as a food, a food additive, and as a traditional medicine. Albuminoids, soluble carbohydrates, woody fibers, and ash are present in TF [240,241]. The plant has wide uses in the traditional medicine and reportedly used to treat diabetes, high cholesterol, wounds, inflammation, and gastrointestinal ailments. Several confections of TF like methi modaka, Svalpamethimodaka etc., are used in rheumatism [51]. Fenugreek seeds have high content of mucilage, choline, and trigonelline. Studies of its extract have shownantihyperglycemic [242], estrogenic [243], antioxidant [244], anticancer [245], anti-inflammatory [246], and antirheumatic activities. The fenugreek mucilage obtained from seeds of the TF at dose 75 mg/kg possess an anti-arthritic activity anddecreased the elevated levels of SGOT, SGPT, CRP, nitrites, ESR, and white blood cell count. The TF may act by decreasing the oxidative stress, cell influx, and release of mediators associated with arthritis. In conclusion, TF showed anti-arthritic activity [247].
Vitex negundo Linn. (VN)(Family-Verbenaceae)
VN is referred to as five leaved chaste tree and a large aromatic shrub or sometimes a smaller slender tree with quadrangular, densely whitish tomentosebranchets. VN is originated in Southern India and Burma [51]. VN have its traditional use in rheumatism, headache, enlarged liver, syphilis, diarrhea, and cholera. Leaves along with garlic, rice and gul is a remedy for rheumatism. In Ayurvedic, Unani and Chinese medicine system the leaves extract of VN was used to treat the rheumatism and inflammation of joints. The Konkan community in Maharashtra used the plant for rheumatism [248]. The chief chemical constituents are nishindine, flavones, luteolin-7-glucoside, casticin, iridoid glycosides, vitamin C, β-sitisterol, and phthalic acid [249]. VN possess different pharmacological activities including anti-inflammatory, analgesic [250-253], anticonvulsant [254], antioxidant [250,255], insecticidal [256,257], and antirheumatic [249]. The active compound agnusideisolated from ethanolic extract of leaves administered at doses of 1.56 mg/10 ml, 3.12 mg/10 ml, 6.25 mg/10 ml and 1.25 mg/10 ml p.o. decreased the elevated levels of ESR, leukotriene B4, PGE2, cytokines, IL-17, TNF-α and interferon gamma. Hence, it can be concluded that the VN possess an anti-arthritic activity [258].
Xanthium strumarium Linn. (XS)(Family-Compositae)
XS commonly known as cochlebur, burweed or burdock datura is anindigenous of tropical parts of India. XS is an annual herb of 1m height with a short, stout, hairy stems, and commonly grows in waste places, roadsides and along river banks in warmer parts. Traditionally, it is used as laxative, anthelmintic, tonic, digestive, antipyretic and also improves appetite, voice, complexion, and memory. XS is also used to cure leukoderma, biliousness, poisonous bites of insects, epilepsy, salivation, and fever. The infusion of plant has been used in treatment of rheumatism in ayurvedic and Chinese medicine system. The active principle of aerial parts of XS are alkaloids; sesquiterpenes lactones such as xanthinin, xanthumin, xanthatin; sulphated glycoside such as xanthostrumarin, atractyloside, carboxyatractyloside; phytosterols, xanthanol, isoxanthanol, xanthosin, 4-oxo-bedfordia acid, hydroquinone, xanthanolides, and deacetylxanthumin [259]. However, recently investigated that XS possess an anti-bacterial [260], anti-tumor [261], anti-cancer [262], anti-tussive [263], anti-fungal [264,265], anti-inflammatory [266,267], vasorelaxant [268], hypoglycaemic [269], antimitotic [270], anti-malarial [271], anti-trypanosomal [272], diuretic [273], anti-allergic [274], and antioxidant activity [275,276]. Oral doses (200 and 400 mg/kg) ofethanolic extract of XS when administered exhibited anti-arthritic activity by inhibiting the release of inflammatory mediators. In conclusion, XS have a potent anti-arthritic activity [277].
DISCUSSION
Since Neanderthal times, the plants had been used for the prevention and cure of various ailments such as RA and other inflammatory diseases. Natural sources such as plants have been considered as the safest and valuable treatment for the disease. From the ethno botanical knowledge, we included the plants that are used in Indian traditional systems such as herbalism, folklore and shamanism. The review article includes more than 485 different plant species that are used for the prevention and cure of RA during last few decades. The botanical name of the plant, family, common name, part used, and various dosage forms studied are summarized in the Table 2. Around more than 100 families are included for 485 plants among them papilionaceae, fabaceae, euphorbiaceae, acanthaceae, compositae, ranunculaceae, malvaceae, rutaceae, liliaceae, labiatae, solanaceae, cruciferae, verbenaceae, lauraceae, and rubiaceae are in major proportion. As shown in Figure 1, around 485 plants have been mentioned in which 19 (4.4%) belongs to family papilonaceae, 17 (4%) to compositae and euphorbiaceae, 15 (3.5%) to rutaceae, 14 (3.3%) to vabenaceae, 13 (3%) to labiatae and fabaceae, 12 (2.7%) to malavaceae and crucuferae, 11 (2.5%) to solanaceae and acanthaceae, 10 (2.3%) to ranunculaceae and liliaceae, 9 (2.1%) to apocynaceae, lauraceae and rubiaceae, 8 (1.8%) to graminae, meliaceae, and umbelliferae, and remaining (48.2%) are categorized as others [Figure 1].
Figure 1.
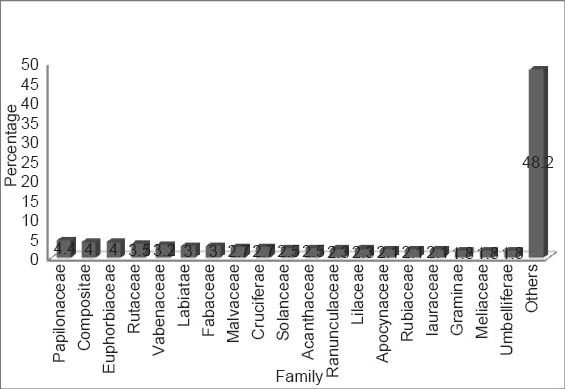
Plants in diverse families with % anti-arthritic activity
From our review, we have noticed that majority of researches were carried mainly in developing countries such as India, China, Korea, and Nigeria. But some developed countries like USA and Japan also continue their research on RA so as to increase the potential benefits [Figure 2].
Figure 2.
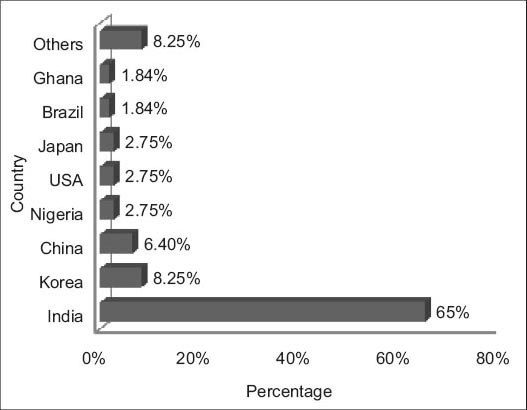
Geographical distribution of researches in the review
For the treatment of RA, various parts of plants are used such as leaves, roots, fruits, rhizomes, and seeds in distinguished dosage forms like extract, decoction, juice, infusion, paste, oil etc. The most potent anti-arthritic plants such as Aconitum ferox, Balsamodendron mukul, BD, Boswellia serrata, CS, CL, PL, Ricinus communis, Plumbago zeylanica, SL, SR, and Strychnos nux vomica have been elaborated in the review article. Among these listed plants, certain plants have been used in acute attack or in chronic pain or chronic rheumatism.
CONCLUSION
Traditional medicines used for the treatment of arthritis are used in various tribal/rural cultures worldwide. At present, investigation of anti-arthritic activity of traditional medicine has led to the development and studies of many herbal remedies employed for such purpose. The information that has been gathered from various sources is helpful in preserving folk indigenous knowledge as well as discovery of potential compounds having promising anti-arthritic activity. The information gathered from the data provides the information on toxicity profile and mechanism of action of tested extracts. Therefore, this review article has been prepared to provide the plants/their parts having specific traditional use in the treatment of arthritis upto year 2013. Moreover, this review has included latest data on new plant species/polyherbal formulations which are not covered in previews reviews on arthritis therapy as per our knowledge.
In conclusion, about 485 plant species mentioned in the list would have a promising anti-arthritic activity in humans. Information about the ethnic proof of the traditionally used anti-arthritic plants was cross-validated from various articles/reviews published in journals. Till know, no such review has analyzed which correlates the plant family, parts used, dosage form with anti-arthritic effects of the plants. Data mentioned in Table 2 show that papilonaceae family contains more plants with anti-arthritic activity whereas among parts, leaves have been maximally used in oil dosage form for the treatment of arthritis. Table 1 provides wealth of information indicates the beneficial effects of polyherbal formulations in the treatment of the arthritis. These includes Rumalaya forte, Rumalaya-liniment, arthacure, ortho joint oil, rheum off gold, Majoon suranjan, HLXL, GHJTY, Sudard, and TBL-II [18,20-24]. The data mentioned in Table 3 in addition provides the dose, toxicity profile, and models with mechanism of action for anti-arthritic activity.
The data discussed in this review might be quite useful in obtaining monographs on plants and recommendations on their use. In this review, we mainly deal with the safety profile, mechanism of action, and toxicity studies of plant extracts. The plant extracts and polyherbal formulations would be served as an alternate therapy for the treatment of arthritis with lesser side effects. Moreover, current knowledge can be helpful in materializing the commercial products, where the evidence can be quite limited.
Future Needs
Majority of traditionally used plants which have been mentioned in Table 2, have not been experimentally proved to have anti-arthritic activity. In addition, data in Table 3 show experimentally, the plants possess anti-arthritic activity only on animals but no clinical data are provided for proving the activity in humans. The data also lack information on exact activity of isolated compounds. However, the emphasis should be given in an area that needs further investigations as studied in animals needs to be translates to humans in order for a natural extract to be recommended for the treatment of arthritis. Therefore, further research of such less explored plants is still needed to determine their anti-arthritic activity.
Limitations
The data studied and prepared had been collected from the literature published in English language only and ignoring the studies published in other languages. The data mentioned in other languages, if had been included, will also be helpful in validating the current data. Further studies on isolated compounds of plants are not included, which otherwise, might be useful in scruitning the cause of anti-arthritic activity of plants.
ACKNOWLEDGMENT
The authors would like to acknowledge UGC, New Delhi for granting Minor Research Project for conducting this study. The authors would also acknowledge Director, Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra for providing necessary facilities for carrying out this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chitme RH, Patel PN. Antiarthritis activity of Aristolochia bracteata extract in experimental animals. Open Natl Pro J. 2009;2:6–15. [Google Scholar]
- 2.Tripathy S, Pradhan D, Anjana M. Anti-inflammatory and antiarthritic potential of Ammania baccifera Linn. Int J Pharm Bio Sci. 2010;1:1–7. [Google Scholar]
- 3.Chunxia C, Peng Z, Huifang P, Hanli R, Zehua H, Jizhou W. Extracts of Arisaema rhizomatum C.E.C. Fischer attenuate inflammatory response on collagen-induced arthritis in BALB/c mice. J Ethnopharmacol. 2011;133:573–82. doi: 10.1016/j.jep.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Babushetty V, Sultanpur MC. Evaluation of anti-arthritis activity of Asystasia dalzelliana leaves. Int J Pharma Biol Arch. 2012;3:377–82. [Google Scholar]
- 5.Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauses SL, Jameson JL. 16th ed. II. United States of America: Mc-Graw Hill Companies; 2005. Harrison's Principle of Internal Medicine. [Google Scholar]
- 6.Ngoc DD, Catrina AI, Lundberg K, Harris HE, Ha NT, Anh PT, et al. Inhibition by Artocarpus tonkinensis of the development of collagen-induced arthritis in rats. Scand J Immunol. 2005;61:234–41. doi: 10.1111/j.1365-3083.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazumder MP, Mondal A, Sasmal D, Arulmozhi S, Rathinavelusamy P. Evaluation of antiarthritic and immunomodulatory activity of Barleria lupulina. Asian Pac J Trop Biomed. 2012;2:S1400–6. [Google Scholar]
- 8.Rajkapoor B, Ravichandran V, Gobinath M, Anbu J, Harikrishnan N, Sumithra M, et al. Effect of Bauhinia variegate on complete freund's adjuvant induced arthritis in rats. J Pharmcol Toxicol. 2007;2:465–72. [Google Scholar]
- 9.Tandon V, Gupta RK. Histomorphological changes induced by Vitex negundo in albino rats. Indian J Pharmacol. 2004;36:176–7. [Google Scholar]
- 10.Vispute S, Khopade A. Glycyrrhiza glabra Linn.-“Klitaka”: A review. Int J Pharm Bio Sci. 2011;2:42–51. [Google Scholar]
- 11.Badami S, Moorkoth S, Suresh B. Caesalpinia sappan a medicinal and dye yielding plant. Nat Prod Rad. 2004;3:75–82. [Google Scholar]
- 12.Kalaria P, Gheewala P, Chakraborty M, Kamath J. A phytopharmacological review of Alstonia scholaris: A panoramic herbal medicine. IJRAP. 2012;3:367–371. [Google Scholar]
- 13.Sudha K, Mathanghi SK. Traditional underutilized green leafy vegetables and its curative properties. Int J Pharm. 2012;2:786–93. [Google Scholar]
- 14.Singh V, Patel H, Suvagiya V, Singh K. Some traditionally used anti-arthritic herbs a review. Int Res J Pharm. 2011;2:43–5. [Google Scholar]
- 15.Kiran D, Rohilla A, Rohilla S, Khan MU. Phyllanthus amarus: An ample therapeutic potential herb. Int J Res Ayur Pharm. 2011;2:1096–9. [Google Scholar]
- 16.Thirumal M, Vadivelan R, Kishore G, Brahmaji VS. Aristolochia bracteolata: An overview on pharmacognostical, phytochemical and pharmacological properties. Earth J. 2012;1:66–78. [Google Scholar]
- 17.Kalita S, Kumar G, Karthik L, Rao BV. A review on medicinal properties of Lantana camara Linn. Res J Pharm Technol. 2012;5:711–5. [Google Scholar]
- 18.Jeoung BR, Lee KD, Na CS, Kim YE, Kim B, Kim YR. Ganghwaljetongyeum, an anti-arthritic remedy, attenuates synoviocyte proliferation and reduces the production of proinflammatory mediators in macrophages: The therapeutic effect of GHJTY on rheumatoid arthritis. BMC Complement Altern Med. 2013;13:47. doi: 10.1186/1472-6882-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel D, Kaur G, Sawant MG, Deshmukh P. Herbal medicine- A natural cure to arthritis. Indian J Nat Prod Res. 2012;4:27–35. [Google Scholar]
- 20.Patel SS, Shah PV. Evaluation of anti-inflammatory potential of the multidrug herbomineral formulation in male Wistar rats against rheumatoid arthritis. J Ayurveda Integr Med. 2013;4:86–93. doi: 10.4103/0975-9476.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S, Nair V, Gupta YK. Antiarthritic activity of majoon suranjan (a polyherbal Unani formulation) in rat. Indian J Med Res. 2011;134:384–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Asad M, Prasad K, Thomas L, Kamath JV. Evaluation of analgesics and anti-inflammatory activity of Sudard, A poly-herbal formulation. Iran J Pharmacol Ther. 2007;6:71–5. [Google Scholar]
- 23.Shen X, Li C, Zhao H, Li S, Chen J, Kobayashi Y, et al. Inhibitory effects of a traditional Chinese herbal formula TBL-II on type II collagen-induced arthritis in mice. J Ethnopharmacol. 2011;134:399–405. doi: 10.1016/j.jep.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Zhang RX, Fan AY, Zhou AN, Moudgil KD, Ma ZZ, Lee DY, et al. Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats. J Ethnopharmacol. 2009;121:366–71. doi: 10.1016/j.jep.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meena AK, Garg N, Nain J, Meena RP, Rao MM. Review on ethanobotany, phytochemical and pharmacological profile of Alstonia scholaris. Int Res J Pharm. 2011;2:49–54. [Google Scholar]
- 26.Khyade MS, Vaikos N. Phytochemical and antibacterial properties of leaves of Alstonia scholaris R. Br. Afr J Biotechnol. 2009;8:6434–6. [Google Scholar]
- 27.Arulmozhi S, Mazumder PM, Narayan LS, Thakurdesai PA. In vitro antioxidant and free radical scavenging activity of fractions from Alstonia scholaris Linn R. Br. Int J Pharm Tech Res. 2010;2:18–25. [Google Scholar]
- 28.Lim-Sylianco CY, Jacano AP, Linn CM. Antimutagenicity of twenty Philippine plants using the micronucleus test in mice. Philipp J Sci. 1990;117:231–5. [Google Scholar]
- 29.Keawpradub N, Kirby GC, Steele JC, Houghton PJ. Antiplasmodial activity of extracts and alkaloids of three Alstonia species from Thailand. Planta Med. 1999;65:690–4. doi: 10.1055/s-1999-14043. [DOI] [PubMed] [Google Scholar]
- 30.Bhogayata K, Sharma PP, Patel BR. A clinical evaluation of Saptaparna (Alstonia scholaris L. R. Br.) on essential hypertension. Ayu. 2009;30:318–322. [Google Scholar]
- 31.Deepti B, Archana J, Manasi J. Antidiabetic and antihyperlipidemic effect of Alstonia scholaris Linn bark in Streptozocin induced diabetic rats. Indian J Pharm Educ. 2011;45:114–120. [Google Scholar]
- 32.Hussain A, Zaman MK, Ramteke AM. Antibacterial activity of trunk bark of Alstonia scholaris. Asian J Pharm Clin Res. 2010;3:46–47. [Google Scholar]
- 33.Shang JH, Cai XH, Feng T, Zhao YL, Luo XD. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J Ethnopharmacol. 2010;129:293–8. doi: 10.1016/j.jep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Iwo MI, Soemardji AA, Retnoningrum DS, Sukrasno Ur. U M. Immunostimulating effect of pule (Alstonia scholaris L. R. Br. Apocynaceae) bark extracts. Clin Hemorheol Microcirc. 2000;23:177–83. [PubMed] [Google Scholar]
- 35.Swafiya J, Ranu C, Kumar PG. Anticancer activity of an Indian medicinal plant, Alstonia scholaris on skin carcinogenesis in mice. Integr Cancer Ther. 2010;9:261–9. doi: 10.1177/1534735409343590. [DOI] [PubMed] [Google Scholar]
- 36.Shang JH, Cai XH, Feng T, Zhao YL, Wang JK, Zhang LY, et al. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J Ethnopharmacol. 2010;129:174–81. doi: 10.1016/j.jep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Lin SC, Lin CC, Lin YH, Supriyatna S, Pan SL. The protective effect of Alstonia scholaris R. Br. on hepatotoxin-induced acute liver damage. Am J Chin Med. 1996;24:153–64. doi: 10.1142/S0192415X96000207. [DOI] [PubMed] [Google Scholar]
- 38.Arulmozhi S, Mazumder PM, Sathiya NP, Thakurdesai A. Antianxiety and antidepressant activity of leaves of Alstonia scholaris Linn R. Br. Pharmacologia. 2012;3:239–248. [Google Scholar]
- 39.Arulmozhi S, Mazumder PM, Sathiyanarayanan L, Ashok P. Anti-arthritic and antioxidant activity of leaves of Alstonia scholaris Linn R Br. Eur J Integr Med. 2011;3:e83–90. [Google Scholar]
- 40.Kalpana Devi B, Kanimozhi S, Suganyadevi P. Phytochemical screening and biological property of Aristolochia bracteata. J Pharm Res. 2011;4:1509–14. [Google Scholar]
- 41.Periyasamy AK, Kumar R, Mahalingam K. Phytochemical screening and antimicrobial activity from five Indian medicinal plants against human pathogens. Middle East J Sci Res. 2010;5:157–162. [Google Scholar]
- 42.Rajamanickam V, Rajasekaram A, Jesupillai M, Darlin Q, Sabitha R. Anti pyretic activity of Aristolochia bracteolate. Internet J Alternat Med. 2009;8:4. [Google Scholar]
- 43.Chitme HR, Malipatil M, Chandrashekhar VM, Prashant PM. Antiallergic activity of Aristolochia bracteolata Lank in animal model. Indian J Exp Biol. 2010;48:46–52. [PubMed] [Google Scholar]
- 44.Niyas MK, Kumar RM, Mani TT, Rahiman FO, Bodhanapu S, Phaneendra P, et al. Anti-ulcer activity of aqueous extracts of Aristolochia bracteolate leaves. Pharmacologyonline. 2011;1:1078–82. [Google Scholar]
- 45.Ramasubramania RR, Niranjan MB. Pharmacognostical phytochemical and antifungal activity of Aristolochia bracteolate Lam in ringworm infection. Res J Pharm Technol. 2011;4:1123. [Google Scholar]
- 46.Parekh J, Chanda S. In vitro anti-microbial activity of some Indian folkfore medicinal plants. J Cell Tissue Res. 2006;6:577–80. [Google Scholar]
- 47.Osawa T. Japan: Japan Scientific Societies Press; 1994. Novel natural antioxidants for utilization in food and biological system; pp. 241–51. [Google Scholar]
- 48.Shirwaikar A, Somashekar AP, Udupa AL, Udupa SL, Somashekar S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Int J Phytother Phytopharmacol. 2003;10:558–62. doi: 10.1078/094471103322331548. [DOI] [PubMed] [Google Scholar]
- 49.Nataraj SK, Puvvada PK, Badami S, Patil SB, Kannan E, Thillainayagam S, et al. Pre-coital and post-coital anti-implantation and abortifacient activities of Aristolochia bracteolata Lam. aerial parts. J Nat Med. 2007;61:302–6. [Google Scholar]
- 50.Rajpoot K, Mishra RN. Boerhaavia diffusa roots (Punarnava mool) - Review as rasayan (rejuvenator/antiaging) Int J Res Pharm Biomed Sci. 2011;2:1451–60. [Google Scholar]
- 51.Nadkarni KM. I. Bombay, India: Poupular Pakashan Pvt. Ltd; 2009. Indian Materia Medica. [Google Scholar]
- 52.Desai SK, Desai SM, Navdeep S, Arya P, Pooja T. Antistress activity of Boerhaavia diffusa root extract and a polyherbal formulation, containing Boerhaavia diffusa using cold restraint stress model. Int J Pharm Pharm Sci. 2011;3:130–32. [Google Scholar]
- 53.Rachh PR, Rachh MR, Modi DC, Shah BN, Bhargava AS, Patel NM, et al. In-vitro evaluation of antioxidant activity of punarnava (Boerhaavia diffusa Linn.) Int J Pharm Res. 2009;1:36–40. [Google Scholar]
- 54.Pandey R, Maurya R, Singh G, Sathiamoorthy B, Naik S. Immunosuppressive properties of flavonoids isolated from Boerhaavia diffusa Linn. Int Immunopharmacol. 2005;5:541–53. doi: 10.1016/j.intimp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Bharali R, Azad MR, Tabassum J. Chemopreventive action of Boerhaavia diffusa on DMBA-induced skin carcinogenesis in mice. Indian J Physiol Pharmacol. 2003;47:459–64. [PubMed] [Google Scholar]
- 56.Mishra JP. Studies on the effect of indigenous drug Boerhaavia diffusa, Rom. on kidney regeneration. Indian J Pharm. 1980;12:59. [Google Scholar]
- 57.Rawat AK, Mehrotra S, Tripathi SC, Shome U. Hepatoprotective activity of Boerhaavia diffusa L. roots – a popular Indian ethnomedicine. J Ethnopharmacol. 1997;56:61–6. doi: 10.1016/s0378-8741(96)01507-3. [DOI] [PubMed] [Google Scholar]
- 58.Gaitonde BB, Kulkarni HJ, Nabar SD. Diuretic activity of punarnava (Boerhaavia diffusa) B Haffkine I. 1974;2:24. [Google Scholar]
- 59.Nalamolu RK, Boini KM, Nammi S. Effect of chronic administration of Boerhaavia diffusa Linn leaf extract on experimental diabetes in rats. Trop J Pharm Res. 2004;3:305–9. [Google Scholar]
- 60.Lohani S, Jan A, Verma HN. In vivo and in vitro resistance induction in tobacco by Boerhaavia diffusa systemic resistance inducing protein and transfer of induced resistance in in vitro tobacco plants. Biotechnology. 2007;3:389–92. [Google Scholar]
- 61.Bhalla TN, Gupta MB, Sheth PK, Bhargava KP. Anti-inflammatory activity of Boerhaavia diffusa. Indian J Physiol Pharmacol. 1968;12:37. [Google Scholar]
- 62.Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP. Anti-inflammatory activity of natural products. I. Triterpenoids. Eur J Pharmacol. 1969;6:67–70. doi: 10.1016/0014-2999(69)90067-3. [DOI] [PubMed] [Google Scholar]
- 63.Dapurkar KV, Sahu KG, Sharma H, Meshram S, Rai G. Anti-arthritic activity of roots extract of Boerhaavia Diffusa in adjuvant induced arthritis rats. Sch Acad J Pharm. 2013;2:107–9. [Google Scholar]
- 64.Upaganlawar A, Ghule B. Pharmacological activities of Boswellia serrata Roxb.- mini review. Ethnobot Leaflets. 2009;13:766–74. [Google Scholar]
- 65.Sane RT. Standardization, quality control, and GMP for herbal drug. Indian Drugs. 2002;39:184–9. [Google Scholar]
- 66.Handa SS. Herbal raw material and traditional remedies. East Pharm. 1995;3:24. [Google Scholar]
- 67.Gupta OP, Sharma N, Chand D. A sensitive and relevant model for evaluating anti-inflammatory activity-papaya latex-induced rat paw inflammation. J Pharmacol Toxicol Methods. 1992;28:15–9. doi: 10.1016/1056-8719(92)90060-e. [DOI] [PubMed] [Google Scholar]
- 68.Menon MK, Kar A. Analgesic and psychopharmacological effects of the gum resin of Boswellia serrata. Planta Med. 1971;19:333–41. doi: 10.1055/s-0028-1099651. [DOI] [PubMed] [Google Scholar]
- 69.Pungle P, Banavalikar M, Suthar A, Biyani M, Mengi S. Immunomodulatory activity of boswellic acids of Boswellia serrata Roxb. Indian J Exp Biol. 2003;41:1460–2. [PubMed] [Google Scholar]
- 70.Tsukada T, Nakashima K, Shirakawa S. Arachidonate 5-lipoxygenase inhibitors show potent antiproliferative effects on human leukemia cell lines. Biochem Biophys Res Commun. 1986;140:832–6. doi: 10.1016/0006-291x(86)90709-6. [DOI] [PubMed] [Google Scholar]
- 71.Huang MT, Badmaev V, Xie JG, Lou YR, Lu YP, Ho CT. Inhibitory effect of an extract of the gum resin exudate of Boswellia serrata on 12-Otetradecanoylphorbol-13-acetate (TPA)-induced skin tumor promotion in mice. P. Am Assoc Cancer Res. 1997;38:368. [Google Scholar]
- 72.Boker DK, Winking M. Die Rolle von Boswellia sauren in der therapie maligner glione. Dtsch Arzteblatt. 1997;94:958–60. [Google Scholar]
- 73.Gerlach U. Methods of Enzymatic Analysis. 3rd ed. III. Weinheim, FL: VCH, W. Germany-Deerfield Beach; 1983. Sorbitol dehydrogenase; pp. 12–117. [Google Scholar]
- 74.Gupta I, Gupta V, Parihar A, Gupta S, Lüdtke R, Safayhi H, et al. Effects of Boswellia serrata gum resin in patients with bronchial asthma: Results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res. 1998;3:511–4. [PubMed] [Google Scholar]
- 75.al-Awadi F, Fatania H, Shamte U. The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res. 1991;18:163–8. [PubMed] [Google Scholar]
- 76.Mishra NK, Bstia S, Mishra G, Chowdary AK, Patra S. Anti-arthritic activity of Glycyrrhiza glabra, Boswellia serrata and their synergistic activity in combined formulation studied in freund's adjuvant induced arthritic rats. J Pharm Educ Res. 2011;2:92–8. [Google Scholar]
- 77.Kirtikar KR, Basu BD. 2nd ed. II. Allahabad: Lalit Mohan Basu; 1989. Indian Medicinal Planta. [Google Scholar]
- 78.Warriers PK, Nambiar VP, Ramankutty C, Vaidhyarathnam PS. Chennai, New-Delhi: Orient Longman Ltd; 1993. Indian Medicinal Plants, A Compendium of 500 Species. [Google Scholar]
- 79.Baek NI, Jeon SG, Ahn EM, Hahn JT, Bahn JH, Jang JS, et al. Anticonvulsant compounds from the wood of Caesalpinia sappan L. Arch Pharm Res. 2000;23:344–8. doi: 10.1007/BF02975445. [DOI] [PubMed] [Google Scholar]
- 80.Kataoka M, Takagaki Y. Effect of the crude drugs on β-hexosaminidase release from rat basophilic leukemia (RBL-2H3) cells. Nat Med. 1995;49:346–9. [Google Scholar]
- 81.Avirutnant W, Pongpan A. The antimicrobial activity of some Thai flowers and plants, The Mahidol University. J Pharm Sci. 1983;10:81–6. [Google Scholar]
- 82.Pongan A, Chumsri P, Taworasate T. The antimicrobial activity of some Thai medicinal plants. Mahidol University. J Pharm Sci. 1982;9:88–91. [Google Scholar]
- 83.Yadava RN, Saxena VK, Nigam SS. Antimicrobial activity of the essential oil of Caesalpinia sappan. Indian Perfume. 1978;22:73–5. [Google Scholar]
- 84.Hikino H, Taguchi T, Fujimura H, Hiramatsu Y. Antiinflammatory principles of Caesalpinia sappan wood and of Haematoxylon campechianum wood. Planta Med. 1977;31:214–20. doi: 10.1055/s-0028-1097516. [DOI] [PubMed] [Google Scholar]
- 85.Itokawa H, Hirayama F, Tsuruoka S, Mizuno K, Takeya K, Nitta A. Screening test for antitumor activity of crude drugs (III). Studies on antitumor activity of Indonesian medicinal plants. Shoyakugaku Zasshi. 1990;44:58–62. [Google Scholar]
- 86.Dhawan BN, Dubey MP, Mehrotra BN, Rastogi RP, Tandon JS. Screening of Indian plants for biological activity: Part IX. Indian J Exp Biol. 1980;18:594–606. [PubMed] [Google Scholar]
- 87.Moon CK, Sim SK, Lee SH, Park SK, Yun YP. Antitumor activity of some phyto based polysaccharides and their effects on the immune function. Arch Pharm Res. 1983;6:123–31. [Google Scholar]
- 88.Kurokawa M, Ochiai H, Nagasaka K, Neki M, Xu H, Kadota S, et al. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Res. 1993;22:175–88. doi: 10.1016/0166-3542(93)90094-y. [DOI] [PubMed] [Google Scholar]
- 89.Chung TH, Kim JC, Kim MK, Choi SC. Investigation of Korean plant extracts for potential phytotherapeutic agents against B-virus Hepatitis. Phytother Res. 1995;9:429–34. [Google Scholar]
- 90.Nikaido T, Ohmoto T, Noguchi H, Kinoshita T, Saitoh H, Sankawa U. Inhibitors of cyclic AMP phosphodiesterase in medicinal plants. Planta Med. 1981;43:18–23. doi: 10.1055/s-2007-971466. [DOI] [PubMed] [Google Scholar]
- 91.Lee KT, Kim JH. Brazilin as a new sunless tanning agent. Sci Conf Asian Soc Cosmet Sci. 1999;3:33–6. [Google Scholar]
- 92.Liu XR, Han WQ, Sun DR. Treatment of intestinal metaplasia and atypical hyperplasia of gastric mucosa with xiao wei yan powder. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1992;12:602–3. 580. [PubMed] [Google Scholar]
- 93.Wang YZ, Sun SQ, Zhou YB. Extract of the dried heartwood of Caesalpinia sappan L. attenuates collagen-induced arthritis. J Ethnopharmacol. 2011;136:271–8. doi: 10.1016/j.jep.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 94.Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimarães FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39:421–9. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
- 95.Silveira FN, Tufik S. Comparative effects between cannabidiol and diazepam on neophobia, food intake and conflict behavior. Res Comm Psychol Psychaitr Behav. 1981;6:25–6. [Google Scholar]
- 96.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82–8. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 97.Moreira FA, Guimarães FS. Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol. 2005;512:199–205. doi: 10.1016/j.ejphar.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 98.Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 2000;97:9561–6. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Das M, Mandal S, Mallick B, Hazra J. Ethanobotany, phytochemical and pharmacological aspects of Cinnamomum zeylanicum Blume. Int Res J Pharm. 2013;4:58–63. [Google Scholar]
- 100.Tang W, Salmeron MC. Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int J Food Microbiol. 2003;85:73–81. doi: 10.1016/s0168-1605(02)00484-1. [DOI] [PubMed] [Google Scholar]
- 101.Shan B, Cai YZ, Brooks JD, Corke H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J Agric Food Chem. 2007;55:5484–90. doi: 10.1021/jf070424d. [DOI] [PubMed] [Google Scholar]
- 102.Valero M, Salmerón MC. Antibacterial activity of 11 essential oils against Bacillus cereus in tyndallized carrot broth. Int J Food Microbiol. 2003;85:73–81. doi: 10.1016/s0168-1605(02)00484-1. [DOI] [PubMed] [Google Scholar]
- 103.Mancini DA, Dias AL, Pinto JR, Mancini FJ. Antioxidant aqueous extract from cinnamon (Cinnamomum zeylanicum, Blume) as inhibitors of influenza virus. Rev Bras Cien Farma. 1999;35:155–60. [Google Scholar]
- 104.Kim SI, Roh JY, Kim DH, Lee HS, Ahn YJ. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae (L.) and Callosobruchus chinensis (L.) J Stored Prod Res. 2003;39:293–303. [Google Scholar]
- 105.Tailing M, Gupta BK, Sharma A. Antidiabetic activity of alcoholic extract of Cinnamomum zeylanicum leaves. People's J Sci Res. 2008;1:9–11. [Google Scholar]
- 106.Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA, et al. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: Implications for cinnamon regulation of insulin signalling. Horm Res. 1998;50:177–82. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]
- 107.Rani P, Venkatesan M, Binilraj J, Sasidha SS, Amma P. Antioxidant and cytotoxic potential of acetone and methanolic extract of C. zeylanicum dry bark. J Cell Tissue Res. 2010;10:2131–8. [Google Scholar]
- 108.Taker M, Deny S, Mohamad SR, Fadzilah A, Abdul M, Hasnah SM, et al. Antioxidant activity of cinnamtannin B1 from Cinnamomum zeylanicum Blume. Phytomedicine. 2007;16:601–8. [Google Scholar]
- 109.Vetal S, Subhash LB, Vishwaraman M, Prasad AT. Anti-inflammatory and anti-arthritic activity of type-A procyanidine polyphenols from bark of Cinnamomum zeylanicum in rats. Food Sci Hum Wellness. 2013;2:59–67. [Google Scholar]
- 110.Verma A, Pandeya SN, Yadav SK, Singh S, Soni P. A review on Coriandrum sativum (Linn.): An ayurvedic medicinal herb of happiness. J Adv Pharm Healthc Res. 2011;1:29–48. [Google Scholar]
- 111.Rajeshwari U, Andulla B. Medicinal benefits of coriander (Coriandrum sativum L.) Spatula DD. 2011;1:51–8. [Google Scholar]
- 112.De Marco A, Senatore F, Capasso F, Iacobellis NS, Cantore PL. Coriandrum sativum and fenugreek has broad antibacterial activity against bacterial diseases of plants. J Agric Food Chem. 2004;52:7862–6. doi: 10.1021/jf0493122. [DOI] [PubMed] [Google Scholar]
- 113.Kubo I, Fujita K, Kubo A, Nihei K, Ogura T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J Agric Food Chem. 2004;52:3329–32. doi: 10.1021/jf0354186. [DOI] [PubMed] [Google Scholar]
- 114.Vejdani R, Shalmani HR, Mir-Fattahi M, Sajed-Nia F, Abdollahi M, Zali MR, et al. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: A pilot study. Dig Dis Sci. 2006;51:1501–7. doi: 10.1007/s10620-006-9079-3. [DOI] [PubMed] [Google Scholar]
- 115.Chithra V, Leelamma S. Coriandrum sativum changes the levels of lipid peroxides and activity of antioxidant enzymes in experimental animals. Indian J Biochem Biophys. 1999;36:59–61. [PubMed] [Google Scholar]
- 116.Sultana S, Ripa FA, Hamid K. Comparative antioxidant activity study of some commonly used spices in Bangladesh. Pak J Biol Sci. 2010;13:340–3. doi: 10.3923/pjbs.2010.340.343. [DOI] [PubMed] [Google Scholar]
- 117.Mishra A, Prakash D, Bajpai M. The aerial parts of coriandrum sativum, spinach and fenugreek have higher values of anti-radical power compared with their seeds. Int J Food Sci Nutr. 2005;56:473–81. doi: 10.1080/09637480500524299. [DOI] [PubMed] [Google Scholar]
- 118.Leelamma S, Chithra V. Coriandrum sativum has a protective role against the deleterious effects in lipid metabolism in experimental colon cancer. J Ethnopharmacol. 2000;71:457–63. doi: 10.1016/s0378-8741(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 119.Suliman SH, Elmahdi B, Abuelgasim AI. The effect of feeding Coriandrum sativum fruits powder on the plasma lipids profile in cholesterol fed rats. Res J Anim Vet Sci. 2008;3:24–8. [Google Scholar]
- 120.Nair V, Singh S, Gupta YK. Evaluation of disease modifying activity of Coriandrum sativum in experimental models. Indian J Med Res. 2012;135:240–5. [PMC free article] [PubMed] [Google Scholar]
- 121.Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–8. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay A, Basu N, Ghatak N, Gujral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–15. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- 123.Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol. 1973;25:447–52. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 124.Araujo CA, Alegrio LV, Gomes DC, Lima ME, Gomes-Cardoso L, Leon LL. Studies on the effectiveness of diarylheptanoids derivatives against Leishmania amazonensis. Mem Inst Oswaldo Cruz. 1999;94:791–4. doi: 10.1590/s0074-02761999000600015. [DOI] [PubMed] [Google Scholar]
- 125.Araújo CAC, Alegrio LV, Castro D, Lima ME, Leon LL. Leishmania amazonensis: In vivo experiments with diarylheptanoids from Leguminosae and Zingiberaceae plants. Mem Inst Oswaldo Cruz. 1998;93:306. [Google Scholar]
- 126.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: Synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 1993;41:1640–3. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 127.Bhavani Shankar TN, Sreenivasa Murthy V. Effect of turmeric (Curcuma longa) fractions on the growth of some intestinal & pathogenic bacteria in vitro. Indian J Exp Biol. 1979;17:1363–6. [PubMed] [Google Scholar]
- 128.Huang HC, Jan TR, Yeh SF. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur J Pharmacol. 1992;221:381–4. doi: 10.1016/0014-2999(92)90727-l. [DOI] [PubMed] [Google Scholar]
- 129.Park EJ, Jeon CH, Ko G, Kim J, Sohn DH. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52:437–40. doi: 10.1211/0022357001774048. [DOI] [PubMed] [Google Scholar]
- 130.Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.) J Agric Food Chem. 2010;58:842–9. doi: 10.1021/jf9027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kokate CK, Purohit AP, Gokhale SB. 43rd ed. New Delhi, India: Nirali Prakashan; 2009. Pharmacognosy. [Google Scholar]
- 132.Sheth A. Ist ed. Vol. 2. Gujarat, India: Hi Scan Pvt. Ltd; 2005. The Herbs of India. [Google Scholar]
- 133.Rastogi RP, Mehrotra BN. Vol. 1. New Delhi: Central Drug Research Institute, Lucknow and National Institute of Science Communication and Information Resources; 1960. Compedium of Indian Medicinal Plants. [Google Scholar]
- 134.Maurya SK, Raj K, Srivastava AK. Antidyslipidaemic activity of Glycyrrhiza glabra in high fructose diet induced dsyslipidaemic Syrian golden hamsters. Indian J Clin Biochem. 2009;24:404–9. doi: 10.1007/s12291-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saxena M, Saxena J, Khare S. A brief review on: Therapeutical values of Lantana camara plant. Int J Pharm Life Sci. 2012;3:1551–4. [Google Scholar]
- 136.Ghisalberti EL. Lantana camara L. (Verbenaceae) Fitoterapia. 2000;71:467–86. doi: 10.1016/s0367-326x(00)00202-1. [DOI] [PubMed] [Google Scholar]
- 137.Ganjewal D, Sam S, Khan KH. Biochemical compositions and antibacterial activities of Lantana camara plants with yellow lavender, red and white flowers. Eurasian J Biol Sci. 2009;3:69–77. [Google Scholar]
- 138.Mayee R, Thosar A. Evaluation of Lantana camara Linn. (Verbenaceae) for antiurolithiatic and antioxidant activities in rats. Int J Pharm Clin Res. 2011;3:10–4. [Google Scholar]
- 139.Ganesh T. Pharmacognostic and anti-hyperglycemic evaluation of Lantana camara (L.). var. aculeate leaves in alloxan-induced hyperglycemic rats. Int J Res Pharm Sci. 2010;1:247–52. [Google Scholar]
- 140.Venkatachalam T. Antidiabetic activity of Lantana camara Linn fruits in normal and streptozotocin-induced diabetic rats. J Pharm Res. 2011;4:1550–2. [Google Scholar]
- 141.Gidwani BK. Analgesic, anti-inflammatory and antihemorrhoidal activity of aqueous extract of Lantana camara Linn. Res J Pharm Technol. 2009;2:378–81. [Google Scholar]
- 142.Sagar L, Sehgal R, Ojha S. Evaluation of antimotility effect of Lantana camara L. var. acuelata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complement Altern Med. 2005;5:18. doi: 10.1186/1472-6882-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Srivastava D, Singh P. Antifungal potential of two common weeds against plant pathogenic fungi- Alternaria sps. Asian J Exp Biol Sci. 2011;2:525–8. [Google Scholar]
- 144.Tripathi S. Potential of Lantana camara Linn weed against wood destroying fungi. Indian Forest. 2009;135:403–11. [Google Scholar]
- 145.Barreto F, Sousa E, Campos A, Costa J, Rodrigues F. Antibacterial activity of Lantana camara Linn and Lantana montevidensis brig extracts from cariri-ceará, Brazil. J Young Pharm. 2010;2:42–4. doi: 10.4103/0975-1483.62211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Badakhshan MP. A comparative study: Antimicrobial activity of methanol extracts of Lantana camara various parts. Pharmacogn Res. 2009;1:348–51. [Google Scholar]
- 147.de Mello FB, Jacobus D, de Carvalho KC, de Mello JR. Effects of Lantana camara (Verbenaceae) on rat fertility. Vet Hum Toxicol. 2003;45:20–3. [PubMed] [Google Scholar]
- 148.Pour BM, Latha LY, Sasidharan S. Cytotoxicity and oral acute toxicity studies of Lantana camara leaf extract. Molecules. 2011;16:3663–74. doi: 10.3390/molecules16053663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar MS, Maneemegalai S. Evaluation of larvicidal effect of Lantana camara Linn. against mosquito species Aedes aegypti and Culex quinquefasciatus. Adv Biol Res. 2008;2:39–43. [Google Scholar]
- 150.Abdulla MA. Acceleration of wound healing potential by Lantana camara leaf extract in experimental rats. Res J Med Sci. 2009;3:75–9. [Google Scholar]
- 151.Gundamaraju R, Sheeba DS, Ramesh C. Evaluation of anti-arthritic effects of Lantana camara var Linn. using acute model on albino rats. Int J Adv Pharm Sci. 2012;3:272–7. [Google Scholar]
- 152.Kiran D, Rohilla A, Rohilla S, Khan MU. Pleiotropic multifaceted therapeutic potential of Phyllanthus amarus. Int J Pharm Biol Arch. 2011;2:610–4. [Google Scholar]
- 153.Kassuya CA, Leite DF, de Melo LV, Rehder VL, Calixto JB. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med. 2005;71:721–6. doi: 10.1055/s-2005-871258. [DOI] [PubMed] [Google Scholar]
- 154.Mazumder A, Mahato A, Mazumder R. Antimicrobial potentiality of Phyllanthus amarus against drug resistant pathogens. Nat Prod Res. 2006;20:323–6. doi: 10.1080/14786410600650404. [DOI] [PubMed] [Google Scholar]
- 155.Okigbo RN, Igwe DI. Antimicrobial effects of Phyllanthus amarus using agar-well diffusion and disc-diffusion methods. Control Acta Microbiol Immunol Hung. 2007;54:353–356. doi: 10.1556/AMicr.54.2007.4.3. [DOI] [PubMed] [Google Scholar]
- 156.Kumar RN, Joy KL, Kuttan G, Ramsewak RS, Nair MG, Kuttan R. Antitumour and anti-carcinogenic activity of Phyllanthus amarus extract. J Ethanopharmacol. 2002;113:17–22. doi: 10.1016/s0378-8741(01)00419-6. [DOI] [PubMed] [Google Scholar]
- 157.Rao MV, Alice KM. Contraceptive effects of Phyllanthus amarus in female mice. Phytother Res. 2001;15:265–7. doi: 10.1002/ptr.735. [DOI] [PubMed] [Google Scholar]
- 158.Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol. 2007;113:503–9. doi: 10.1016/j.jep.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 159.Ali H, Houghton PJ, Soumyanath A. Alpha-Amylase inhibitory activity of some Malaysian plants used to treat diabetes;with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006;107:449–55. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Odetola AA, Akojenu SM. Anti-diarrhoeal and gastro-intestinal potentials of the aqueous extract of Phyllanthus amarus (Euphorbiaceae) Afr J Med Med Sci. 2000;29:119–22. [PubMed] [Google Scholar]
- 161.Harikumar KB, Kuttan R. Protective effect of Phyllanthus amarus against radiation-induced changes in the intestine and mouse chromosomal damage. J Radiat Res. 2007;48:469–76. doi: 10.1269/jrr.07039. [DOI] [PubMed] [Google Scholar]
- 162.Kassuya CA, Silvestre AA, Rehder VL, Calixto JB. Anti-allodynic and anti-oedematogenic properties of the extract and lignans from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. Eur J Pharmacol. 2003;478:145–53. doi: 10.1016/j.ejphar.2003.08.079. [DOI] [PubMed] [Google Scholar]
- 163.Wright CL, Van-Buren L, Kroner CI, Koning MM. Anti-allodynic and anti-oedematogenic properties of the extract and lignans from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. J Ethnopharmacol. 2007;114:1–31. [Google Scholar]
- 164.Kumar KB, Kuttan R. Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomedicine. 2005;12:494–500. doi: 10.1016/j.phymed.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 165.Malia SM, Sinnathambia A, Kapasea CU, Bodhankara SL, Mahadik KR. Anti-arthritic activity of standardised extract of Phyllanthus amarus in freund's complete adjuvant induced arthritis. Biomed Aging Pathol. 2011;1:85–190. [Google Scholar]
- 166.Chauhan K, Solanki R, Patel A, Macwan C, Patel M. Phytochemical and therapeutic potential of Piper longum Linn: A review. Int J Res Ayur Pharm. 2011;2:157–61. [Google Scholar]
- 167.Chatterjee A, Dutta C. The structure of piper longumine. A new alkaloid isolated from the roots of Piper longum Linn. (Piperaceae) Sci Cult. 1963;29:568. [Google Scholar]
- 168.Sharma A, Singh R. Screening of anti-inflammatory activity of certain indigenous drugs on carragenin induced hind paw oedema in rats. Bull Med Ethnobot Res. 1980;2:262. [Google Scholar]
- 169.Rao C, Nigam S. Antimicrobial activity of essential oils. Indian J Pharm Sci. 1968;30:150. [Google Scholar]
- 170.Banga S, Garg L, Atal C. Effects of piplartine and crude extracts of Piper longum on the ciliary movements. Indian J Pharm Sci. 1964;26:139. [Google Scholar]
- 171.Mananvalan G, Singh J. Chemical and some pharmacological studies on leaves of P. longum Linn. Indian J Pharm Sci. 1979;41:190. [Google Scholar]
- 172.Yende SR, Sannapuri VD, Vyawahare SN, Harle UN. Antirheumatoid activity of aqueous extract of Piper longum on freund's adjuvant-induced arthritis in rats. Int J Pharm Sci Res. 2010;1:129–33. [Google Scholar]
- 173.Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.). A review. Altern Med Rev. 2008;13:128–44. [PubMed] [Google Scholar]
- 174.Balbir-Gurman A, Fuhrman B, Braun-Moscovici Y, Markovits D, Aviram M. Consumption of pomegranate decreases serum oxidative stress and reduces disease activity in patients with active rheumatoid arthritis: A pilot study. Isr Med Assoc J. 2011;13:474–9. [PubMed] [Google Scholar]
- 175.Ahmed S, Wang N, Hafeez BB, Cheruvu VK, Haqqi TM. Punica granatum L. extract inhibits IL-1βeta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J Nutr. 2005;135:2096–102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Albrecht M, Jiang W, Kumi-Diaka J, Lansky EP, Gommersall LM, Patel A, et al. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J Med Food. 2004;7:274–83. doi: 10.1089/jmf.2004.7.274. [DOI] [PubMed] [Google Scholar]
- 177.Lansky EP, Jiang W, Mo H, Bravo L, Froom P, Yu W, et al. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Invest New Drugs. 2005;23:11–20. doi: 10.1023/B:DRUG.0000047101.02178.07. [DOI] [PubMed] [Google Scholar]
- 178.Rosenblat M, Hayek T, Aviram M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis. 2006;187:363–71. doi: 10.1016/j.atherosclerosis.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 179.Chidambara Murthy KN, Jayaprakasha GK, Singh RP. Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem. 2002;50:4791–5. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 180.Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–8. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 181.Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, et al. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: Role of lowering circulating lipids. Br J Pharmacol. 2005;145:767–74. doi: 10.1038/sj.bjp.0706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ignarro LJ, Byrns RE, Sumi D, de Nigris F, Napoli C. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide. 2006;15:93–102. doi: 10.1016/j.niox.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 183.Rosenblat M, Volkova N, Coleman R, Aviram M. Pomegranate byproduct administration to apolipoprotein e-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem. 2006;54:1928–35. doi: 10.1021/jf0528207. [DOI] [PubMed] [Google Scholar]
- 184.Sumner MD, Elliott-Eller M, Weidner G, Daubenmier JJ, Chew MH, Marlin R, et al. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am J Cardiol. 2005;96:810–4. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 185.Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci U S A. 2005;102:14813–8. doi: 10.1073/pnas.0505870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Malik A, Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;5:371–3. doi: 10.4161/cc.5.4.2486. [DOI] [PubMed] [Google Scholar]
- 187.Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. 2006;6:79–92. [PubMed] [Google Scholar]
- 188.Vasconcelos LC, Sampaio MC, Sampaio FC, Higino JS. Use of Punica granatum as an antifungal agent against candidosis associated with denture stomatitis. Mycoses. 2003;46:192–6. doi: 10.1046/j.1439-0507.2003.00884.x. [DOI] [PubMed] [Google Scholar]
- 189.Voravuthikunchai SP, Limsuwan S. Medicinal plant extracts as anti- Escherichia coli O157: H7 agents and their effects on bacterial cell aggregation. J Food Prot. 2006;69:2336–41. doi: 10.4315/0362-028x-69.10.2336. [DOI] [PubMed] [Google Scholar]
- 190.Machado TB, Leal IC, Amaral AC. Antimicrobial ellagitannin of Punica granatum fruits. J Braz Chem Soc. 2002;13:606–10. [Google Scholar]
- 191.Azadzoi KM, Schulman RN, Aviram M, Siroky MB. Oxidative stress in arteriogenic erectile dysfunction: Prophylactic role of antioxidants. J Urol. 2005;174:386–93. doi: 10.1097/01.ju.0000161209.39959.67. [DOI] [PubMed] [Google Scholar]
- 192.Türk G, Sönmez M, Aydin M, Yüce A, Gür S, Yüksel M, et al. Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clin Nutr. 2008;27:289–96. doi: 10.1016/j.clnu.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 193.Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–15. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 194.Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr Res. 2005;57:858–64. doi: 10.1203/01.PDR.0000157722.07810.15. [DOI] [PubMed] [Google Scholar]
- 195.West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci. 2007;29:363–72. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–43. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Asgarpanah J, Khoshkam R. Phytochemistry and pharmacological properties of Ruta graveolens L. J Med Plants Res. 2012;6:3942–9. [Google Scholar]
- 198.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 199.Ratheesh M, Helen A. Anti-inflammatory activity of Ruta graveolens Linn on carrageenan induced paw edema in wistar male rats. Afr J Biotechnol. 2007;6:1209–11. [Google Scholar]
- 200.Endale M, Lee WM, Kwak YS, Kim NM, Kim BK, Kim SH, et al. Torilin ameliorates type II collagen-induced arthritis in mouse model of rheumatoid arthritis. Int Immunopharmacol. 2013;16:232–42. doi: 10.1016/j.intimp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 201.Chowdhury AK, Steinberger E. Effect of 5alpha reduced androgens on sex accessory organs, initiation and maintenance of spermatogenesis in the rat. Biol Reprod. 1975;12:609–17. doi: 10.1095/biolreprod12.5.609. [DOI] [PubMed] [Google Scholar]
- 202.Khouri NA, El-Akawi Z. Antiandrogenic activity of Ruta graveolens L in male Albino rats with emphasis on sexual and aggressive behavior. Neuro Endocrinol Lett. 2005;26:823–9. [PubMed] [Google Scholar]
- 203.Ahmed OM, Moneim AA, Yazid IA, Mahmoud AM. Antihyperglycemic, anti-hyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozocin induced diabetic rats. Diabetol Croat. 2010;39:15–35. [Google Scholar]
- 204.Liao K, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: Importance of the partition coefficient. J Agric Food Chem. 2000;48:2266–70. doi: 10.1021/jf990946w. [DOI] [PubMed] [Google Scholar]
- 205.Raz I, Eldor R, Cernea S, Shafrir E. Diabetes: Insulin resistance and derangements in lipid metabolism. Cure through intervention in fat transport and storage. Diabetes Metab Res Rev. 2005;21:3–14. doi: 10.1002/dmrr.493. [DOI] [PubMed] [Google Scholar]
- 206.Pathak S, Multani AS, Banerji P, Banerji P. Ruta 6 selectively induces cell death in brain cancer cells but proliferation in normal peripheral blood lymphocytes: A novel treatment for human brain cancer. Int J Oncol. 2003;23:975–82. [PubMed] [Google Scholar]
- 207.Ratheesh M, Shyni GL, Sindhu G, Helen A. Protective effects of isolated polyphenolic and alkaloid fractions of Ruta graveolens L. on acute and chronic models of inflammation. Inflammation. 2010;33:18–24. doi: 10.1007/s10753-009-9154-y. [DOI] [PubMed] [Google Scholar]
- 208.Shah NC. Herbal folk medicines in Northern India. J Ethnopharmacol. 1982;6:293–301. doi: 10.1016/0378-8741(82)90052-6. [DOI] [PubMed] [Google Scholar]
- 209.Altman R. Capsaicin cream 0.625% as monothrapy for osteoarthritis: A double blind study. Semin Arthritis Rheum. 1994;23:25–33. [Google Scholar]
- 210.Negi JS, Biht VK, Bhandari AK, Bhatt VP, Sati MK, Mohanty JP, et al. Antidiarrheal activity of methanol extract and major essential oil contents of Saussurea lappa Clarke. Afr J Pharm Pharmacol. 2013;7:474–7. [Google Scholar]
- 211.Mitra SK, Gopumadhavan S, Hemavathi TS, Muralidhar TS, Venkataranganna MV. Protective effect of UL-409, a herbal formulation against physical and chemical factor induced gastric and duodenal ulcers in experimental animals. J Ethnopharmacol. 1996;52:165–9. doi: 10.1016/0378-8741(95)01414-4. [DOI] [PubMed] [Google Scholar]
- 212.Sutar N, Garai R, Sharma US, Singh N, Roy SD. Antiulcerogenic activity of Saussurea lappa root. Int J Pharm Life Sci. 2011;2:516–20. [Google Scholar]
- 213.Hasson SS, Al-Balushi MS, Alharthy K, Al-Busaidi JZ, Aldaihani MS, Othman MS, et al. Evaluation of anti-resistant activity of Auklandia (Saussurea lappa) root against some human pathogens. Asian Pac J Trop Biomed. 2013;3:557–62. doi: 10.1016/S2221-1691(13)60113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Robinson A, Kumar TV, Sreedhar E, Naidu VG, Krishna SR, Babu KS, et al. Anew sesquiterpene lactone from the roots of Saussurea lappa: Structure-anticancer activity study. Bioorg Med Chem Lett. 2008;18:4015–7. doi: 10.1016/j.bmcl.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 215.Yaeesh S, Jamal Q, Shah AJ, Gilani AH. Antihepatotoxic activity of Saussurea lappa extract on D-galactosamine and lipopolysaccharide-induced hepatitis in mice. Phytother Res. 2010;24(Suppl 2):S229–32. doi: 10.1002/ptr.3089. [DOI] [PubMed] [Google Scholar]
- 216.Chen HC, Chou CK, Lee SD, Wang JC, Yeh SF. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995;27:99–109. doi: 10.1016/0166-3542(94)00083-k. [DOI] [PubMed] [Google Scholar]
- 217.Gokhale AB, Damre AS, Kulkami KR, Saraf MN. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine. 2002;9:433–7. doi: 10.1078/09447110260571689. [DOI] [PubMed] [Google Scholar]
- 218.Gupta SR, Nirmal SA, Patil RY, Asane GS. Anti-arthritic activity of various extracts of Sida rhombifolia aerial parts. Nat Prod Res. 2009;23:689–95. doi: 10.1080/14786410802242778. [DOI] [PubMed] [Google Scholar]
- 219.Rastogi RP, Mehrotra BN. III. Lucknow: CDRI; 1993. Compendium of Indian Medicinal Plants. [Google Scholar]
- 220.Islam ME, Haque ME, Mosaddik MA. Cytotoxicity and antibacterial activity of Sida rhombifolia (Malvaceae) grown in Bangladesh. Phytother Res. 2003;17:973–5. doi: 10.1002/ptr.1294. [DOI] [PubMed] [Google Scholar]
- 221.Alam M, Joy S, Ali SU. Antibacterial activity of Sida cordifolia Linn Sida rhomboidea Roxb. and Triumfetta rotundifolia Lam. Indian Drugs. 1991a;28:570–2. [Google Scholar]
- 222.Bhatt DJ, Baxi AJ, Parikh AR. Chemical investigations of leaves of Sida rhombifolia Linn. J Indian Chem Soc. 1983;60:98. [Google Scholar]
- 223.Alam M, Joy S, Ali SU. Screening of Sida cordifolia Linn, Sida rhombifolia and Triumfetta rotundifolia for anti-inflammatory and anti-pyretic drugs. Indian Drugs. 1991b;28:397–9. [Google Scholar]
- 224.Prasad L, Husain Khan T, Jahangir T, Sultana S. Chemomodulatory effects of Terminalia chebula against nickel chloride induced oxidative stress and tumor promotion response in male Wistar rats. J Trace Elem Med Biol. 2006;20:233–9. doi: 10.1016/j.jtemb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 225.Kannan P, Ramadevi SR, Hopper W. Antibacterial activity of Terminalia chebula fruit extract. Afr J Microbiol Res. 2009;3:180–4. [Google Scholar]
- 226.Li G, Liu D, Zhang Y, Qian Y, Zhang H, Guo S, et al. Hydrolysable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 2011;85:4386–98. doi: 10.1128/JVI.01492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kamaraj C, Rahuman AA. Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res Vet Sci. 2011;91:400–4. doi: 10.1016/j.rvsc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 228.Shinde SL, More SM, Junne SB, Wadje SS. The antifungal activity of five Terminalia species checked by paper disk method. Int J Pharma Res Dev. 2011;3:36–40. [Google Scholar]
- 229.Sohni YR, Bhatt RM. Activity of a crude extract formulation in experimental hepatic amoebiasis and in immunomodulation studies. J Ethnopharmacol. 1996;54:119–24. doi: 10.1016/s0378-8741(96)01457-2. [DOI] [PubMed] [Google Scholar]
- 230.Ponnusankar S, Pandit S, Babu R, Bandyopadhyay A, Mukherjee PK. Cytochrome P450 inhibitory potential of Triphala – a Rasayana from Ayurveda. J Ethnopharmacol. 2011;133:120–5. doi: 10.1016/j.jep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 231.Pinmai K, Hiriote W, Soonthornchareonnon N, Jongsakul K, Sireeratawong S, Tor-Udom S. In vitro and in vivo antiplasmodial activity and cytotoxicity of water extracts of Phyllanthus emblica, Terminalia chebula, and Terminalia bellerica. J Med Assoc Thai. 2010;93(Suppl 7):S120–6. [PubMed] [Google Scholar]
- 232.Chen X, Sun F, Ma L, Wang J, Qin H, Du G. In vitro evaluation on the antioxidant capacity of triethylchebulate, an aglycone from Terminalia chebula Retz fruit. Indian J Pharmacol. 2011;43:320–3. doi: 10.4103/0253-7613.81508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Murali YK, Anand P, Tandon V, Singh R, Chandra R, Murthy PS. Long-term effects of Terminalia chebula Retz. on hyperglycemia and associated hyperlipidemia, tissue glycogen content and in vitro release of insulin in streptozotocin induced diabetic rats. Exp Clin Endocrinol Diabetes. 2007;115:641–6. doi: 10.1055/s-2007-982500. [DOI] [PubMed] [Google Scholar]
- 234.Sharma P, Prakash T, Kotresha D, Ansari MA, Sahrm UR, Kumar B, et al. Antiulcerogenic activity of Terminalia chebula fruit in experimentally induced ulcer in rats. Pharm Biol. 2011;49:262–8. doi: 10.3109/13880209.2010.503709. [DOI] [PubMed] [Google Scholar]
- 235.Gandhi NM, Nair CK. Radiation protection by Terminalia chebula: Some mechanistic aspects. Mol Cell Biochem. 2005;277:43–8. doi: 10.1007/s11010-005-4819-9. [DOI] [PubMed] [Google Scholar]
- 236.Hamada S, Kataoka T, Woo JT, Yamada A, Yoshida T, Nishimura T, et al. Immunosuppressive effects of gallic acid and chebulagic acid on CTL-mediated cytotoxicity. Biol Pharm Bull. 1997;20:1017–9. doi: 10.1248/bpb.20.1017. [DOI] [PubMed] [Google Scholar]
- 237.Suchalatha S, Shyamala Devi CS. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J Exp Biol. 2004;42:174–8. [PubMed] [Google Scholar]
- 238.Tasduq SA, Singh K, Satti NK, Gupta DK, Suri KA, Johri RK. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum Exp Toxicol. 2006;25:111–8. doi: 10.1191/0960327106ht601oa. [DOI] [PubMed] [Google Scholar]
- 239.Nair V, Singh S, Gupta YK. Anti-arthritic and disease modifying activity of Terminalia chebula Retz. in experimental models. J Pharm Pharmacol. 2010;62:1801–6. doi: 10.1111/j.2042-7158.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- 240.Jayaweera DM. Sri Lanka: Royal Botanic Garden, Peradeniya; 1981. Medicinal Plant. Part III; p. 225. [Google Scholar]
- 241.Yoshikawa M, Murakami T, Komatsu H, Murakami N, Yamahara J, Matsuda H. Medicinal foodstuffs. IV. Fenugreek seed. (1): Structures of trigoneosides Ia, Ib, IIa, IIb, IIIa, and IIIb, new furostanol saponins from the seeds of Indian Trigonella foenum - graecum L. Chem Pharm Bull (Tokyo) 1997;45:81–7. doi: 10.1248/cpb.45.81. [DOI] [PubMed] [Google Scholar]
- 242.Kulkarni CP, Bodhankar SL, Ghule AK, Mohan V, Thakurdeai PA. Antidiabetic activity of Trigonella foenum graecum L. seeds extract in neonatal streptocotocin-induced (N-STZ) rats. Diabetol Croat. 2012;41:29–40. [Google Scholar]
- 243.Sreeja S, Anju VS, Sreeja S. In vitro estrogenic activities of fenugreek Trigonella foenum graecum seeds. Indian J Med Res. 2010;131:814–9. [PubMed] [Google Scholar]
- 244.Subhashini N, Thangathirupathi A, Lavanya N. Antioxidant activity of Trigonella foenum greacum using various in vitro and ex vivo models. Int J Pharm Pharm Sci. 2011;3:96–102. [Google Scholar]
- 245.Alizadeh S, Jahanmehr SA, Ardjmand AR, Rezian M, Dargahi H, Einolahi N, et al. Antineoplastic effect of fenugreek (Trigonella foenum graecum) seed extract against acute myeloblastic leukemia cell line (KG-1) Iran J Blood Cancer. 2009;1:139–46. [Google Scholar]
- 246.Pandian RS, Anuradha CV, Viswanathan P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J Ethnopharmacol. 2002;81:393–7. doi: 10.1016/s0378-8741(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 247.Sindhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 248.Ladda PL, Magdum CS. Vitex negundo Linn.: Ethnobotany, phytochemistry and pharmacology-A review. Int J Adv Pharm Biol Chem. 2012;1:111–20. [Google Scholar]
- 249.Tandon VR. Medical uses and biological activities of Vitex negundo. Nat Prod Radiance. 2005;4:162–5. [Google Scholar]
- 250.Tandon V, Gupta RK. Effect of Vitex negundo on oxidative stress. Indian J Pharmacol. 2005;37:38–40. [Google Scholar]
- 251.Ravishankar B, Bhaskaran NR, Sasikala CK. Pharmacological evaluation of Vitex negundo (Nirgundi) leaves. Bull Med Ethano Bot Res. 1985;6:72–92. [Google Scholar]
- 252.Ravishankar B, Bhaskaran NR, Sasikala CK. Pharmacology of Vitex negundo Linn (Nirgundi) root. J Res Ayurv Siddha. 1986;7:62–77. [Google Scholar]
- 253.Telang RS, Chatterjee S, Varshneya C. Studies on analgesic and anti-inflammatory activities of Vitex negundo Linn. Indian J Pharmacol. 1999;31:363–6. [Google Scholar]
- 254.Gupta RK, Tandon V. An experimental evaluation of anticonvulsant activity of Vitex negundo Linn. Indian J Physiol Pharmacol. 2002;46:82. [PubMed] [Google Scholar]
- 255.Munasinghe TC, Seneviratne CK, Thabrew MI, Abeysekera AM. Anti-radical and antilipoperoxidative effect of some plant extracts used by Sri Lankan traditional medical practitioner for cardioprotection. Phytother Res. 2001;15:519–23. doi: 10.1002/ptr.994. [DOI] [PubMed] [Google Scholar]
- 256.Deshmukh PB, Chavan SR, Renapurkar DM. A study of Insecticidal activity of twenty indigenous plants. Pesticides. 1982;16:7. [Google Scholar]
- 257.Hebbalkar DS, Hebbalkar GD, Sharma RN, Joshi VS, Bhat VS. Mosquito repellent activity of oils from Vitex negundo Linn. leaves. Indian J Med Res. 1992;95:200–3. [PubMed] [Google Scholar]
- 258.Pandey A, Bani S, Satti NK, Gupta BD, Suri KA. Anti-arthritic activity of agnuside mediated through the down-regulation of inflammatory mediators and cytokines. Inflamm Res. 2012;61:293–304. doi: 10.1007/s00011-011-0410-x. [DOI] [PubMed] [Google Scholar]
- 259.Kamboj P, Kalia AN. Hepatoprotective effect of Drynaria quercifolia Fronds hydroalcoholic extract and isolated constituent against CCl4-induced hepatocellular damge. Br J Pharm Res. 2013;3:3563–78. [Google Scholar]
- 260.Gautam R, Saklani A, Jachak SM. Indian medicinal plants as a source of antimycobacterial agents. J Ethnopharmacol. 2007;110:200–34. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 261.Kim HS, Lee TS, Yeo SW, Seong LS, Yu TS. Isolation and characterization of antitumor agents from Xanthium strumarium L. Korean J Biotechnol Bioeng. 2003;18:324–8. [Google Scholar]
- 262.Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J. In vitro anticancer screening of South African plants. J Ethnopharmacol. 2008;119:455–61. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 263.Mandal SC, Boominathan R, Devi BP, Panda S. Studies on antitussive activity of Xanthium strumarium L. extract. ISHS Acta Horticulturae 678: III WOCMAP Congress on Medicinal and Aromatic Plants. Vol. 4. Targeted Screening of Medicinal and Aromatic Plants, Economics and Law. Acta Hort. 2005;678:149–152. [Google Scholar]
- 264.Dong KK, Chang KS, Dong WB, Yeon SK, Min-Suk Y, He KK. Identification and biological characteristics of an antifungal compound extracted from Cocklebur (Xanthium Strumarium) against Phytophthora drechsleri. Plant Pathol J. 2002;18:288–92. [Google Scholar]
- 265.Kishore N, Dubey NK, Tripathi RD, Singh SK. Fungitoxicity of the leaf extracts of some higher plants against Fusarium Moniliforme. Natl Acad Sci Lett. 1982;5:9–10. [Google Scholar]
- 266.Jongwon C, Kyung TL, Hyun J, Hee JP, Intae K, Young MP. Methanolic extract of Xanthium strumarium L. possesses anti-inflammatory and antinociceptive activities. Biol Pharm Bull. 2005;28:94–100. doi: 10.1248/bpb.28.94. [DOI] [PubMed] [Google Scholar]
- 267.Han T, Li HL, Zhang QY, Han P, Zheng HC, Rahman K, et al. Bioactivity-guided fractionation for anti-inflammatory and analgesic properties and constituents of Xanthium strumarium L. Phytomedicine. 2007;14:825–9. doi: 10.1016/j.phymed.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 268.Yin MH, Kang DG, Choi DH, Kwon TO, Lee HS. Screening of vasorelaxant activity of some medicinal plants used in Oriental medicines. J Ethnopharmacol. 2005;99:113–7. doi: 10.1016/j.jep.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 269.Hsu FL, Chen YC, Cheng JT. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med. 2000;66:228–30. doi: 10.1055/s-2000-8561. [DOI] [PubMed] [Google Scholar]
- 270.Menon GS, Kuchroo K, Dasgupta D. Interaction of microtubules with active principles of Xanthium strumarium. Physiol Chem Phys Med NMR. 2001;33:153–62. [PubMed] [Google Scholar]
- 271.Tran QL, Tezuka Y, Ueda JY, Nguyen NT, Maruyama Y, Begum K, et al. In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J Ethnopharmacol. 2003;86:249–52. doi: 10.1016/s0378-8741(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 272.Talakal TS, Dwivedi SK, Sharma SR. In vitro and in vivo antitrypanosomal activity of Xanthium strumarium leaves. J Ethnopharmacol. 1995;49:141–5. doi: 10.1016/0378-8741(95)01313-x. [DOI] [PubMed] [Google Scholar]
- 273.Nieves LJ, Padilla MC, Rodríguez RH, Simón LG, Freixas JL. Effecto diuretico del Xanthium strumarium L. (guizazo de caballo) Rev Cubana Plant Med. 1999;1:22–5. [Google Scholar]
- 274.Hong SH, Jeong HJ, Kim HM. Inhibitory effects of Xanthii fructus extract on mast cell-mediated allergic reaction in murine model. J Ethnopharmacol. 2003;88:229–34. doi: 10.1016/s0378-8741(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 275.Lee SJ, Lee KW, Chung YS, Hong EK, Lee JH, Wee WR. Antioxidant assay of extracted fractions Xanthium strumarium L. using lens protein crosslink activity. J Korean Ophthalmol Soc. 2001;42:152–9. [Google Scholar]
- 276.Kang DG, Yun Ck, Lee HS. Screening and comparison of antioxidant activity of solvent extracts of herbal medicines used in Korea. J Ethnopharmacol. 2003;87:231–6. doi: 10.1016/s0378-8741(03)00142-9. [DOI] [PubMed] [Google Scholar]
- 277.Patil MV, Kandhare AD, Bhise SD. Anti-arthritic and anti-inflammatory activity of Xanthium srtumarium L. ethanolic extract in freund's complete adjuvant induced arthritis. Biomed Aging Pathol. 2012;2:6–15. [Google Scholar]
- 278.Kirtikar KR, Basu BD. 2nd ed. Uttranchal, India: Oriental Enterprises; 2003. Indian Medicinal Plants. [Google Scholar]
- 279.Pushpan R, Nishteswar K, Kumari H. Anti-arthritic natural medicine: Classical ayurveda and ethanomedical source. BMC Musculoskelet Disord. 2013;1:32–40. [Google Scholar]
- 280.Mikaili P, Shayegh J, Asghari MH. Review on the indigenous use and ethnopharmacology of hot and cold natures of phytomedicines in the Iranian traditional medicine. Asian Pac J Trop Med. 2012;2:S1189–93. [Google Scholar]
- 281.Neogi NC, Rathor RS, Shrestha AD, Banerjee DK. Studies on the anti-inflammatory and anti-arthritic activity of Achyranthine. Indian J Pharmacol. 1969;1:37–47. [Google Scholar]
- 282.Bentley R, Trimen H. Reprint Indian Edition. Delhi, India: Asiatic Publishing House; 2002. Medicinal Plants. [Google Scholar]
- 283.Vijayan V, Shyni GL, Helen A. Efficacy of Bacopa monniera (L.). Wettst in alleviating lysosomal instability in adjuvant-induced arthritis in rats. Inflammation. 2011;34:630–8. doi: 10.1007/s10753-010-9272-6. [DOI] [PubMed] [Google Scholar]
- 284.Ramachandran J, Thilagar S, Angappan R, Lakshmanan DK. Anti-arthritic activity of the Indian leafy vegetable Cardiospermum halicacabum in Wistar rats and UPLC–QTOF–MS/MS identification of the putative active phenolic components. Inflamm Res. 2013;62:115–26. doi: 10.1007/s00011-012-0558-z. [DOI] [PubMed] [Google Scholar]
- 285.Chippada CS, Vangalapati M. Antioxidant, an anti-inflammatory and anti-arthritic activity of Centella asiatica extracts. J Chem Biol Physical Sci. 2011;1:260–9. [Google Scholar]
- 286.Narendhirakannan RT, Subramanian S, Kandaswamy M. Anti-inflammatory and lysosomal stability actions of Cleome gynandra L. studied in adjuvant induced arthritic rats. Food Chem Toxicol. 2007;45:1001–12. doi: 10.1016/j.fct.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 287.Srivastava S, Singh P, Jha KK, Mishra G, Srivastava S, Khosa RL. Evaluation of anti-arthritic potential of the methanolic extract of the aerial parts of Costus speciosus. J Ayurveda Integr Med. 2012;3:204–8. doi: 10.4103/0975-9476.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Murugananthan G, Mohan S. Anti-inflammatory and anti-arthritic activities of Delonix elata bark extracts. Int J Res Ayur Pharm. 2011;2:1819–21. [Google Scholar]
- 289.Yeom MJ, Lee HC, Kim GH, Lee HJ, Shim I, Oh SK, et al. Anti-arthritic effects of Ephedra sinica STAPF herb-acupuncture: Inhibition of lipopolysaccharide-induced inflammation and adjuvant-induced polyarthritis. J Pharmacol Sci. 2006;100:41–50. doi: 10.1254/jphs.fp0050637. [DOI] [PubMed] [Google Scholar]
- 290.Harpalani AN, Taranalli AD, Otari KV, Karadi RV, Shete RV. An anti-inflammatory and anti-arthritic potential of aqueous and alcoholic extract of Euphorbia antiquorum Linn. Pharmacologyonline. 2011;2:287–98. [Google Scholar]
- 291.Manocha N, Chandra SK, Sharma V, Sangameswaran B, Saluja M. Anti-rheumatic and antioxidant activity of extract of stem bark of Ficus bengalensis. Res J Chem Sci. 2011;1:2–8. [Google Scholar]
- 292.Mehta A, Sethiya NK, Mehta C, Shah GB. Anti-arthritis activity of roots of Hemidesmus indicus R. Br. (Anantmul) in rats. Asian Pac J Trop Med. 2012;5:130–5. doi: 10.1016/S1995-7645(12)60011-X. [DOI] [PubMed] [Google Scholar]
- 293.Silva CR, Fröhlich JK, Oliveira SM, Cabreira TN, Rossato MF, Trevisan G, et al. The antinociceptive and anti-inflammatory effects of the crude extract of Jatropha isabellei in a rat gout model. J Ethnopharmacol. 2013;145:205–13. doi: 10.1016/j.jep.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 294.Paval J, Kaitheri SK, Potu BK, Govindan S, Kumar RS, Narayanan SN, et al. Anti-arthritic potential of the plant Justicia gendarussa Burm F. Clinics (Sao Paulo) 2009;64:357–62. doi: 10.1590/S1807-59322009000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kore KJ, Shete RV, Desai NV. Anti-Arthritic activity of hydroalcoholic extract of Lawsonia Innermis. Int J Drug Dev Res. 2011;3:217–24. [Google Scholar]
- 296.Kripa KG, Chamundeeswari D, Thanka J, Uma Maheswara, Reddy C. Modulation of inflammatory markers by the ethanolic extract of Leucas aspera in adjuvant arthritis. J Ethnopharmacol. 2011;134:1024–7. doi: 10.1016/j.jep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 297.Kaithwas G, Majumdar DK. Therapeutic effect of Linum usitatissimum (flaxseed/linseed) fixed oil on acute and chronic arthritic models in albino rats. Inflammopharmacology. 2010;18:127–36. doi: 10.1007/s10787-010-0033-9. [DOI] [PubMed] [Google Scholar]
- 298.Kamalutheen M, Gopalkrishnan M, Ismail TS. Anti-inflammatory and anti-arthritic activities of Merremia tridentata (L.). Hall. E J Chem. 2009;6:943–8. [Google Scholar]
- 299.Rajendran R, Krishnakumar E. Anti-arthritic activity of Premna serratifolia linn. wood against adjuvant induced arthritis. Avicenna J Med Biotechnol. 2010;2:101–6. [PMC free article] [PubMed] [Google Scholar]
- 300.Sekiguchi Y, Mano H, Nakatani S, Shimizu J, Kobata K, Wada M. Anti-proliferative effects of Salacia reticulata leaves hot-water extract on interleukin-1ß-activated cells derived from the synovium of rheumatoid arthritis model mice. BMC Res Notes. 2012;5:198. doi: 10.1186/1756-0500-5-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sharma S, Sahu D, Das HR, Sharma D. Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem Toxicol. 2011;49:3395–406. doi: 10.1016/j.fct.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 302.Ramprasath VR, Shanthi P, Sachdanandam P. Immunomodulatory and anti-inflammatory effects of Semecarpus anacardium Linn. Nut milk extract in experimental inflammatory conditions. Biol Pharm Bull. 2006;29:693–700. doi: 10.1248/bpb.29.693. [DOI] [PubMed] [Google Scholar]
- 303.Ekambaram S, Perumal SS, Subramanian V. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund's adjuvant induced arthritic rat model. BMC Complement Altern Med. 2010;10:56. doi: 10.1186/1472-6882-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8:208–13. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Manjunatha BK, Abhilasha N, Hedge V, Suchitra MN, Vidya SM. Hepatoprotective potency of Achyranthes aspera: An in-vitro study. Int J Pharm Phytopharm Res. 2012;1:387–90. [Google Scholar]
- 306.Li M, He J, Jiang LL, Ng ES, Wang H, Lam FF, et al. The anti-arthritic effects of Aconitum vilmorinianum, a folk herbal medicine in Southwestern China. J Ethnopharmacol. 2013;147:122–7. doi: 10.1016/j.jep.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 307.Kaithwas G, Gautam R, Jachak SM, Saklani A. Antiarthritic effects of Ajuga bracteosa Wall ex Benth. in acute and chronic models of arthritis in albino rats. Asian Pac J Trop Biomed. 2012;2:185–8. doi: 10.1016/S2221-1691(12)60039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chandel S, Bagai U. Antiplasmodial activity of Ajuga bracteosa against Plasmodium berghei infected BALB/c mice. Indian J Med Res. 2010;131:440–4. [PubMed] [Google Scholar]
- 309.Ono Y, Fukaya Y, Imai S, Yamakuni T. Beneficial effects of Ajuga decumbens on osteoporosis and arthritis. Biol Pharm Bull. 2008;31:1199–204. doi: 10.1248/bpb.31.1199. [DOI] [PubMed] [Google Scholar]
- 310.Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, et al. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol. 2000;71:179–86. doi: 10.1016/s0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 311.Kamaljeet Tomar S, Thakur N. Antipyretic activity of whole aerial part from Argyreia nervosa. Int J Pharm Pharm Sci. 2012;4:1–2. [Google Scholar]
- 312.Fan H, Yang M, Che X, Zhang Z, Xu H, Liu K, et al. Activity study of a hydroxynaphthoquinone fraction from Arnebia euchroma in experimental arthritis. Fitoterapia. 2012;83:1226–37. doi: 10.1016/j.fitote.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 313.Coelho MG, Reis PA, Gava VB, Marques PR, Gayer CR, Laranja GA, et al. Anti-arthritic effect and subacute toxicological evaluation of Baccharis genistelloides aqueous extract. Toxicol Lett. 2004;154:69–80. doi: 10.1016/j.toxlet.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 314.Ghosh T, Maity TK, Das M, Bose A, Dash DK. In vitro antioxidant and hepatoprotective activity of ethanol extract of Bacopa monnieri Linn. aerial parts. Iranian J Pharmacol Ther. 2007;6:77–85. [Google Scholar]
- 315.Singh B, Bani S, Gupta DK, Chandan BK, Kaul A. Anti-inflammatory activity of ‘TAF’ an active fraction from the plant Barleria prionitis Linn. J Ethnopharmacol. 2003;85:187–93. doi: 10.1016/s0378-8741(02)00358-6. [DOI] [PubMed] [Google Scholar]
- 316.Bodakhe SH, Ram A. Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku Zasshi. 2007;127:1503–7. doi: 10.1248/yakushi.127.1503. [DOI] [PubMed] [Google Scholar]
- 317.Nazir N, Koul S, Qurishi MA, Taneja SC, Ahmad SF, Bani S, et al. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis – a flow cytometric study. J Ethnopharmacol. 2007;112:401–5. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 318.Fan AY, Lao L, Zhang RX, Zhou AN, Wang LB, Moudgil KD, et al. Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in lewis rats. J Ethnopharmacol. 2005;101:104–9. doi: 10.1016/j.jep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 319.Muralidhar A, Babu KS, Sankar TR, Reddanna P, Latha J. Evaluation of wound healing properties of bioactive fractions from the extract of Butea monosperma (Lam.). stem bark. Int J Phytomed. 2011;3:41–9. [Google Scholar]
- 320.Yerragunta V, Perusomula R, Bhangale J, Chaudhari R, Alluri R. Evaluation of anti-inflammatory and antiarthritic activity of Butea monosperma L. in laboratory animals. J Pharmacol Toxicol. 2011;1:53–8. [Google Scholar]
- 321.Wu SQ, Otero M, Unger FM, Goldring MB, Phrutivorapongkul A, Chiari C, et al. Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. J Ethnopharmacol. 2011;138:364–72. doi: 10.1016/j.jep.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Sireeratawong S, Piyabhan P, Singhalak T, Wongkrajang Y, Temsiririrkkul R, Punsrirat J, et al. Toxicity evaluation of sappan wood extract in rats. J Med Assoc Thai. 2010;93(Suppl 7):S50–7. [PubMed] [Google Scholar]
- 323.Saratha V, Subramanian SP. Lupeol, a triterpenoid isolated from Calotropis gigantea latex ameliorates the primary and secondary complications of FCA induced adjuvant disease in experimental rats. Inflammopharmacology. 2012;20:27–37. doi: 10.1007/s10787-011-0095-3. [DOI] [PubMed] [Google Scholar]
- 324.Bulani V, Biyani K, Kale R, Joshi U, Charhate K, Kumar D, Pagore R. Inhibitory effect of Calatropis gigantean extract on ovalbumin-induced airway inflammation and arachidonic acid induced inflammation in murine model of asthma. Int J Curr Biol Med Sci. 2011;1:19–25. [Google Scholar]
- 325.Kumar VL, Roy S. Calotropis procera latex extract affords protection against inflammation and oxidative stress in Freund's complete adjuvant-induced monoarthritis in rats. Mediators Inflamm. 2007;2007:47523. doi: 10.1155/2007/47523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ouedraogo GG, Ouedraogo M, Lamien-Sanou A, Lompo M, Goumbri-Lompo OM, Guissou PI. Acute and subchronic toxicity studies of roots braks extracts of Calotropis procera (Ait.). R. Br. used in the treatment of sickle cell disease in Burkina Faso. Br J Pharmacol Toxicol. 2013;4:194–200. [Google Scholar]
- 327.Suszko A, Obminska-Mrukowicz B. Influence of polysaccharide fractions isolated from Caltha palustris L. on the cellular immune response in collagen-induced arthritis (CIA) in mice. A comparison with methotrexate. J Ethnopharmacol. 2013;145:109–17. doi: 10.1016/j.jep.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 328.Danquah AC, Woode E, Boakye-Gyasi E. Anti-arthritic effects of an ethanolic extract of Capparis erythrocarpus Isert roots in freund's adjuvant-induced arthritis in rats. J Pharmacol Toxicol. 2011;6:201–7. [Google Scholar]
- 329.Feng X, Lu J, Xin H, Zhang L, Wang Y, Tang K. Anti-arthritic active fraction of Capparis spinosa L. fruits and its chemical constituents. Yakugaku Zasshi. 2011;131:423–9. doi: 10.1248/yakushi.131.423. [DOI] [PubMed] [Google Scholar]
- 330.Chaudhari SS, Chaudhari RS, Chavan JM. Analgesic, anti-inflammatory and anti-arthritic activity of Cassia uniflora Mill. Asian Pac J Trop Med. 2012:S181–S186. [Google Scholar]
- 331.Escandell JM, Recio MC, Máñez S, Giner RM, Cerdá-Nicolás M, Ríos JL. Dihydrocucurbitacin B, isolated from Cayaponia tayuya reduces damage in adjuvant-induced arthritis. Eur J Pharmacol. 2006;532:145–54. doi: 10.1016/j.ejphar.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 332.Venkatesha SH, Yu H, Rajaiah R, Tong L, Moudgil KD. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J Biol Chem. 2011;286:15138–46. doi: 10.1074/jbc.M111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Li Y. Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kB-mediated matrix metalloproteinase-9 expression. Plos One. 2013;8:1–13. doi: 10.1371/journal.pone.0068905. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 334.Amresh, Reddy GD, Rao CV, Shirwaikar A. Ethnomedical value of Cissampelos pareira extract in experimentally induced diarrhoea. Acta Pharm. 2004;54:27–35. [PubMed] [Google Scholar]
- 335.Amresh G, Singh PN, Rao CV. Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J Ethnopharmacol. 2007;22(111):531–6. doi: 10.1016/j.jep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 336.Lee YC, Kim SH, Roh SS, Choi HY, Seo YB. Suppressive effects of Chelidonium majus methanol extract in knee joint, regional lymph nodes, and spleen on collagen-induced arthritis in mice. J Ethnopharmacol. 2007;112:40–8. doi: 10.1016/j.jep.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 337.Hsieh MS, Wang KT, Tseng SH, Lee CJ, Chen CH, Wang CC. Using 18F-FDG microPET imaging to measure the inhibitory effects of Clematis chinensis Osbeck on the pro-inflammatory and degradative mediators associated with inflammatory arthritis. J Ethnopharmacol. 2011;136:511–7. doi: 10.1016/j.jep.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 338.Shaik K, Shaik A, Kumar D, Kadirvel D. Evaluation of preliminary phytochemical properties and hypoglycemic activity of Cleome gyandra L. Int J Pharm Pharm Sci. 2013;5:824–8. [Google Scholar]
- 339.Mazhar J, Mazumder A. Evaluation of anti-diabetic activity of methanoloc leaf extract of Coriandrum sativum alloxan induced diabetic rats. Res J Pharm Biol Chem Sci. 2013;4:500–7. [Google Scholar]
- 340.Choudhary N, Kalita JC, Haque A. Effect of Costus speciosus Keon on reproductive organ of female albino mice. Int Res J Pharm. 2012;3:200–2. [Google Scholar]
- 341.Kaushik ML, Jalalpure SS. Effect of Curcuma zedoaria Rosc root extracts on behavioral and radiology changes in arthritic rats. J Adv Pharm Technol Res. 2011;2:170–6. doi: 10.4103/2231-4040.85537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Jung HW, Jung JK, Son KH, Lee DH, Kang TM, Kim YS, et al. Inhibitory effects of the root extract of Dipsacus asperoides C.Y. Cheng et al T.M. Ai on collagen-induced arthritis in mice. J Ethnopharmacol. 2012;139:98–103. doi: 10.1016/j.jep.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 343.Saravanan S, Mutheeswaran S, Saravanan M, Chellappandian M, Gabriel Paulraj M, Karunai Raj M, et al. Ameliorative effect of Drynaria quercifolia (L.). J. Sm. an ethnomedicinal plant, in arthritic animals. Food Chem Toxicol. 2013;51:356–63. doi: 10.1016/j.fct.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 344.Ramasamy SK, Rajendran VK, Rangaraj RK, Chinnayan V, Palanisamy R, Prasannan D, et al. Effect of Elaeocarpus sphaericus in freund's complete adjuvant induced rheumatoid arthritis in albino rats. Indo Glob Res J Pharm Sci. 2012;2:378–82. [Google Scholar]
- 345.Han Y. Ginkgo terpene component has an anti-inflammatory effect on Candida albicans-caused arthritic inflammation. Int Immunopharmacol. 2005;5:1049–56. doi: 10.1016/j.intimp.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 346.Ramesh RP, Vijaya C. Anti-diabetic and anti-arthritic potential of Glycosmis pentaphylla stem bark in FCA induced arthritis and streptozotocin induced diabetic rats. Int J Pharm Bio Sci. 2012;3:328–36. [Google Scholar]
- 347.Rai A. The anti-inflammatory and antiarthritic properties of ethanol extract of Herdera helix. Indian J Pharm Sci. 2013;75:99–102. doi: 10.4103/0250-474X.113537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Debella A, Taye A, Abebe D, Mudi K, Melaku D, Taye G. Screening of some Ethiopian medicinal plants for mosquito larvicidal effects and phytichemical constituents. Pharmacologyonline. 2007;3:231–43. [Google Scholar]
- 349.Perez RM, Perez S, Zavala MA, Salazar M. Anti-inflammatory activity of the bark of Hippocratea excelsa. J Ethnopharmacol. 1995;47:85–90. doi: 10.1016/0378-8741(95)01257-e. [DOI] [PubMed] [Google Scholar]
- 350.Tripathy S, Sahoo SP, Pradhan D, Sahoo S, Satapathy DK. Evaluation of anti-arthritic potential of Hybanthus enneaspermus. Afr J Pharm Pharm. 2009;3:611–4. [Google Scholar]
- 351.Luo X, Li LL, Zhang SS, Lu JL, Zeng Y, Zhang HY, et al. Therapeutic effects of total coumarins from Urtica dentata Hand on collagen-induced arthritis in Balb/c mice. J Ethnopharmacol. 2011;138:523–9. doi: 10.1016/j.jep.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 352.Zanwar AA, Hegde MV, Bodhankar SL. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol. 2011;4:90–7. doi: 10.2478/v10102-011-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lee JH, Han Y. Antiarthritic effect of lonicerin on Candida albicans arthritis in mice. Arch Pharm Res. 2011;34:853–9. doi: 10.1007/s12272-011-0520-6. [DOI] [PubMed] [Google Scholar]
- 354.Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A. Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. J Ethnopharmacol. 2006;107:370–3. doi: 10.1016/j.jep.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 355.Nwaehujor CO, Ezeja MI, Udeh NE, Okoye DN, Udegbunam RI. Anti-inflammatory and anti-oxidant activities of Mallotus oppositifolius (Geisel) methanol leaf extracts. Arab J Chem. 2012;7:1–6. [Google Scholar]
- 356.Kukuia KE, Ameyaw EO, Mante PK, Adongo DW, Woode E. Screening of central effects of the leaves of Mallotus opposttifolus (Geiseler) Mull Arg. in mice. Pharmacologia. 2012;3:683–92. [Google Scholar]
- 357.Purushoth Prabhu T, Panneerselvam P, Vijaykumar R, Atlee CW, Balasubramanian S. Anti-inflammatory, anti-arthritis and analgesic effect of ethanolic extract of whole plant of Merremia emarginata Burm. F. Cent Eur J Exp Biol. 2012;1:94–9. [Google Scholar]
- 358.Sharma V, Singh M. In vitro antiarthritic and hemolysis preventive: Membrane stabilizing efficacy of ethanolic root extract of Operculina turpethum. World J Pharm Pharm Sci. 2013;2:302–12. [Google Scholar]
- 359.Pulipaka S, Challa SR, Pingili RB. Comparative anti-diabetic activity of methanolic extract of Operculina turpethum stem and root against healthy and streptozocin inducd diabetic rats. Int Curr Pharm J. 2012;1:272–8. [Google Scholar]
- 360.Kim KR, Chung TY, Shin H, Son SH, Park KK, Choi JH, et al. Red ginseng saponin extract attenuates murine collagen-induced arthritis by reducing pro-inflammatory responses and matrix metalloproteinase-3 expression. Biol Pharm Bull. 2010;33:604–10. doi: 10.1248/bpb.33.604. [DOI] [PubMed] [Google Scholar]
- 361.Kumar SN, Kishore G, Kumar SG, Sindhu PE. In vitro anti-inflammatory and anti-arthritic activity of leaves of Physalis angulata L. Int J Pharm Ind Res. 2011;1:211–3. [Google Scholar]
- 362.Nanumala SK, Kannadhasan R, Gunda K, Sivakumar G, Somasekhar P. Anti-ulcer activity of the ethanolic extract of leaves Physalis angulata L. Int J Pharm Pharm Sci. 2012;4:226–8. [Google Scholar]
- 363.Tsubata M, Takagaki K, Hirano S, Iwatani K, Abe C. Effects of flavangenol, an extract of French maritime pine bark on collagen-induced arthritis in rats. J Nutr Sci Vitaminol (Tokyo) 2011;57:251–7. doi: 10.3177/jnsv.57.251. [DOI] [PubMed] [Google Scholar]
- 364.Schulz V, Hansel R, Tyler VE. Berlin: Springer; 1997. Rational Phytotherapy: A Physician's Guide to Herbal Medicine; p. 306. [Google Scholar]
- 365.Pandey A, Bani S, Dutt P, Suri KA. Modulation of Th1/Th2 cytokines and inflammatory mediators by hydroxychavicol in adjuvant induced arthritic tissues. Cytokine. 2010;49:114–21. doi: 10.1016/j.cyto.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 366.Elumalai A, Prakash YG. Evaluation of anti-arthritic activity of ethanolic extract of Pisonia grandis R. Br. Asian J Pharm Clin Res. 2012;2:91–3. [Google Scholar]
- 367.Kyei S, Koffuor GA, Boampong JN. Antiarthritic effect of aqueous and ethanolic leaf extracts of Pistia stratiotes in adjuvant-induced arthritis in Sprague-dawley rats. J Exp Pharmacol. 2012;4:41–51. doi: 10.2147/JEP.S29792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ali KM, Paul P, Torquel IM, Nath BN, Kumar SS. Cytotoxicity, antimicrobial and neuropharmacological evaluation of ethanoloc extract of Pistia stratiotes L. Int Res J Pharm. 2011;2:82–91. [Google Scholar]
- 369.Patel P, Patel D, Patel N. Experimental investigation of anti-rheumatoid activity of Pleurotus sajorcaju in adjuvant-induced arthritic rats. Chin J Nat Med. 2012;10:0269–74. [Google Scholar]
- 370.Georgewill AO, Georgewill UO. Antiarthritic activity of Pseudocdrea kotschyi in albino rats. Afr J Appl Zool Environ Biol. 2008;10:70–2. [Google Scholar]
- 371.Bhandary BS, Sharmila KP, Kumari NS, Bhat SV. Acute and subacute study of ethanolic extract of Punuca granatum (L) whole fruits and seeds and synthetic ellagic acid in swiss albino mice. Asian J Pharm Clin Res. 2013;6:192–8. [Google Scholar]
- 372.Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, et al. Flavonol-rich RVHxR from Rhus verniciflua stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int Immunopharmacol. 2009;9:268–76. doi: 10.1016/j.intimp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 373.Freire RB, Borba HR, Coelho CD. Ruta graveolens L. toxicity in Vampirolepis nana infected mice. Indian J Pharmacol. 2010;42:345–50. doi: 10.4103/0253-7613.71898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Mukhopadhyay MK, Nath D. Phytochemical screening and toxicity study of Saraca asoca bark methanolic extract. Int J Phytomed. 2011;3:498–505. [Google Scholar]
- 375.Saleem TS, Lokanath N, Prasanthi A, Madhavi M, Mallika G, Vishnu MN. Aqueous extract of Saussurea lappa root ameliorate oxidative myocardial injury induced by isoproterenol in rats. J Adv Pharm Technol Res. 2013;4:94–100. doi: 10.4103/2231-4040.111525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chakraborty M, Asdaq SM. Interaction of Semecarpus anacardium L. with propranolol against isoproterenol induced myocardial damage in rats. Indian J Exp Biol. 2011;49:200–6. [PubMed] [Google Scholar]
- 377.Sireeratawong S, Lertprasertsuke N, Srisawat U, Thuppia A, Ngamjariyawat A, Suwanlikhid N, et al. Acute and subchronic toxicity study of the water extract from root of Sida rhombifolia Linn. in rats. Songklanakarin J Sci Technol. 2008;30:729–37. [Google Scholar]
- 378.Liu L, Buchner E, Beitze D, Schmidt-Weber CB, Kaever V, Emmrich F, et al. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int J Immunopharmacol. 1996;18:529–43. doi: 10.1016/s0192-0561(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 379.Sreena K, Mathew M, Nair SS. Anti-inflammatory and anti-arthritic activity of Smithia sensitive. Int J Pharm Chem Sci. 2012;1:1401–4. [Google Scholar]
- 380.Jin JH, Kim JS, Kang SS, Son KH, Chang HW, Kim HP. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J Ethnopharmacol. 2010;127:589–95. doi: 10.1016/j.jep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 381.Agarwal RB, Rangari VD. Anti-inflammatory and anti-arthritic activities of Lupeol and 19a-H Lupeol isolated from Strobilanthus callosus and Strobilanthus ixiocephala roots. Indian J Pharmacol. 2003;35:384–7. [PubMed] [Google Scholar]
- 382.Desu BS, Elango K, Satish Kumar MN, Suresh B, Manimaran S, Nanjan MJ. Investigation of selected medicinal plants (Strobilanthes kunthianus, Strobilanthes cuspidatus) and marketed formulation (Shallaki) for their anti-inflammatory and anti-osteoarthritic activity. Pharmanest. 2011;2:492–9. [Google Scholar]
- 383.Patil CR, Rambhade AD, Jadhav RB, Patil KR, Dubey VK, Sonara BM, et al. Modulation of arthritis in rats by Toxicodendron pubescens and its homeopathic dilutions. Homeopathy. 2011;100:131–7. doi: 10.1016/j.homp.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 384.Patil CR, Gadekar AR, Patel PN, Rambhade A, Surana SJ, Gaushal MH. Dual effect of Toxicodendron pubescens on Carrageenan induced paw edema in rats. Homeopathy. 2009;98:88–91. doi: 10.1016/j.homp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 385.Chamundeeswari D, Vasantha J, Gopalakrishnan S, Sukumar E. Free radical scavenging activity of the alcoholic extract of Trewia polycarpa roots in arthritic rats. J Ethnopharmacol. 2003;88:51–6. doi: 10.1016/s0378-8741(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 386.Jain DK, Patel NS, Nagar H, Patel A, Chandel HS. Anti-arthritic activity of Tridax procumbens ethanolic extract of leaves. J Pharm Sci. 2012;2:80–6. [Google Scholar]
- 387.Abudoleh S, Disi A, Qunaibi E, Aburjai T. Anti-arthritic activity of the methanolic leaf extract of Urtica pilulifera L. on albino rats. Am J Pharmacol Toxicol. 2011;6:27–32. [Google Scholar]
- 388.Latha RM, Geetha T, Varalakshmi P. Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen Pharmacol. 1998;31:601–6. doi: 10.1016/s0306-3623(98)00049-4. [DOI] [PubMed] [Google Scholar]
- 389.Choudhary S, Sharma M, Tripathi J, Mishra P. Antihyperglycemic activity of Vernonia cinerea L. on alloxan-induced diabetic mice. Int J Adv Res. 2013;1:35–42. [Google Scholar]
- 390.Das S, Kanodia L. Effect of ethanolic extract of leaves of Vitex negundo L. on acetic acid induced colitis in albino rats. Asian J Pharm Clin Res. 2013;6:138–41. doi: 10.4103/0253-7613.117728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Cheeke PR, Piacente S, Oleszek W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J Inflamm (Lond) 2006;3:6. doi: 10.1186/1476-9255-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


