Abstract:
A wide range of pharmacological, surgical, and mechanical pump approaches have been studied to attenuate the systemic inflammatory response to cardiopulmonary bypass, yet no systematically based review exists to cover the scope of anti-inflammatory interventions deployed. We therefore conducted an evidence-based review to capture “self-identified” anti-inflammatory interventions among adult cardiopulmonary bypass procedures. To be included, trials had to measure at least one inflammatory mediator and one clinical outcome, specified in the “Outcomes 2010” consensus statement. Ninety-eight papers satisfied inclusion criteria and formed the basis of the review. The review identified 33 different interventions and approaches to attenuate the systemic inflammatory response. However, only a minority of papers (35 of 98 [35.7%]) demonstrated any clinical improvement to one or more of the predefined outcome measures (most frequently myocardial protection or length of intensive care unit stay). No single intervention was supported by strong level A evidence (multiple randomized controlled trials [RCTs] or meta-analysis) for clinical benefit. Interventions at level A evidence included off-pump surgery, minimized circuits, biocompatible circuit coatings, leukocyte filtration, complement C5 inhibition, preoperative aspirin, and corticosteroid prophylaxis. Interventions at level B evidence (single RCT) for minimizing inflammation included nitric oxide donors, C1 esterase inhibition, neutrophil elastase inhibition, propofol, propionyl-L-carnitine, and intensive insulin therapy. A secondary analysis revealed that suppression of at least one inflammatory marker was necessary but not sufficient to confer clinical benefit. The most effective interventions were those that targeted multiple inflammatory pathways. These observations are consistent with a “multiple hit” hypothesis, whereby clinically effective suppression of the systemic inflammatory response requires hitting multiple inflammatory targets simultaneously. Further research is warranted to evaluate if combinations of interventions that target multiple inflammatory pathways are capable of synergistically reducing inflammation and improving outcomes after cardiopulmonary bypass.
Keywords: Inflammation, systemic–CPB, inflammatory response, complications and management–CPB, equipment, inflammatory inhibitors, outcomes
A systemic inflammatory response is triggered in patients undergoing cardiothoracic surgery with cardiopulmonary bypass (CPB) as a result of the combination of surgical trauma, activation of blood components in the extracorporeal circuit, ischemia/reperfusion injury, and endotoxin release (1–4). There is evidence for activation of all the body’s major host defensive pathways, including complement, coagulation, kinins, fibrinolysis, leukocytes, platelets, and inflammatory cytokines (4–13). This broad wave of systemic activation has been linked to adverse clinical outcomes ranging from mild adverse effects (fever or diffuse tissue edema), to moderate adverse effects (pathological hemodynamic instability or coagulopathy), to severe complications (acute organ injury requiring mechanical support), and even mortality (14–16).
A variety of approaches have been adopted in an attempt to limit the systemic inflammatory response to CPB. These include modifications to CPB equipment such as filters to remove inflammatory leukocytes or soluble mediators, minimized circuits to reduce surface area, coatings to improve the biocompatibility of extracorporeal surfaces, or the elimination of CPB altogether with off-pump coronary revascularization procedures. A range of pharmaceutical interventions has also been investigated such as steroidal and nonsteroidal anti-inflammatories, complement inhibitors, protease inhibitors, antifibrinolytics, anesthetic regimens, antioxidants, and others. Previous evaluations of the evidence base have been limited to meta-analyses or database reviews for a single intervention such as steroids or biocompatible surface coating, but there has been no systematic evaluation of the literature covering the complete range of anti-inflammatory strategies deployed.
The Society of Thoracic Surgeons (STS) Perfusion Guideline Writing Group is a multicountry, multi-institution initiative tasked with producing evidence-based clinical guidelines on a range of practices affecting outcomes in CPB such as temperature management, renal protection, blood conservation, and anti-inflammatory interventions. An immediate challenge faced by the Inflammation Writing Group was the fact that a previous analysis of the evidence base of pharmacological strategies to attenuate the inflammatory response (17) had reported that only a small minority of papers measured any traditional clinical end points (e.g., death, myocardial infarction, stroke). The problem is compounded by small sample sizes pervading the inflammation literature (median sample size: n = 40) (18), implying that there may not be adequate statistical power to analyze hard clinical end points. A review process covering multiple peer reviews from cardiothoracic, cardiac anesthesia, and perfusion journals as well as guideline writing committees of the STS, The Society of Cardiovascular Anesthesiologists, and The American Society of Extracorporeal Technology eventually concluded that the evidence base would be insufficient to recommend clinical practice guidelines for anti-inflammatory interventions based on traditional clinical end points.
The Inflammation Writing Group has therefore undertaken a critical review of the literature to illustrate the scope of interventions and approaches being deployed in the field to highlight promising areas of research and identify gaps in the literature. In this review, surrogate markers of organ dysfunction and measures of hospital resource use, defined in the “Outcomes 2010” consensus statement, were accepted as outcomes (19), and studies had to “self-identify” as being related to the systemic inflammatory response. Finally, studies had to measure at least one inflammatory marker using a relaxed definition of that term to include markers of complement activation, coagulation, kinins, oxidative stress, endothelial activation, white cell activation, white cell count, or a range of soluble inflammatory mediators. These inclusion criteria yielded a rich evidence base consisting of 98 papers covering a wide range of interventions and approaches to reduce the inflammatory response after adult CPB.
The purpose of this review was to identify gaps in the literature, inform further research, and to critically evaluate the strength of the evidence base for practices capable of attenuating the systematic inflammatory response and their relationship to clinical outcomes.
MATERIALS AND METHODS
Literature Search
The literature search was designed to capture clinical trials reporting on the inflammatory response to adult CPB together with clinical outcomes or surrogate markers for organ injury to five index organs: heart, lung, brain, kidney, and gut. The search terms (Appendix A) recovered >1600 articles in PubMed.
Abstract and Paper Reviews
A title review narrowed the search to 602 abstracts that were submitted for more detailed analysis using the Guideliner™ reviewing software (www.Guideliner.org/default.aspx, accessed September 15, 2014). All abstracts were reviewed in duplicate by independent reviewers, of which 236 were selected for full paper review. To be included, trials had to measure at least one clinical outcome and one inflammatory mediator. The inclusion criteria were as follows: randomized clinical trial (RCT) or meta-analysis, adult CPB, cardiac surgery, perioperative intervention, published 2002–2011, measured at least one inflammatory marker or used an established anti-inflammatory strategy (e.g., steroid or nonsteroidal anti-inflammatory drug), and measured at least one prespecified clinical outcome (defined by “Outcomes 2010” Consensus Statement) (19). The rules for deciding on inclusion/exclusion of papers were based on the Methodology Manual and Policies From the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Task Force on Practice Guideline (http://assets.cardiosource.com/Methodology_Manual_for_ACC_AHA_Writing_Committees.pdf); hence, any one reviewer could select an abstract for inclusion in a full paper review, but at least two reviewers had to agree to exclude a paper. The same rules applied at the paper review stage with two reviewers needing to agree whether to exclude a paper. These rules were incorporated into Guideliner™ and the following reviewers performed abstract and paper reviews: R.C.L., J.R.B., D.S.L., and D.F. These same individuals decided on whether an intervention achieved a clinical benefit. The assessment of clinical benefit was derived from review of each paper with regard to number of subjects studied, quality of the biomarker data, clinical outcomes achieved, and the strength of the statistical associations for reduced inflammation and clinical outcomes. The assignment of Level of Evidence used ACC/AHA guidelines (20). Any discordance between two reviewers was discussed and resolved if necessary by an independent third reviewer (J.H. and L.S.L.).
RESULTS
Synthesis of the Evidence Base
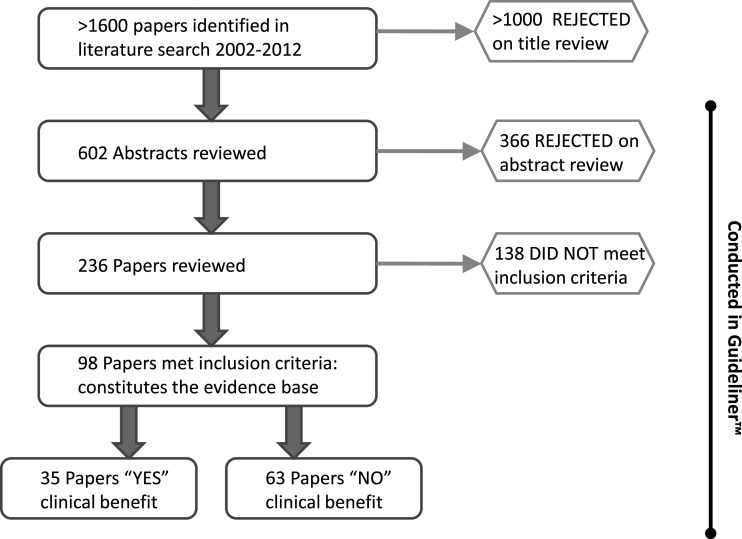
The literature search identified over 1600 papers, of which 602 abstracts were selected based on title review for entry into the Guideliner™ database. The Guideliner™ reviewing software was used in all subsequent steps for synthesizing the evidence base. Of 602 abstracts, 236 were selected for full paper review (Figure 1). After verifying inclusion/exclusion criteria 98 papers made up the final evidence base (listed in the Appendix B). These covered three broad categories of intervention: surgical and perioperative management (19 papers), perfusion-related (35 papers), and pharmacological (44 papers). Thirty-five of the 98 papers demonstrated a clinical benefit and were distributed approximately evenly across the three categories (Table 1). The total number of patients forming the evidence base was 17,676. The complete list of interventions and their associated level of evidence is summarized in Table 2. An analysis of the most relevant interventions according to the strength of evidence follows.
Figure 1.
Synthesis of the evidence base. This illustration shows how the final 98 articles comprising the evidence base were derived from the original search strategy and what proportion achieved a clinical benefit. Guideliner™ is a bespoke reviewing software specifically purposed for this review process.
Table 1.
Summary of evidence base.
| Category of Intervention | Number of Papers | Percentage with Clinical Benefit (%) |
|---|---|---|
| Surgical/perioperative management | 19 | 6/19 = 31.6% |
| Perfusion-related | 35 | 11/35 = 31.4% |
| Pharmacological | 44 | 18/44 = 40.9% |
| Total | 98 | 35/98 = 35.7% |
Table 2.
Summary of interventions and level of evidence.
| Category of Intervention | Intervention | Level of Evidence |
|---|---|---|
| Surgical/perioperative management | Off-pump coronary revascularization | Level A |
| Preoperative aspirin | Level A | |
| Preoperative fluvastatin | Level B | |
| Left ventricular assist | Level B | |
| Intensive insulin therapy | Level B | |
| Continuous ventilation | Level B | |
| No cardioplegic arrest | Level B | |
| Perfusion-related | Minimized extracorporeal circuit | Level A |
| Biocompatible circuit coating | Level A | |
| Leukocyte-depleting filter | Level A | |
| Ultrafiltration | Level B | |
| Pericardial blood processing | Level B | |
| Discard mediastinal blood | Level B | |
| Pharmacological | Steroids | Level A |
| Complement inhibitors | Level A | |
| C1 esterase inhibitors | Level B | |
| Neutrophil elastase inhibitors | Level B | |
| Nitric oxide donors | Level B | |
| Propofol | Level B | |
| Sevoflurane | Level B | |
| Aminophylline | Level B | |
| Propionyl-L-carnitine | Level B | |
| Hydroxyethyl starch in prime | Level B | |
| Gelatin colloid | Level B | |
| Aprotinin | Level B | |
| Adenosine | Level B | |
| Ethyl pyruvate | Level B | |
| Erythropoietin | Level B | |
| Taurine | Level B | |
| Glutamine | Level B | |
| N-acetyl cysteine | Level B | |
| Dual-dose tranexamic acid | Level B | |
| Lidocaine | Level B |
Evidence Level A (multiple randomized controlled trials)
Off-Pump Surgery:
There were 10 RCTs on off-pump coronary revascularization surgery (Table 2). Four of the 10 papers demonstrated a clinical benefit. The studies were not uniform, however, in that they differed with respect to heparin dosing, and the control CPB groups differed with respect to circuit volume and use of cardioplegia. There was considerable heterogeneity in the reporting of inflammatory biomarkers with each of the 10 studies measuring a different set of markers that reflected inflammation. A best available summary of the evidence suggests that the use of OPCAB may reduce myocardial injury (troponin and CKMB), but this could not be linked in an obligate relationship with inflammatory suppression. Whereas the four studies with improved clinical outcome did show inflammatory suppression, three other studies with suppressed inflammation did not achieve a clinical benefit. The median sample size for the OPCAB studies was 50, reinforcing the thin evidence base even for this relatively well studied intervention.
Minimized Extracorporeal Circulation:
There were eight RCTs on approaches to minimize the surface area of the extracorporeal circuit, and three of these eight achieved clinical benefit (Table 3). Again there was heterogeneity in the range of inflammatory biomarkers studied and again there was no clear linkage between suppression of inflammation and clinical outcome; hence, the three studies with improved clinical outcome had suppressed inflammation, yet three other studies with suppressed inflammation did not show clinical improvement. The median sample size for this intervention was 45.
Table 3.
Summary of surgical and perioperative management interventions.
| Intervention (no. of papers) | Type of Study, Surgery | Author (year) [reference]1* | No.2† | Inflammatory Biomarker(s) Suppressed Yes–No: Biomarker3‡ | Clinical Benefit Yes–No: Outcome Modified | Comment |
|---|---|---|---|---|---|---|
| Off-pump (n = 10) | RCT, CABG | Rastan (2005) [1] | 194† | Yes: CRP5‡ | Yes: lactate, CK-MB | Off-pump verus on-pump beating heart as control group; CK-MB and lactate significantly improved by off-pump |
| RCT, CABG | Nesher (2006) [2] | 60 | Yes: IL-6, IL-8 | Yes: CK-MB, cTnI | Significant myocardial protection (CK-MB and TnI) off-pump | |
| RCT, CABG | Serrano (2009) [3] | 40 | Yes: IL-8, CRP, WBC, sP-selectin | Yes: CK-MB, cTnI | Significant myocardial protection off-pump (CK-MB and TnI) but no improvement in other clinical end points | |
| RCT, CABG | Tsai (2010) [4] | 12 | Yes: IL-6, TNFα, thrombomodulin | Yes: ICU stay | ICU stay and fever significantly improved off-pump | |
| RCT, CABG | Sahlman (2003) [5] | 25 | Yes; oxidative stress | No | No improvement in myocardial injury, inotrope use, or ICU stay in off-pump group; no benefit | |
| RCT, CABG | Wan (2004) [6] | 18 | Yes: IL-8, TNFα | No | Off-pump versus on-pump beating heart as control group; no improvement in ICU stay or other clinical end points | |
| RCT, CABG | Velissaris (2004) [7] | 26 | No | No | No improvement in ICU stay or other clinical end points off-pump | |
| RCT, CABG | Quaniers (2006) [8] | 20 | Yes: C5b-9 | No | Two off-pump groups, receiving heparin at 1 or 3 mg/kg; no change ICU stay or hard end points in either treatment group | |
| RCT, CABG | Paulitsch (2009) [9] | 50 | No | No | No statistically significant changes in ICU stay or other hard clinical end points in off-pump group | |
| RCT, CABG | Formica (2009) [10] | 30 | No | No | Comparison of off-pump with miniaturized extracorporeal circuit; no change in myocardial protection or ICU stay | |
| Preoperative aspirin (1) | Meta-analysis valve/CABG | Sun (2008) [11] | 412 | N/D6§ | No | Preoperative aspirin is statistically significantly associated with worsened reoperation rates (p <.02), at 325-mg dose |
| Preoperative aspirin + Clopidogrel, perioperative aprotinin (1) | RCT, CABG | Akowuah (2005) [12] | 25 | Yes: platelet aggregation | No | Aspirin and Clopidogrel given preoperative, with perioperative aprotinin; concern regarding two deaths resulting from intestinal embolism |
| Preoperative fluvastatin (1) | RCT, CABG | Berkan (2009) [13] | 23 | Yes: soluble P-selectin | Yes: ICU stay, cTnI, inotropes | Fluvastatin (80 mg/day 3 weeks before surgery) significantly improved ICU stay, TnI marker, and need for inotropes |
| Left ventricular assist (3) | RCT, CABG | Meyns (2002) [14] | 105 | Yes: NE, C3 | No | CABG supported with intracardiac axial pump did not improve any hard clinical endpoints or ICU stay |
| RCT, CABG | Stassano (2009) [15] | 38 | Yes: IL-6, TNFα, CRP, NE | No | LVA (minus oxygenator or heat exchanger) compared to MECC. No myocardial protection or other clinical benefit | |
| RCT, CABG | Stassano (2010) [16] | 21 | Yes: IL-6, IL-8, TNFα | No | LVA assisted beating heart surgery vs. conventional CPB. No significant clinical changes | |
| Intensive insulin therapy (1) | RCT, valve | Zheng (2010) [17] | 50 | Yes: IL-6, IL-10, TNFα | Yes: ICU stay, cTnI | Insulin therapy (Portland Protocol) in patients with no history of diabetes: improved ICU stay and TnI marker |
| Continuous ventilation (1) | RCT, CABG | Ng (2009) [18] | 23 | No | No | Continuous ventilation throughout CPB had no effect on bronchoalveolar lavage cell activation status or ICU stay |
| No cardioplegic arrest (1) | RCT, CABG | Narayan (2011) [19] | 41 | No | No | CPB without cardioplegic arrest did not alter myocardial injury marker (TnI), neural marker (S100), or ICU stay |
Number in square brackets [ ] refers to reference number in the Appendix B: “98 References Comprising the Evidence Base.”
N = number of subjects in treatment group.
Abbreviations used: RCT, randomized controlled trial; CABG, coronary artery bypass grafting; CRP, C-reactive protein; IL, interleukin; WBC, white blood cell count; TNF, tumor necrosis factor; ICU, intensive care unit; NE, neutrophil elastase; C, complement; C5b-9, terminal complement complex; CK-MB, creatine kinase MB fraction; cTn, cardiac-specific troponin; MECC, minimal extracorporeal circulation.
N/D, not done.
Biocompatible Circuit Coating:
There were 14 RCTs examining different biocompatible surface coatings of which six indicated a clinical benefit (Table 3). Heparin was the most widely studied biocompatible coating plus one paper was a direct comparison of two different types of heparin-coated circuits. Poly-2-methoxyethylacrylate and amphophilic silicone–caprolactone oligomer were also studied as surface coatings. The same pattern between inflammatory suppression and clinical outcome was observed with 12 papers reporting inflammatory suppression but only six reporting a clinical benefit. Median sample size was 38.
Leukocyte-Depleting Filters:
There were eight RCTs studying leukocyte depletion, seven of which used the same arterial line leuko-depleting filter. Two of these eight studies were assigned a clinical benefit. Although leukocyte numbers were diminished by leukofiltration, inflammatory markers and leukocyte activation status were increased. Median sample size for this intervention was 36.
Corticosteroids:
There were 13 RCTs and one Cochrane database review (21) on steroid interventions. The Cochrane meta-analysis was adequately powered (n = 3615) to study hard clinical end points and reported no clinical benefit on mortality or myocardial and pulmonary complications (21). Among the 13 RCTs, three were assigned a clinical benefit (Table 4). Individual steroid regimens showed a clinical benefit in only two of six studies of methylprednisolone, one of six studies of dexamethasone, and not at all in a hydrocortisone trial. The steroid RCTs were relatively small (median n = 30) and lacked uniformity with distinct steroid dosing regimens used in 12 of the 13 studies. The dissociation between inflammation and clinical outcome was quite marked with all but one study showing inflammatory suppression but only three studies able to demonstrate a clinical benefit.
Table 4.
Summary of perfusion-related interventions.
| Intervention (no. of papers) | Type of Study, Surgery | Author (year) [reference]1* | No.2† | Inflammatory Biomarker(S) Suppressed Yes–No: Biomarker3‡ | Clinical Benefit Yes–No: Outcome Modified | Comment |
| Heparin coating (9) | RCT, CABG | Heyer (2002) [20]4* | 265† | Yes: C3a6‡ | Yes: cognitive function | Significantly improved cognitive dysfunction but no other clinical changes; mild benefit |
| RCT, CABG | Svenmarker (2002) [21] | 256 | Yes: WBC | Yes: neurologic deviation | No difference in mortality, ventilator time or ICU stay, but significantly less neurological deviation; mild benefit | |
| RCT, CABG | De Vroege (2004) [22] | 26 | Yes: C3b | Yes: pulmonary shunt | Decreased pulmonary vascular resistance and pulmonary shunt; no other clinical changes | |
| RCT, mixed valve/CABG | Ueyama (2004) [23] | 10 | Yes: IL-6, CRP, bradykinin | Yes: A-a O2 gradient | Two intervention groups: heparin and poly-2-methoxyethyl acrylate (PMEA) coating; both improved the A-a O2 gradient | |
| RCT, mixed valve/CABG | Lindholm (2004) [24] | 21 | Yes: IL-8, NE, C5b-9 | No | No statistically significant change in ICU or respirator time or inotrope support; no myocardial protection (Tn T) | |
| RCT, CABG | Baufreton (2005) [25] | 12 | Yes: C5b-9 | No | No statistically significant improvements in prespecified neurological outcomes | |
| RCT, mixed valve/CABG | Vanden Eynden (2008) [26] | 99 | No | No | No myocardial protection (CK-MB); no change in ICU stay, intubation time, or other hard clinical end points | |
| RCT, CABG | Mirow (2011) [27] | 21 | Yes: TAT | No | No change in neurological lesions by diffusion-weighted imaging; no change in ICU stay or other clinical outcomes | |
| RCT, CABG | Baufreton (2011) [28] | 12 | Yes: C5b-9 | No | No statistically significant differences between groups in hard clinical end points | |
| Heparin head-to-head | RCT, mixed valve/CABG | Hoel (2004) [29] | 15 | N/A7§ | N/A | No control group in this heparin head-to-head trial; no difference in inflammatory markers or clinical outcomes |
| Poly-2-methoxyethylacrylate (4) | RCT, mixed valve/CABG | Ueyama (2004) [23] | 10 | Yes: IL-6, CRP, bradykinin | Yes: A-a O2 gradient | Two intervention groups: heparin and PMEA coating; both improved the A-a O2 gradient significantly |
| RCT, CABG | Skrabal (2006) [30] | 19 | Yes: NE | Yes: neurocognitive function | Significant improvement in neurocognitive function (Go/NoGo and Mini-Mental-test); Nn change in ICU stay; mild benefit | |
| RCT, mixed valve/CABG | Ninomiya (2003) [31] | 11 | Yes: C3a, NE | No | No improvement in ICU stay or intubation time; no clinical benefit | |
| RCT, CABG | Thiara (2011) [32] | 15 | No | No | No benefit of PMEA over phosphorylcholine as control; no difference in ICU stay | |
| Amphophilic silicone-caprolactone oligomer (1) | RCT, CABG | Allen (2005) [33] | 20 | Yes: IL-10 | No | No difference between groups in creatinine, urea, or N-acetyl glucosamine; no change in intubation time or ICU stay |
| Minimized extracorporeal circuit (8) | RCT, CABG | Kofidis (2008) [34] | 50 | Yes: IL-8 | Yes: ventilator time, cTnI | Improved myocardial protection (Tn I) and ventilator time in minigroup |
| RCT, CABG | Gunaydin (2009) [35] | 20 | Yes: IL-6, C3a, CD11b | Yes: ICU, O2sat. cerebral, CK-MB | Significantly improved ICU stay, myocardial protection (CK-MB), cerebral oxygen saturation, and intubation time | |
| RCT, CABG | Rimpilainen (2011) [36] | 18 | Yes: IL-6, IL-8, TNFα, NE, C3a | Yes: ICU stay, cerebral emboli | Significantly decreased ICU time and microemboli by retinal fluorography in minigroup | |
| RCT, valve | Tanaka (2003) [37] | 9 | No | No | Closed cardiopulmonary bypass circuit does not improve any prespecified clinical endpoints in this small study | |
| Intervention (no. of papers) | Type of Study, Surgery | Author (year) [reference]8* | No.9† | Inflammatory Biomarker(S) Suppressed Yes–No: Biomarker10‡ | Clinical Benefit Yes–No: Outcome Modified | Comment |
| RCT, CABG | Abdel-Rahman (2005) [38] | 101 | Yes: NE, C5b-9 | No | No change in ICU duration or other clinical end points in mini-group | |
| RCT, CABG | Rex (2006) [39] | 15 | No | No | No improvement in myocardial injury marker (Tn T) in minigroup | |
| RCT, CABG | Huybregts (2007) [40] | 25 | Yes: IL-6, thromboxane B2 | No | No statistically significant improvements in ICU stay or prespecified clinical end points | |
| RCT, CABG | Ohata (2008) [41] | 34 | Yes: IL-8, NE | No | No change in any reported clinical outcomes | |
| Leukocyte-depleting filter (8) | RCT, CABG | Alexiou (2004) [42] | 25 | Yes: WBC, oxidative burst | Yes: A-a O2 gradient | Arterial line leuko-depleting filter; improved A-a O2 gradient; no other clinical changes; mild benefit |
| RCT, CABG | Gunaydin (2009) [43] | 10 | Yes: IL-6, C3a, CD11b | Yes: CK-MB | Dual filter approach (continuous and after x-clamp) provided significant myocardial protection (CK-MB) | |
| RCT, valve | Zhang (2010) [44] | 26 | No | No | Arterial line filter improved myocardial protection and respiratory index but increased inflammatory cytokines | |
| RCT, valve | Hayashi (2003) [45] | 10 | Yes: NE, oxidative stress | No | Cardioplegia leukocyte-depleting filter placed after oxygenator reservoir did not change clinical outcomes | |
| RCT, mixed valve/CABG | Chen (2004) [46] | 16 | Yes: IL-8, sP-sel., sICAM, ox. stress | No | Arterial line filter did not improve prespecified clinical endpoints | |
| RCT, valve/CABG | Koskenkari (2006) [47] | 10 | No | No | Arterial line filter did not improve ICU stay or clinical end points; neutrophil activation noted in filter group | |
| RCT, valve/CABG | Soo (2010) [48] | 20 | No | No | Arterial line filter did not improve ICU stay or clinical end points; leukocyte activation noted in filter group | |
| RCT, CABG | Rubino (2011) [49] | 41 | No | No | Continuous arterial line plus cardioplegia filters did not improve myocardial markers or ICU stay | |
| Ultrafiltration (3) | RCT, CABG | Tallman (2002) [50] | 15 | No | No | Zero balance ultrafiltration; no change ventilator or ICU time; paradoxical rise in plasma inflammatory markers |
| RCT, mixed valve/CABG | Oliver (2004) [51] | 62 | No | No | Ultrafiltration; no clinical benefit in pulmonary function or other prespecified outcomes | |
| RCT, CABG | Torina (2010) [52] | 20 | No | No | Modified ultrafiltration demonstrated no change in A-a O2 gradient; no benefit | |
| Pericardial blood processing (1) | RCT, CABG | Marcheix (2008) [53] | 25 | No | No | Blood processing with cell saving device did not significantly improve ICU length of stay or other hard clinical end points |
| Discard mediastinal blood (1) | RCT, CABG | Westerberg (2004) [54] | 17 | Yes: IL-8, TNFα | No | Discarding mediastinal and cardiotomy suction blood had no effect on adverse events or myocardial marker (TnT) |
Number in square bracket refers to reference number in the Appendix B.
N, number of subjects in treatment group.
Abbreviations used: RCT, randomized controlled trial; CABG, coronary artery bypass grafting; WBC, white blood cell count; IL, interleukin; CRP, C-reactive protein; TNF, tumor necrosis factor; NE, neutrophil elastase; TAT, thrombin antithrombin; sP-sel., soluble P-selectin; sICAM, soluble ICAM-1; A-a O2, arterial–alveolar oxygen gradient; CK-MB, creatine kinase MB fraction; cTn, cardiac-specific troponin.
N/A, not appropriate.
Complement Inhibitors:
Five RCTs investigated the use of complement inhibitors, three of which found a clinical benefit. Differences were noted between different complement targets. The C1 esterase inhibitor studies were notable for their study design, using patients undergoing emergency coronary artery bypass grafting (CABG) and for the compelling myocardial protection observed despite a modest sample size (median n = 66). The multicenter PRIMO CABG trial for C5 inhibition missed its primary composite end point of 30-day mortality/myocardial infarction in patients undergoing CABG but was able to demonstrate significant improvement in the highest risk patient group for the combined PRIMO CABG I and II studies (n = 7353 in total).
Aspirin:
There was one RCT and one meta-analysis for aspirin given preoperatively (Table 3), and neither study was able to demonstrate a clinical benefit. The meta-analysis for preoperative aspirin use (n = 824) was statistically significantly associated with worsened reoperative rates. Aspirin and clopidogrel given until surgery in combination with aprotinin during surgery did not affect clinical outcome statistically, but there was concern about two deaths in the treatment arm resulting from intestinal embolism in this relatively small trial (n = 50).
Evidence Level B (single randomized controlled trial)
The full list of interventions at evidence level B is included in Tables 3–5, but the most noteworthy interventions are discussed.
Table 5.
Summary of pharmacological interventions.
| Intervention (no. of papers) | Type of Study, Surgery | Author (year) [reference]1* | No.2† | Inflammatory Biomarker(S) Suppressed Yes–No: Biomarker3‡ | Clinical Benefit Yes–No: Outcome Modified | Comment |
| Methylprednisolone (6) | RCT, CABG | Giomarelli (2003) [55]4* | 105† | Yes: IL-6, IL-8, IL-10, TNFα6‡ | Yes: CK-MB, A-a O2 gradient | 1 g preoperatively, 5 × 125 mg postoperatively; improved myocardial protection creatine kinase MB fraction (CK-MB) and A-a O2 gradient |
| RCT, CABG | Demir (2009) [56] | 15 | Yes: IL-6, IL-10 | Yes: ICU stay, NSE | 1 g before CPB; improved intensive care unit (ICU) length of stay and levels of neuron specific enolase (NSE) | |
| RCT, CABG | Fillinger (2002) [57] | 15 | Yes: IL-6, IL-10 | No | 15 mg/kg preoperatively, 4 × .3 mg/kg postoperatively; less nausea but no other changes in clinical outcomes | |
| RCT, CABG | McBride (2004) [58] | 18 | Yes: IL-8, IL-10, TNF | No | 30 mg/kg before induction; no changes in clinical outcomes | |
| RCT, CABG | Bourbon (2004) [59] | 12 | Yes: IL-6, TNF | No | 10 mg/kg preoperatively; no clinical changes, no adverse events | |
| RCT, CABG | Liakopoulos (2007) [60] | 40 | Yes: IL-6, IL-18, IL-10, TNF, CRP | No | 15 mg/kg bolus preoperatively; improved troponin (Tn) T but worsened pulmonary shunt, hyperglycemia, and lactic acidosis | |
| Dexamethasone (6) | RCT, CABG | Von Spiegel (2004) [61] | 10 | N/D7§ | Yes: lung water | 1 mg/kg after induction; improvement in lung water; mild benefit |
| RCT, CABG | Halvorsen (2003) [62] | 147 | N/D | No | 4 mg perioperatively + 4 mg postoperatively; no change in hard clinical end points, inotrope use, or reoperation rates | |
| RCT, CABG | Loef (2004) [63] | 10 | N/D | No | 1 mg/kg at induction + .5 mg/kg postoperatively; significant glycosuria, controlled through insulin; no clinical benefit | |
| RCT, mixed valve/CABG | Yared (2007) [64] | 37 | No | No | .6-mg/kg bolus; no clinical benefit and could not demonstrate inflammatory suppression to a range of markers | |
| RCT, CABG | Sobiesky (2008) [65] | 13 | Yes: IL-6 | No | 100-mg bolus after induction; no change A-a O2 gradient or ICU stay; no clinical benefit | |
| RCT, CABG | Amr (2009) [66] | 50 | Yes: IL-6, IL-8 | No | 1 mg/kg at induction + .5 mg/kg postoperatively; significant hyperglycemia; no change in hard end points; no benefit | |
| Hydrocortisone (1) | RCT, mixed valve/CABG | Halonen (2007) [67] | 120 | Yes: CRP | No | 100 mg days 1–4 of operation; no change to any prespecified clinical end points |
| Cochrane review (1) | meta-analysis valve/CABG | Dieleman (2011) [68] | 1807 | N/D | No | No beneficial effect of corticosteroid use on mortality, cardiac, and pulmonary complications |
| C1 esterase inhibitor (2) | RCT, emergency CABG | Thielmann (2006) [69] | 28 | Yes: C3, C4 | Yes: cTnI | 40-IU/kg bolus + 20 IU/kg infusion showed myocardial protection (Trop. I) with earlier treatment from acute STEMI |
| RCT, emergency CABG | Fattouch (2007) [70] | 38 | Yes: C3a, C4 | Yes: ICU stay, cTnI | 1000 IU Cl INH provided convincing improvement in ICU length of stay and cardiac function (cTnI and wall motion) | |
| Complement C5 inhibitor (2) | RCT, mixed valve/CABG | Verrier (2004) [71] | 1378 | Yes: serum complement activity | No | Multicenter trial; missed primary end point 30-day death/Ml in CABG, but significant risk reduction in intent to treat analysis |
| RCT, mixed valve/CABG | Smith (2011) [72] | 2156 | Yes: serum complement activit1 | Yes: mortality | Analysis of combined PRIMO-CABG I and II trials; highest risk patients showed significant benefit at 30-day mortality | |
| Complement receptor 1 inhibitor (1) | RCT, mixed valve/CABG | Lazar (2004) [73] | 72 | Yes: C3a, C5b-9 | No | Striking gender bias; soluble CR1 significantly inhibited death/MI in males but not females; overall no benefit |
| Neutrophil elastase inhibitor (1) | RCT, valve | Fujii (2010) [74] | 6 | Yes: IL-6, IL-8, NE | Yes: PaO2/FiO2 ratio | Infusion of neutrophil elastase inhibitor significantly improved PaO2/FiO2 ratio |
| Urinary protease inhibitor (2) | RCT, CABG | Bingyang (2007) [75] | 15 | Yes: IL-6, IL-8, NE, TNFα | Yes: ventilator time, A-a O2 gradient | Documented antiprotease activity is against neutrophil elastase; improved ventilator time and A-a O2 gradient |
| RCT, valve | Song (2011) [76] | 24 | No | Yes: ICU stay | Infusion or urintary proteas inhibitor significantly improved ICU stay; no change in inflammatory mediators | |
| Sodium nitroprusside (1) | RCT, CABG | Gol (2002) [77] | 10 | Yes: IL-6 | Yes: ICU stay, inotropes | Na nitroprusside (NO donor) intravenously after x-clamp release; convincing improvement ICU stay and need for inotropes |
| Intervention (no. of papers) | Type of Study, Surgery | Author (year) [reference]8* | No.9† | Inflammatory Biomarker(S) Suppressed Yes–No: Biomarker10‡ | Clinical Benefit Yes–No: Outcome Modified | Comment |
| Aminophylline (2) | RCT, valve | Luo (2004) [78] | 15 | Yes: IL-8, IL-10, TNFα | Yes: ICU stay, ventilator time | Perioperative aminophylline infusion significantly improved ventilator time and ICU length of stay |
| RCT, valve | Luo (2007) [79] | 15 | Yes: neutrophil myeloperoxidase | Yes: cTnI | Significant myocardial protection (TnI); fewer neutrophils in coronary sinus with less myeloperoxidase on biopsies | |
| Glutamine (1) | RCT, mixed valve/CABG | Engel (2009) [80] | 31 | Yes: IL-2, IFN-γ, NE | No | Infusion of glutamine after induction to postoperative day 3 did not affect ventilation time, ICU stay, or organ dysfunction |
| Taurine (1) | RCT, CABG | Doddakula (2010) [81] | 15 | Yes: IL-6 | No | Infusion of a 2% solution of the antioxidant agent taurine did not affect hard clinical end points or ICU stay |
| Erythropoietin (1) | RCT, mixed valve/CABG | Poulsen (2009) [82] | 22 | No | No | 2 × 500 IU/kg doses at days −1 and +1; no change in prespecified clinical outcomes |
| Aprotinin (1) | RCT, CABG | Kipfer (2003) [83] | 15 | No | No | Bleeding and transfusion benefits; no change in permitted clinical outcomes |
| Dual-dose tranexamic acid (1) | RCT, CABG | Jimenez (2011) [84] | 80 | No | No | Double-dose TXA (40 + 40 mg/kg) versus single-dose control; unwanted trend towards increased mortality and seizures |
| N-acetyl cysteine (1) | RCT, CABG | El-Hamamsy (2007) [85] | 50 | No | No | Intravenous infusion; concerning trend toward increased death and MI; also trend toward myocardial injury (CK-MB and TnT) |
| Lidocaine (1) | RCT, mixed valve/CABG | Mathew (2009) [86] | 114 | No | No | Interaction of lidocaine with diabetes caused significant postoperative cognitive decline |
| Ethylpyruvate (1) | RCT, mixed valve/CABG | Bennett-Guerrero (2009) [87] | 49 | No | No | Infusion of ethyl pyruvate did not affect death, MI, ARF, or a raft of clinical markers or hospital resource end points |
| Propofol (1) | RCT, valve | An (2008) [88] | 15 | Yes: IL-8, oxidative stress | Yes: ICU stay, ventilation time | Propofol targeted infusion during cross-clamp improved ventilation time, lung compliance and ICU stay |
| Isoflurane + propofol (1) | RCT, CABG | Huang (2011) [89] | 30 | Yes:IL-6, TNFα, oxidative stress | Yes: CK-MB, cTnI | Synergistic protective effect of isoflurane with propofol on ICU stay and myocardial protection (CK-MB and TnI) |
| Sevoflurane (2) | RCT, valve | Kawamura (2006) [90] | 13 | Yes: IL-6, IL-8 | Yes: CK-MB, TnT | Fentanyl + sevoflurane anesthesia versus fentanyl + propofol control; convincing myocardial protection (CK-MB and TnT) |
| RCT, valve | Cho (2009) [91] | 15 | Yes: IL-6, IL-10 | No | Sevoflurane anesthesia versus fentanyl + midazolam control; no change in ICU stay or other clinical end points | |
| NO gas (1) | RCT, valve | Gianetti (2004) [92] | 14 | Yes: soluble P-selectin | Yes: CK-MB, cTnI, BNP | Continuous NO inhalation perioperatively and in ICU; protection of myocardial injury markers (CK-MB, TnI, and BNP) |
| L-Arginine in cardioplegia (1) | RCT, CABG | Colagrande (2006) [93] | 33 | Yes: IL-6, TNFα | Yes: ICU stay, CK-MB, cTnI | L-arginine (nitric oxide substrate) added to cardioplegia; convincing improvement in ICU stay, CK-MB, and TnI |
| Propionyl L-Carnitine (1) | RCT, CABG | Lango (2005) [94] | 21 | Yes: endothelin, oxidative stress | Yes: lactate | Diabetic cohort; propionyl L-carnitine in cardioplegia significantly improved cardiac index, SVR, PVR, and lactate |
| Adenosine (1) | RCT, valve | Koksal (2008) [95] | 15 | Yes: neutrophil count | No | Adenosine as an adjunct to cardioplegia did not improve clinical end points or confer significant myocardial protection |
| Hydroxyethyl starch in prime (1) | RCT, valve | Choi (2010) [96] | 28 | Yes: NE | No | Hydroxyethyl starch used as priming solution, versus albumin control, did not affect ICU stay or inotrope use |
| Gelatin colloid (1) | RCT, CABG | Tamayo (2008) [97] | 22 | No | No | Priming with gelatin colloid, versus crystalloid control, had no effect on ventilation time, ICU stay, or need for inotropes |
| N-acetyl cysteine (1) | RCT, CABG | Liu (2009) [98] | 15 | No | No | In cardioplegia; no change in hard clinical end points but same trend as with intravenous infusion toward myocardial injury (CK-MB) |
Number in square bracket refers to reference number in the Appendix B.
N = number of subjects in treatment group.
Abbreviations used: RCT, randomized controlled trial; CABG, coronary artery bypass grafting; IL, interleukin; TNF, tumor necrosis factor; NE, neutrophil elastase; CRP, C-reactive protein; A-a O2, arterial–alevolar oxygen gradient; CK-MB, creatine kinase MB fraction; cTn, cardiac-specific troponin; ICU, intensive care unit; STEMI, ST-elevation myocardial infarction.
N/D, not done.
Nitric Oxide:
There were three RCTs examining different methods to deliver nitric oxide (NO) to patients perioperatively: direct inhalation of NO gas, infusion of sodium nitroprusside (NO donor), or L-arginine (NO substrate) added to cardioplegia. Taken together, these small NO trials (median sample size n = 28) all demonstrated clinical benefit.
Neutrophil Elastase Inhibitors:
There were three RCTs examining administration of neutrophil elastase inhibitors, two of which used urinary protease inhibitor and one human elastase inhibitor. Taken together, these small neutrophil elastase interventions (median sample size n = 30) were adjudged clinically beneficial.
Propofol:
There were two RCTs studying propofol anesthesia. Both studies demonstrated a clinical outcome improvement. One study examined the interaction between isoflurane preconditioning and propofol postconditioning and was able to demonstrate a clinical benefit for this combination.
Aminophylline:
There were two RCTs from the same research group on the bronchodilator aminophylline. Both of these small studies (n = 30) demonstrated a clinical benefit.
Sevoflurane:
There were two RCTs on sevoflurane anesthesia, only one of which demonstrated an improvement in a clinical outcome.
Intensive Insulin Therapy:
There was one RCT on intensive insulin therapy using the Portland Protocol (22) in patients with no history of diabetes. This well-designed study (n = 100) showed a clinical benefit with significantly shortened intensive care unit stay and myocardial protection.
Fluvastatin:
There was one RCT on fluvastatin administered for 3 weeks up to the day of surgery; this study demonstrated clinical benefit.
Propionyl-L-Carnitine:
There was one RCT on propionyl L-carnitine administration in a diabetic cohort assigned a clinical benefit for this intervention.
Ultrafiltration:
There were three RCTs on ultrafiltration using three different ultrafiltration techniques. However, none of the trials recorded a significant depletion of inflammatory biomarkers and none achieved a clinical benefit. There were no indications of negative patient outcomes.
There was little evidence across the many varied interventions for causing harm, but lidocaine infusion exhibited an interaction with diabetes leading to significantly worsened postoperative cognitive dysfunction. Two trials on N-acetyl cysteine manifested a concerning trend for death or myocardial injury in the treatment arm, whether given intravenously or added to cardioplegia. Aspirin given preoperatively was associated with worsened reoperative rates. Finally, double-dose tranexamic acid revealed a trend toward increased mortality and seizures.
Methodological Quality
Variable quality in methodological descriptions was noted across the evidence base, particularly in areas of blood management and the description of perfusion equipment used: 31.6% of papers provided inadequate or nonexistent protocols for blood management and 29.6% of papers were classified as poor for equipment. Surgical protocols were described more adequately with only 12.2% of papers classified as poor in the evidence base.
Association between Inflammatory Suppression and Clinical Benefit
The five most frequently monitored biomarkers for inflammation (interleukin [IL]-6, IL-8, tumor necrosis factor α, neutrophil elastase and complement component C3a) were also the five most often suppressed and most likely to be associated with a clinical benefit. Suppression of biomarkers occurred with similar frequency across the three categories of intervention. It occurred in 68.4% of the studies in surgical/perioperative management, 65.7% of the studies of perfusion-related interventions, and 68.2% of the investigations of pharmacological interventions.
DISCUSSION
The review strategy adopted in this systematic analysis, combining self-identified anti-inflammatory studies with inclusion criteria specified in the Outcomes consensus statement (19), yielded a rich evidence base for anti-inflammatory interventions covering surgical, pharmacological, and perfusion approaches. The overall clinical dividend from this large body of literature yielded only 35.7% of papers capable of demonstrating a clinical benefit. The most widely studied intervention, steroid use, showed no evidence for improvement of clinical outcomes according to our analysis, which supports a recent Cochrane review that concluded corticosteroids exerted no clinical benefit on mortality, cardiac, or pulmonary complications (21). On the positive side, there was little evidence for harm associated with the many varied attempts to inhibit the systemic inflammatory response with only certain isolated interventions earning a precautionary negative recommendation (aspirin, lidocaine in persons with diabetes, N-acetylcysteine, and double-dose tranexamic acid).
Various surface coating and surface reduction strategies showed some promise but no compelling clinical benefit, including biocompatible surface coatings, minimized circuits, and off-pump coronary revascularization. The rationale for heparin coating, however, targeting factor IIa at the terminus of the coagulation cascade, must remain questionable. It is likely that whatever clinical benefit heparin confers is the result of “part-time” inhibition higher up the cascade at the level of factor IXa (23). We hypothesize that upstream interdictions might provide a better opportunity for anti-inflammatory protection by blocking the avalanche of activated factors at the top of the cascade. On this basis, we highlight interventions using C1 esterase inhibitor (Appendix B: references [69,70]), a pleiotropic agent with upstream targets in coagulation, complement, and fibrinolytic pathways (24). Both C1 esterase inhibitor studies showed a clinical benefit in emergency CABG populations and both demonstrated a gradient effect with improved myocardial protection associated with earlier treatment from the point of acute ST-elevation myocardial infarction. Other promising interventions such as NO therapy (Appendix B: references [77,92,93]) may also have benefited from a pleiotropic mechanism of action, because NO acts to promote vasodilation (25), has antiadhesive effects on leukocytes (26), and anticoagulant properties against platelets (27). We hypothesize that pleiotropic agents like NO or C1 esterase inhibitor are able to achieve a consistent clinical benefit by blocking multiple targets in multiple pathways (13,28,29).
Filtration strategies to remove leukocytes or soluble cytokines were not typically associated with any clinical benefit. This contrasted with arterial line filters for removing microemboli, which earned a class I recommendation in a previous evidence-based review (30). Some studies noted exacerbation of leukocyte activation, presumably as a result of adherence and activation of leukocytes at the filter surface. Our observations are consistent with the findings of other meta-analyses, which concluded that leukocyte-depleting filters or zero-balanced ultrafiltration did not confer significant clinical benefits (31,32).
The authors recognize some limitations to this critical review. First, the review is not intended as a critical care guide to manage patients after they have experienced a severe systemic inflammatory response, but as a guide to clinical practices aimed at limiting the inflammatory response from the outset. We acknowledge that patient distribution in the evidence base was highly skewed with one Cochrane review on steroid use and one multicenter trial on C5 inhibition accounting for over 60% of the total number. We further recognize that a different “mix” of papers was captured by our reviewing strategy compared with other meta-analyses. For example, a previous metaanalysis on biocompatible surface coatings identified 36 papers, whereas only 14 qualified by our inclusion criteria (33). This was attributable to the fact our search was limited to a 10-year window and used more stringent inclusion criteria. Nonetheless, we reached the same conclusion as that meta-analysis: that biocompatible surface coatings without other measures to limit blood activation conferred only limited clinical benefit. In adopting the minimal reporting criteria of the consensus statement, we recognize that clinical benefit may be based on a single clinical variable. Improvement of a single variable may not on its own guarantee improvement in clinical outcome and benefits might best be accrued when treatments are used in combination. Because few such combinations have been evaluated systematically, this becomes an important area for future research. The overall validity of our approach was supported across several interventions with the current review reaching similar conclusions as contemporary meta-analyses in the areas of: corticosteroid use, filtration, and biocompatible surface coating (21,31–33). Regular updates are warranted to capture changing practice and emerging evidence outside the 10-year window of this review (34–36) that may change clinical practice in the future.
The review highlighted several shortcomings in methodological quality. In addition to problems with small sample size and heterogeneity of interventions and biomarkers studied, there were fundamental shortcomings in methodological descriptions. This was particularly apparent for blood management protocols and description of perfusion equipment. Inadequate descriptions of these practices are important, because they can impact inflammatory processes and clinical outcomes (30). We therefore advocate the use of tables in the Methods section to itemize in detail the system components of the bypass circuit and blood management protocols used (37).
The broad scope of the current review yielded novel insight into the relationship between anti-inflammatory action and clinical efficacy. This arose from a secondary analysis showing that 97% of papers with a clinical benefit achieved suppression of at least one inflammatory marker (Tables 3–5). However, over half the papers (57%) that did not find a clinical benefit also did demonstrate suppression of an inflammatory biomarker. This suggests that suppression of one inflammatory biomarker is therefore necessary but not sufficient to translate into a clinical benefit.
CONCLUSIONS
This critical review concludes that no single intervention used on its own demonstrates strong evidence for limiting adverse outcomes as a result of the systemic inflammatory response. A secondary analysis showed that suppression of a single inflammatory biomarker was required but was not sufficient to confer a clinical benefit. The most promising interventions were those that targeted multiple inflammatory pathways. These results are consistent with a “multiple hit” hypothesis, whereby clinically effective suppression of the systemic inflammatory response requires hitting multiple inflammatory targets. Further research is warranted to evaluate combinations of interventions capable of achieving synergy by targeting the many pathways that are activated.
ACKNOWLEDGMENTS
We acknowledge Ms. Suzanne Burge who assisted with sourcing of articles in the literature review.
APPENDIX A. PubMed search structure.
The following search terms recovered >1600 articles in PubMed: ((cardiac surgery OR (((“cardiopulmonary bypass”[TIAB] NOT Medline[SB]) OR “cardiopulmonary bypass” [MeSH Terms] OR (“coronary artery bypass” [TIAB] NOT Medline[SB]) OR “coronary artery bypass” [MeSH Terms]) OR (valve OR valvular) AND surgery) OR “Heart-lung machine” [MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]))) NOT (cardiac surgery OR (((“cardiopulmonary bypass” [TIAB] NOT Medline[SB]) OR “cardiopulmonary bypass” [MeSH Terms] OR (“coronary artery bypass” [TIAB] NOT Medline[SB]) OR “coronary artery bypass” [MeSH Terms]) OR (valve OR valvular) AND surgery) OR “Heart-lung machine” [MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child [MeSH] OR adolescent[MeSH]))))) OR ((cardiac surgery OR (((“cardiopulmonary bypass” [TIAB] NOT Medline [SB]) OR “cardiopulmonary bypass” [MeSH Terms] OR (“coronary artery bypass” [TIAB] NOT Medline[SB]) OR “coronary artery bypass” [MeSH Terms]) OR (valve OR valvular) AND surgery) OR “Heart-lung machine” [MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English[lang]) AND ((infant[MeSH] OR child[MeSH] OR adolescent[MeSH])))) AND (cardiac surgery OR (((“cardiopulmonary bypass” [TIAB] NOT Medline[SB]) OR “cardiopulmonary bypass” [MeSH Terms] OR (“coronary artery bypass” [TIAB] NOT Medline[SB]) OR “coronary artery bypass” [MeSH Terms]) OR (valve OR valvular) AND surgery) OR “Heart-lung machine” [MeSH Terms] OR ((hemofiltration OR ultrafiltration) AND (cardiac OR heart)) AND ((Humans[Mesh]) AND (English [lang]) AND (adult[MeSH])))) NOT (Editorial[ptyp] OR Letter[ptyp] OR Comment[ptyp] OR Festschrift[ptyp] OR Historical Article[ptyp] OR Lectures[ptyp] OR Legal Cases[ptyp] OR Legislation[ptyp] OR News[ptyp] OR Newspaper Article[ptyp] OR Patient Education Handout [ptyp]) nerv* OR cogniti* OR cerebr* OR brain OR neurolog* OR neurocognitive OR “cerebral arteries” [mesh] OR “brain chemistry” [mesh] OR “cognition disorders” [mesh] OR “cerebrovascular circulation” [mesh] OR “brain” [mesh] OR “nervous system diseases” [mesh] OR embolism[ mesh] OR “Cerebrovascular Disorders” [mesh] inflammatory OR inflammation OR Anti-Inflammatory Agents OR immunology OR SIRS death OR mi OR infarction OR lung OR kidney OR heart OR icu OR dialysis OR patency.
APPENDIX B. References comprising the evidence base.
References in order of appearance in Tables 3, 4, and 5
- [1].Rastan AJ, Bittner HB, Gummert JF, et al. On-pump beating heart versus off-pump coronary artery bypass surgery—Evidence of pump-induced myocardial injury. Eur J Cardiothorac Surg. 2005;27:1057–1064. [DOI] [PubMed] [Google Scholar]
- [2].Nesher N, Frolkis I, Vardi M, et al. Higher levels of serum cytokines and myocardial tissue markers during on-pump versus off-pump coronary artery bypass surgery. J Card Surg. 2006;21:395–402. [DOI] [PubMed] [Google Scholar]
- [3].Serrano CV Jr, Souza JA, Lopes NH, et al. Reduced expression of systemic proinflammatory and myocardial biomarkers after off-pump versus on-pump coronary artery bypass surgery: A prospective randomized study. J Crit Care. 2010;25:305–312. [DOI] [PubMed] [Google Scholar]
- [4].Tsai CS, Tsai YT, Lin CY, et al. Expression of thrombomodulin on monocytes is associated with early outcomes in patients with coronary artery bypass graft surgery. Shock. 2010;34:31–39. [DOI] [PubMed] [Google Scholar]
- [5].Sahlman A, Ahonen J, Nemlander A, et al. Myocardial metabolism on off-pump surgery; a randomized study of 50 cases. Scand Cardiovasc J. 2003;37:211–215. [DOI] [PubMed] [Google Scholar]
- [6].Wan IY, Arifi AA, Wan S, et al. Beating heart revascularization with or without cardiopulmonary bypass: Evaluation of inflammatory response in a prospective randomized study. J Thorac Cardiovasc Surg. 2004;127:1624–1631. [DOI] [PubMed] [Google Scholar]
- [7].Velissaris T, Tang AT, Murray M, et al. A prospective randomized study to evaluate stress response during beating-heart and conventional coronary revascularization. Ann Thorac Surg. 2004;78:506–512. [DOI] [PubMed] [Google Scholar]
- [8].Quaniers JM, Leruth J, Albert A, Limet RR, Defraigne JO.. Comparison of inflammatory responses after off-pump and on-pump coronary surgery using surface modifying additives circuit. Ann Thorac Surg. 2006;81:1683–1690. [DOI] [PubMed] [Google Scholar]
- [9].Paulitsch FS, Schneider D, Sobel BE, et al. Hemostatic changes and clinical sequelae after on-pump compared with off-pump coronary artery bypass surgery: A prospective randomized study. Coron Artery Dis. 2009;20:100–105. [DOI] [PubMed] [Google Scholar]
- [10].Formica F, Broccolo F, Martino A, et al. Myocardial revascularization with miniaturized extracorporeal circulation versus off pump: Evaluation of systemic and myocardial inflammatory response in a prospective randomized study. J Thorac Cardiovasc Surg. 2009;137:1206–1212. [DOI] [PubMed] [Google Scholar]
- [11].Sun JC, Whitlock R, Cheng J, et al. The effect of pre-operative aspirin on bleeding, transfusion, myocardial infarction, and mortality in coronary artery bypass surgery: A systematic review of randomized and observational studies. Eur Heart J. 2008;29:1057–1071. [DOI] [PubMed] [Google Scholar]
- [12].Akowuah E, Shrivastava V, Jamnadas B, et al. Comparison of two strategies for the management of antiplatelet therapy during urgent surgery. Ann Thorac Surg. 2005;80:149–152. [DOI] [PubMed] [Google Scholar]
- [13].Berkan O, Katrancioglu N, Ozker E, Ozerdem G, Bakici Z, Yilmaz MB.. Reduced P-selectin in hearts pretreated with fluvastatin: A novel benefit for patients undergoing open heart surgery. Thorac Cardiovasc Surg. 2009;57:91–95. [DOI] [PubMed] [Google Scholar]
- [14].Meyns B, Autschbach R, Boning A, et al. Coronary artery bypass grafting supported with intracardiac microaxial pumps versus normothermic cardiopulmonary bypass: A prospective randomized trial. Eur J Cardiothorac Surg. 2002;22:112–117. [DOI] [PubMed] [Google Scholar]
- [15].Stassano P, Di TL, Monaco M, et al. Myocardial revascularization by left ventricular assisted beating heart is associated with reduced systemic inflammatory response. Ann Thorac Surg. 2009;87:46–52. [DOI] [PubMed] [Google Scholar]
- [16].Stassano P, Di TL, Monaco M, et al. Left heart pump-assisted myocardial revascularization favorably affects neutrophil apoptosis. World J Surg. 2010;34:652–657. [DOI] [PubMed] [Google Scholar]
- [17].Zheng R, Gu C, Wang Y, et al. Impacts of intensive insulin therapy in patients undergoing heart valve replacement. Heart Surg Forum. 2010;13:E292–E298. [DOI] [PubMed] [Google Scholar]
- [18].Ng CS, Wan S, Wan IY, et al. Ventilation during cardiopulmonary bypass: Impact on neutrophil activation and pulmonary sequestration. J Invest Surg. 2009;22:333–339. [DOI] [PubMed] [Google Scholar]
- [19].Narayan P, Rogers CA, Bayliss KM, et al. On-pump coronary surgery with and without cardioplegic arrest: Comparison of inflammation, myocardial, cerebral and renal injury and early and late health outcome in a single-centre randomised controlled trial. Eur J Cardiothorac Surg. 2011;39:675–683. [DOI] [PubMed] [Google Scholar]
- [20].Heyer EJ, Lee KS, Manspeizer HE, et al. Heparin-bonded cardiopulmonary bypass circuits reduce cognitive dysfunction. J Cardiothorac Vasc Anesth. 2002;16:37–42. [DOI] [PubMed] [Google Scholar]
- [21].Svenmarker S, Haggmark S, Jansson E, et al. Use of heparin-bonded circuits in cardiopulmonary bypass improves clinical outcome. Scand Cardiovasc J. 2002;36:241–246. [DOI] [PubMed] [Google Scholar]
- [22].de Vroege R van Oeveren W, van Klarenbosch J, et al. The impact of heparin-coated cardiopulmonary bypass circuits on pulmonary function and the release of inflammatory mediators. Anesth Analg. 2004;98:1586–1594, table. [DOI] [PubMed] [Google Scholar]
- [23].Ueyama K, Nishimura K, Nishina T, Nakamura T, Ikeda T, Komeda M.. PMEA coating of pump circuit and oxygenator may attenuate the early systemic inflammatory response in cardiopulmonary bypass surgery. ASAIO J. 2004;50:369–372. [DOI] [PubMed] [Google Scholar]
- [24].Lindholm L, Westerberg M, Bengtsson A, Ekroth R, Jensen E, Jeppsson A.. A closed perfusion system with heparin coating and centrifugal pump improves cardiopulmonary bypass biocompatibility in elderly patients. Ann Thorac Surg. 2004;78:2131–2138. [DOI] [PubMed] [Google Scholar]
- [25].Baufreton C, Allain P, Chevailler A, et al. Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. Ann Thorac Surg. 2005;79:1597–1605. [DOI] [PubMed] [Google Scholar]
- [26].Vanden Eynden F, Carrier M, Ouellet S, et al. Avecor Trillium oxygenator versus noncoated Monolyth oxygenator: A prospective randomized controlled study. J Card Surg. 2008;23:288–293. [DOI] [PubMed] [Google Scholar]
- [27].Mirow N, Zittermann A, Korperich H, et al. Diffusion-weighted magnetic resonance imaging for the detection of ischemic brain lesions in coronary artery bypass graft surgery: Relation to extracorporeal circulation and heparinization. J Cardiovasc Surg (Torino). 2011;52:117–126. [PubMed] [Google Scholar]
- [28].Baufreton C, Pinaud F, Corbeau JJ, et al. Increased cerebral blood flow velocities assessed by transcranial Doppler examination is associated with complement activation after cardiopulmonary bypass. Perfusion. 2011;26:91–98. [DOI] [PubMed] [Google Scholar]
- [29].Hoel TN, Videm V, Baksaas ST, Mollnes TE, Brosstad F, Svennevig JL.. Comparison of a Duraflo II-coated cardiopulmonary bypass circuit and a trillium-coated oxygenator during open-heart surgery. Perfusion. 2004;19:177–184. [DOI] [PubMed] [Google Scholar]
- [30].Skrabal CA, Khosravi A, Westphal B, Steinhoff G, Liebold A.. Effects of poly-2-methoxyethylacrylate (PMEA)-coating on CPB circuits. Scand Cardiovasc J. 2006;40:224–229. [DOI] [PubMed] [Google Scholar]
- [31].Ninomiya M, Miyaji K, Takamoto S.. Influence of PMEA-coated bypass circuits on perioperative inflammatory response. Ann Thorac Surg. 2003;75:913–917. [DOI] [PubMed] [Google Scholar]
- [32].Thiara AS, Mollnes TE, Videm V, et al. Biocompatibility and pathways of initial complement pathway activation with Phisio- and PMEA-coated cardiopulmonary bypass circuits during open-heart surgery. Perfusion. 2011;26:107–114. [DOI] [PubMed] [Google Scholar]
- [33].Allen S, McBride WT, Young IS, et al. A clinical, renal and immunological assessment of surface modifying additive treated (SMART) cardiopulmonary bypass circuits. Perfusion. 2005;20:255–262. [DOI] [PubMed] [Google Scholar]
- [34].Kofidis T, Baraki H, Singh H, et al. The minimized extracorporeal circulation system causes less inflammation and organ damage. Perfusion. 2008;23:147–151. [DOI] [PubMed] [Google Scholar]
- [35].Gunaydin S, Sari T, McCusker K, Schonrock U, Zorlutuna Y.. Clinical evaluation of minimized extracorporeal circulation in high-risk coronary revascularization: Impact on air handling, inflammation, hemodilution and myocardial function. Perfusion. 2009;24:153–162. [DOI] [PubMed] [Google Scholar]
- [36].Rimpilainen R, Hautala N, Koskenkari JK, et al. Minimized cardiopulmonary bypass reduces retinal microembolization: A randomized clinical study using fluorescein angiography. Ann Thorac Surg. 2011;91:16–22. [DOI] [PubMed] [Google Scholar]
- [37].Tanaka H, Oshiyama T, Narisawa T, et al. Clinical study of biocompatibility between open and closed heparin-coated cardiopulmonary bypass circuits. J Artif Organs. 2003;6:245–252. [DOI] [PubMed] [Google Scholar]
- [38].Abdel-Rahman U, Ozaslan F, Risteski PS, et al. Initial experience with a minimized extracorporeal bypass system: Is there a clinical benefit? Ann Thorac Surg. 2005;80:238–243. [DOI] [PubMed] [Google Scholar]
- [39].Rex S, Brose S, Metzelder S, et al. Normothermic beating heart surgery with assistance of miniaturized bypass systems: The effects on intraoperative hemodynamics and inflammatory response. Anesth Analg. 2006;102:352–362. [DOI] [PubMed] [Google Scholar]
- [40].Huybregts RA, Morariu AM, Rakhorst G, et al. Attenuated renal and intestinal injury after use of a mini-cardiopulmonary bypass system. Ann Thorac Surg. 2007;83:1760–1766. [DOI] [PubMed] [Google Scholar]
- [41].Ohata T, Mitsuno M, Yamamura M, et al. Beneficial effects of minicardiopulmonary bypass on hemostasis in coronary artery bypass grafting: Analysis of inflammatory response and hemodilution. ASAIO J. 2008;54:207–209. [DOI] [PubMed] [Google Scholar]
- [42].Alexiou C, Tang AA, Sheppard SV, et al. The effect of leucodepletion on leucocyte activation, pulmonary inflammation and respiratory index in surgery for coronary revascularisation: A prospective randomised study. Eur J Cardiothorac Surg. 2004;26:294–300. [DOI] [PubMed] [Google Scholar]
- [43].Gunaydin S, Modine T, Sari T, Zorlutuna Y, Gourlay T.. Clinical efficacy of two-phase leukocyte filtration in high-risk patients undergoing coronary revascularization with cardiopulmonary bypass. J Extra Corpor Technol. 2009;41:149–156. [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang X, Zhou C, Zhuang J, et al. Effects of leukocyte depletion on cardiopulmonary protection and inflammation after valve surgery. Int J Artif Organs. 2010;33:812–818. [PubMed] [Google Scholar]
- [45].Hayashi Y, Sawa Y, Fukuyama N, et al. Leukocyte-depleted terminal blood cardioplegia provides superior myocardial protective effects in association with myocardium-derived nitric oxide and peroxynitrite production for patients undergoing prolonged aortic crossclamping for more than 120 minutes. J Thorac Cardiovasc Surg. 2003;126:1813–1821. [DOI] [PubMed] [Google Scholar]
- [46].Chen YF, Tsai WC, Lin CC, et al. Effect of leukocyte depletion on endothelial cell activation and transendothelial migration of leukocytes during cardiopulmonary bypass. Ann Thorac Surg. 2004;78:634–642. [DOI] [PubMed] [Google Scholar]
- [47].Koskenkari JK, Rimpilainen J, Ohman H, et al. Leukocyte filter enhances neutrophil activation during combined aortic valve and coronary artery bypass surgery. Heart Surg Forum. 2006;9:E693–E699. [DOI] [PubMed] [Google Scholar]
- [48].Soo AW, Maher BM, Daly L, Wood AE, Watson WR.. Preoperative neutrophil response as a predictive marker of clinical outcome following open heart surgery and the impact of leukocyte filtration. Interact Cardiovasc Thorac Surg. 2010;11:604–611. [DOI] [PubMed] [Google Scholar]
- [49].Rubino AS, Serraino GF, Mariscalco G, Marsico R, Sala A, Renzulli A.. Leukocyte depletion during extracorporeal circulation allows better organ protection but does not change hospital outcomes. Ann Thorac Surg. 2011;91:534–540. [DOI] [PubMed] [Google Scholar]
- [50].Tallman RD, Dumond M, Brown D.. Inflammatory mediator removal by zero-balance ultrafiltration during cardiopulmonary bypass. Perfusion. 2002;17:111–115. [DOI] [PubMed] [Google Scholar]
- [51].Oliver WC Jr, Nuttall GA, Orszulak TA, et al. Hemofiltration but not steroids results in earlier tracheal extubation following cardiopulmonary bypass: A prospective, randomized double-blind trial. Anesthesiology. 2004;101:327–339. [DOI] [PubMed] [Google Scholar]
- [52].Torina AG, Petrucci O, Oliveira PP, et al. The effects of modified ultrafiltration on pulmonary function and transfusion requirements in patients underwent coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2010;25:59–65. [DOI] [PubMed] [Google Scholar]
- [53].Marcheix B, Carrier M, Martel C, et al. Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann Thorac Surg. 2008;85:530–535. [DOI] [PubMed] [Google Scholar]
- [54].Westerberg M, Bengtsson A, Jeppsson A.. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg. 2004;78:54–59. [DOI] [PubMed] [Google Scholar]
- [55].Giomarelli P, Scolletta S, Borrelli E, Biagioli B.. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Ann Thorac Surg. 2003;76:117–123. [DOI] [PubMed] [Google Scholar]
- [56].Demir T, Demir H, Tansel T, et al. Influence of methylprednisolone on levels of neuron-specific enolase in cardiac surgery: A corticosteroid derivative to decrease possible neuronal damage. J Card Surg. 2009;24:397–403. [DOI] [PubMed] [Google Scholar]
- [57].Fillinger MP, Rassias AJ, Guyre PM, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:163–169. [DOI] [PubMed] [Google Scholar]
- [58].McBride WT, Allen S, Gormley SM, et al. Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery. Cytokine. 2004;27:81–89. [DOI] [PubMed] [Google Scholar]
- [59].Bourbon A, Vionnet M, Leprince P, et al. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardiothorac Surg. 2004;26:932–938. [DOI] [PubMed] [Google Scholar]
- [60].Liakopoulos OJ, Schmitto JD, Kazmaier S, et al. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. 2007;84:110–118. [DOI] [PubMed] [Google Scholar]
- [61].von Spiegel T, Giannaris S, Wietasch GJ, et al. Effects of dexamethasone on intravascular and extravascular fluid balance in patients undergoing coronary bypass surgery with cardiopulmonary bypass. Anesthesiology. 2002;96:827–834. [DOI] [PubMed] [Google Scholar]
- [62].Halvorsen P, Raeder J, White PF, et al. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesth Analg. 2003;96:1578–1583, table. [DOI] [PubMed] [Google Scholar]
- [63].Loef BG, Henning RH, Epema AH, Rietman GW, et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2004;93:793–798. [DOI] [PubMed] [Google Scholar]
- [64].Yared JP, Bakri MH, Erzurum SC, et al. Effect of dexamethasone on atrial fibrillation after cardiac surgery: Prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2007;21:68–75. [DOI] [PubMed] [Google Scholar]
- [65].Sobieski MA, Graham JD, Pappas PS, Tatooles AJ, Slaughter MS.. Reducing the effects of the systemic inflammatory response to cardiopulmonary bypass: Can single dose steroids blunt systemic inflammatory response syndrome? ASAIO J. 2008;54:203–206. [DOI] [PubMed] [Google Scholar]
- [66].Yasser MA, Elmistekawy E, El-Serogy H.. Effects of dexamethasone on pulmonary and renal functions in patients undergoing CABG with cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2009;13:231–237. [DOI] [PubMed] [Google Scholar]
- [67].Halonen J, Halonen P, Jarvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. JAMA. 2007;297:1562–1567. [DOI] [PubMed] [Google Scholar]
- [68].Dieleman JM, van Paassen J, van Dijk D, et al. Prophylactic corticosteroids for cardiopulmonary bypass in adults. Cochrane Database Syst Rev. 2011;5:CD005566. [DOI] [PubMed] [Google Scholar]
- [69].Thielmann M, Marggraf G, Neuhauser M, et al. Administration of C1-esterase inhibitor during emergency coronary artery bypass surgery in acute ST-elevation myocardial infarction. Eur J Cardiothorac Surg. 2006;30:285–293. [DOI] [PubMed] [Google Scholar]
- [70].Fattouch K, Bianco G, Speziale G, et al. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: A randomised double-blind study. Eur J Cardiothorac Surg. 2007;32:326–332. [DOI] [PubMed] [Google Scholar]
- [71].Verrier ED, Shernan SK, Taylor KM, et al. Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: A randomized trial. JAMA. 2004;291:2319–2327. [DOI] [PubMed] [Google Scholar]
- [72].Smith PK, Shernan SK, Chen JC, et al. Effects of C5 complement inhibitor pexelizumab on outcome in high-risk coronary artery bypass grafting: Combined results from the PRIMO-CABG I and II trials. J Thorac Cardiovasc Surg. 2011;142:89–98. [DOI] [PubMed] [Google Scholar]
- [73].Lazar HL, Bokesch PM, van Lenta F, et al. Soluble human complement receptor 1 limits ischemic damage in cardiac surgery patients at high risk requiring cardiopulmonary bypass. Circulation. 2004;110Suppl 1:II274–II279. [DOI] [PubMed] [Google Scholar]
- [74].Fujii M, Miyagi Y, Bessho R, Nitta T, Ochi M, Shimizu K.. Effect of a neutrophil elastase inhibitor on acute lung injury after cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10:859–862. [DOI] [PubMed] [Google Scholar]
- [75].Bingyang J, Jinping L, Mingzheng L, Guyan W, Zhengyi F.. Effects of urinary protease inhibitor on inflammatory response during onpump coronary revascularisation. Effect of ulinastatin on inflammatory response. J Cardiovasc Surg (Torino). 2007;48:497–503. [PubMed] [Google Scholar]
- [76].Song JE, Kang WS, Kim DK, et al. The effect of ulinastatin on postoperative blood loss in patients undergoing open heart surgery with cardiopulmonary bypass. J Int Med Res. 2011;39:1201–1210. [DOI] [PubMed] [Google Scholar]
- [77].Gol MK, Nisanoglu V, Iscan Z, Balci M, Kandemir O, Tasdemir O.. Inhibition of systemic inflammatory response with sodium nitroprusside in open heart surgery. J Cardiovasc Surg (Torino). 2002;43:803–809. [PubMed] [Google Scholar]
- [78].Luo WJ, Ling X, Huang RM.. Effects of aminophylline on cytokines and pulmonary function in patients undergoing valve replacement. Eur J Cardiothorac Surg. 2004;25:766–771. [DOI] [PubMed] [Google Scholar]
- [79].Luo WJ, Qian JF, Jiang HH.. Pretreatment with aminophylline reduces release of Troponin I and neutrophil activation in the myocardium of patients undergoing cardioplegic arrest. Eur J Cardiothorac Surg. 2007;31:360–365. [DOI] [PubMed] [Google Scholar]
- [80].Engel JM, Pitz S, Muhling J, et al. Role of glutamine administration on T-cell derived inflammatory response after cardiopulmonary bypass. Clin Nutr. 2009;28:15–20. [DOI] [PubMed] [Google Scholar]
- [81].Doddakula KK, Neary PM, Wang JH, et al. The antiendotoxin agent taurolidine potentially reduces ischemia/reperfusion injury through its metabolite taurine. Surgery. 2010;148:567–572. [DOI] [PubMed] [Google Scholar]
- [82].Poulsen TD, Andersen LW, Steinbruchel D, Gotze JP, Jorgensen OS, Olsen NV.. Two large preoperative doses of erythropoietin do not reduce the systemic inflammatory response to cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:316–323. [DOI] [PubMed] [Google Scholar]
- [83].Kipfer B, Englberger L, Gygax E, Nydegger U, Carrel T.. Is reduced systemic heparinization justified with heparin-bonded bypass circuits in cardiac surgery? Experience with and without aprotinin. Transfus Apheresis Sci. 2003;29:17–24. [DOI] [PubMed] [Google Scholar]
- [84].Jimenez JJ, Iribarren JL, Brouard M, et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: A randomized double-blind, dose-dependent, phase IV clinical trial. J Cardiothorac Surg. 2011;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].El-Hamamsy I, Stevens LM, Carrier M, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: A randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2007;133:7–12. [DOI] [PubMed] [Google Scholar]
- [86].Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Benett-Guerrero E, Swaminathan M, Grigore AM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:324–329. [DOI] [PubMed] [Google Scholar]
- [88].An K, Shu H, Huang W, et al. Effects of propofol on pulmonary inflammatory response and dysfunction induced by cardiopulmonary bypass. Anaesthesia. 2008;63:1187–1192. [DOI] [PubMed] [Google Scholar]
- [89].Huang Z, Zhong X, Irwin MG, et al. Synergy of isoflurane preconditioning and propofol postconditioning reduces myocardial reperfusion injury in patients. Clin Sci (Lond). 2011;121:57–69. [DOI] [PubMed] [Google Scholar]
- [90].Kawamura T, Kadosaki M, Nara N, et al. Effects of sevoflurane on cytokine balance in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2006;20:503–508. [DOI] [PubMed] [Google Scholar]
- [91].Cho EJ, Yoon JH, Hong SJ, Lee SH, Sim SB.. The effects of sevoflurane on systemic and pulmonary inflammatory responses after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:639–645. [DOI] [PubMed] [Google Scholar]
- [92].Gianetti J, Del SP, Bevilacqua S, et al. Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg. 2004;127:44–50. [DOI] [PubMed] [Google Scholar]
- [93].Colagrande L, Formica F, Porta F, et al. Reduced cytokines release and myocardial damage in coronary artery bypass patients due to L-arginine cardioplegia supplementation. Ann Thorac Surg. 2006;81:1256–1261. [DOI] [PubMed] [Google Scholar]
- [94].Lango R, Smolenski RT, Rogowski J, et al. Propionyl-L-carnitine improves hemodynamics and metabolic markers of cardiac perfusion during coronary surgery in diabetic patients. Cardiovasc Drugs Ther. 2005;19:267–275. [DOI] [PubMed] [Google Scholar]
- [95].Koksal H, Rahman A, Burma O, Halifeoglu I, Bayar MK.. The effects of low dose N-acetylcysteine (NAC) as an adjunct to cardioplegia in coronary artery bypass surgery. Anadolu Kardiyol Derg. 2008;8:437–443. [PubMed] [Google Scholar]
- [96].Choi YS, Shim JK, Hong SW, Kim JC, Kwak YL.. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 on coagulation and inflammatory response when used as priming solutions for cardiopulmonary bypass. Minerva Anestesiol. 2010;76:584–591. [PubMed] [Google Scholar]
- [97].Tamayo E, Alvarez FJ, Alonso O, et al. The inflammatory response to colloids and crystalloids used for pump priming during cardiopulmonary bypass. Acta Anaesthesiol Scand. 2008;52:1204–1212. [DOI] [PubMed] [Google Scholar]
- [98].Liu R, Xing J, Miao N, et al. The myocardial protective effect of adenosine as an adjunct to intermittent blood cardioplegia during open heart surgery. Eur J Cardiothorac Surg. 2009;36:1018–1023. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Prondzinsky R, Knupfer A, Loppnow H, et al. Surgical trauma affects the proinflammatory status after cardiac surgery to a higher degree than cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:760–766. [DOI] [PubMed] [Google Scholar]
- 2.Bittar N, Koke JR, Berkoff HA, Kahn DR.. Histochemical and structural changes in human myocardial cells after cardiopulmonary bypass. Circulation. 1975;52Suppl:I16–I25. [PubMed] [Google Scholar]
- 3.Freeman R, Gould FK.. Rises in antibody to enteric gram negative bacilli after open heart surgery: A possible mechanism for postoperative pyrexia. Thorax. 1985;40:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW.. Complement activation during cardiopulmonary bypass: Evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304:497–503. [DOI] [PubMed] [Google Scholar]
- 5.Brister SJ, Ofosu FA, Buchanan MR.. Thrombin generation during cardiac surgery: Is heparin the ideal anticoagulant? Thromb Haemost. 1993;70:259–262. [PubMed] [Google Scholar]
- 6.Kang HM, Kalnoski MH, Frederick M, Chandler WL.. The kinetics of plasmin inhibition by aprotinin in vivo. Thromb Res. 2005;115:327–340. [DOI] [PubMed] [Google Scholar]
- 7.Wachtfogel YT, Kucich U, Hack CE, et al. Aprotinin inhibits the contact, neutrophil, and platelet activation systems during simulated extracorporeal perfusion. J Thorac Cardiovasc Surg. 1993;106:1–9. [PubMed] [Google Scholar]
- 8.McBride WT, Armstrong MA, Crockard AD, McMurray TJ, Rea JM.. Cytokine balance and immunosuppressive changes at cardiac surgery: Contrasting response between patients and isolated CPB circuits. Br J Anaesth. 1995;75:724–733. [DOI] [PubMed] [Google Scholar]
- 9.Christen S, Finckh B, Lykkesfeldt J, et al. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38:1323–1332. [DOI] [PubMed] [Google Scholar]
- 10.Philippidis P, Mason JC, Evans BJ, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: Antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. [DOI] [PubMed] [Google Scholar]
- 11.Hill GE, Alonso A, Spurzem JR, Stammers AH, Robbins RA.. Aprotinin and methylprednisolone equally blunt cardiopulmonary bypass-induced inflammation in humans. J Thorac Cardiovasc Surg. 1995;110:1658–6162. [DOI] [PubMed] [Google Scholar]
- 12.Ferraris VA, Ferraris SP, Singh A, et al. The platelet thrombin receptor and postoperative bleeding. Ann Thorac Surg. 1998;65:352–358. [DOI] [PubMed] [Google Scholar]
- 13.Edmunds LH Jr.. Blood-surface interactions during cardiopulmonary bypass. J Card Surg. 1993;8:404–410. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Rocker GM, Westaby S.. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55:552–559. [DOI] [PubMed] [Google Scholar]
- 15.Mojcik CF, Levy JH.. Aprotinin and the systemic inflammatory response after cardiopulmonary bypass. Ann Thorac Surg. 2001;71:745–754. [DOI] [PubMed] [Google Scholar]
- 16.Boyle EM Jr, Pohlman TH, Johnson MC, Verrier ED.. Endothelial cell injury in cardiovascular surgery: The systemic inflammatory response. Ann Thorac Surg. 1997;63:277–284. [DOI] [PubMed] [Google Scholar]
- 17.Landis RC, Brown JR, Murkin JM, Likosky DS, Baker RA.. An evidence based review of pharmaceutical interventions to limit the systemic inflammatory response in coronary surgery. Heart Surg Forum. 2008;11:E307. [Google Scholar]
- 18.Landis RC.. 20 years on: Is it time to redefine the systemic inflammatory response to cardiothoracic surgery? J Extra Corpor Technol. (in press). [PMC free article] [PubMed] [Google Scholar]
- 19.Clive Landis R, Murkin JM, Stump DA, et al. Consensus statement: Minimal criteria for reporting the systemic inflammatory response to cardiopulmonary bypass. Heart Surg Forum. 2010;13:E116–E123. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons RJ, Smith S, Antman E.. American College of Cardiology/American Heart Association clinical practice guidelines: Part I: Where do they come from? Circulation. 2003;107:2979–2986. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman JM. van Paassen J, van Dijk D, et al. Prophylactic corticosteroids for cardiopulmonary bypass in adults. Cochrane Database Syst Rev. 2011;5:CD005566. [DOI] [PubMed] [Google Scholar]
- 22.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A.. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–360. [DOI] [PubMed] [Google Scholar]
- 23.McNeely TB, Griffith MJ.. The anticoagulant mechanism of action of heparin in contact-activated plasma: Inhibition of factor X activation. Blood. 1985;65:1226–1231. [PubMed] [Google Scholar]
- 24.Harpel PC, Cooper NR.. Studies on human plasma C1 inactivator–enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975;55:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer RM, Ferrige AG, Moncada S.. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. [DOI] [PubMed] [Google Scholar]
- 26.Kubes P, Suzuki M, Granger DN.. Nitric oxide: An endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glusa E, Markwardt F, Sturzebecher J.. Effects of sodium nitroprusside and other pentacyanonitrosyl complexes on platelet aggregation. Haemostasis. 1974;3:249–256. [DOI] [PubMed] [Google Scholar]
- 28.Landis RC, de Silva RJ.. The systemic inflammatory response to cardiopulmonary bypass. In: Mackay JH, Arrowsmith JE, eds. Core Topics in Cardiac Anaesthesia. 2nd ed. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 29.Miller BE, Levy JH.. The inflammatory response to cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:355–366. [DOI] [PubMed] [Google Scholar]
- 30.Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–290. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Ji B, Wang G, Liu J, Long C.. The effects of zero-balance ultrafiltration on postoperative recovery after cardiopulmonary bypass: A meta-analysis of randomized controlled trials. Perfusion. 2012;27:386–392. [DOI] [PubMed] [Google Scholar]
- 32.Warren O, Wallace S, Massey R, et al. Does systemic leukocyte filtration affect perioperative hemorrhage in cardiac surgery? A systematic review and meta-analysis. ASAIO J. 2007;53:514–521. [DOI] [PubMed] [Google Scholar]
- 33.Ranucci M, Balduini A, Ditta A, Boncilli A, Brozzi S.. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann Thorac Surg. 2009;87:1311–1319. [DOI] [PubMed] [Google Scholar]
- 34.Anastasiadis K, Antonitsis P, Haidich AB, Argiriadou H, Deliopoulos A, Papakonstantinou C.. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2013;164:158–169. [DOI] [PubMed] [Google Scholar]
- 35.Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery: A randomized controlled trial. JAMA. 2012;308:1761–1767. [DOI] [PubMed] [Google Scholar]
- 36.Moller CH, Penninga L, Wetterslev J, Steinbruchel DA, Gluud C.. Off-pump versus on-pump coronary artery bypass grafting for ischaemic heart disease. Cochrane Database Syst Rev. 2012;3:CD007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rimpilainen R, Hautala N, Koskenkari JK, et al. Minimized cardiopulmonary bypass reduces retinal microembolization: A randomized clinical study using fluorescein angiography. Ann Thorac Surg. 2011;91:16–22. [DOI] [PubMed] [Google Scholar]