Abstract:
Arterial filters used in the extracorporeal circuit (ECC) have been shown to minimize cerebral injury by capturing particulate matter and microbubbles. We clinically use the Affinity NT oxygenator with an Affinity arterial filter attached (“Affinity system”). The new Affinity Fusion oxygenator (“Fusion”) incorporates integrated arterial filtering. Our aim was to determine if the Fusion oxygenator was as safe as the Affinity system in terms of relative microbubble transmission of introduced air. A recirculating in vitro circuit primed with blood was used to compare the Fusion with the Affinity system. Microbubbles were detected using a GAMPT BC100 Doppler in the oxygenator–arterial filter outflow line. Measurements were taken 1 minute before and 3 minutes after bolusing 30 mL air proximal to the venous reservoir while altering pump flow rates (3 L/min; 5 L/min). Both the Fusion and Affinity system transmitted microbubbles during air injection. Microbubble volume transmitted at 5 L/min pump flow was significantly greater than at 3 L/min in both systems. The Fusion tended to transmit fewer bubbles, less bubble volume, and smaller sized bubbles than the Affinity system. Under the parameters of this in vitro study, the Affinity Fusion oxygenator with an integrated arterial filter is as safe as the Affinity NT oxygenator with a separate arterial filter in terms of microbubble transmission. However, more research is needed to confirm this study’s findings and generalizability to the clinical environment. As both oxygenator–arterial filter systems transmitted microbubbles during air introduction, it is important to develop strategies to minimize microbubble entry into the ECC.
Keywords: extracorporeal circuit, oxygenator, arterial filter, microbubbles
Microemboli are an important cause of postoperative cognitive impairment after cardiac surgery (1). However, a significant source of microemboli during cardiopulmonary bypass may be the extracorporeal circuit (ECC) itself (2). Commonly integrated within the ECC are arterial filters, which have been shown to minimize cerebral injury by capturing particulate matter and microbubbles (3,4).
An arterial filter is traditionally located some distance away from the outlet of the oxygenator, being the last component in the circuit to capture potential emboli before entering the patient’s systemic circulation. We clinically use the Affinity NT oxygenator (CB511; Medtronic Inc.) with an Affinity 38-μm adult arterial filter (CB351; Medtronic Inc., Minneapolis, MN) installed in the ECC distal to the oxygenator outlet.
With the aim of reducing hemodilution and improving hemocompatibility, some of the latest oxygenators are being designed with an integrated arterial filter. The new Affinity Fusion oxygenator (Medtronic Inc.) has an integrated 25-μm filtration system that incorporates a premembrane bubble trap and also uses progressively denser bundling of its microfibers to filter out gas and particulate matter (5).
As part of our departmental consideration of a replacement oxygenator, we wanted to determine if the Fusion oxygenator (with its integrated arterial filter) was as safe as the Affinity NT (with a separate arterial filter) in terms of relative microbubble transmission of introduced air because no independent studies were available to inform us. This was investigated by comparing the Affinity NT oxygenator with a separate arterial filter (“Affinity system”) to the Affinity Fusion oxygenator (“Fusion”) in terms of relative microbubble transmission during air introduction, in vitro, over a range of flow rates. More specifically, we wanted to determine if these two oxygenator–arterial filter systems transmit microbubbles when air is introduced into their respective oxygenator inlets and, if they do, which of the oxygenator–arterial filter systems transmits more air.
MATERIALS AND METHODS
Test Circuit
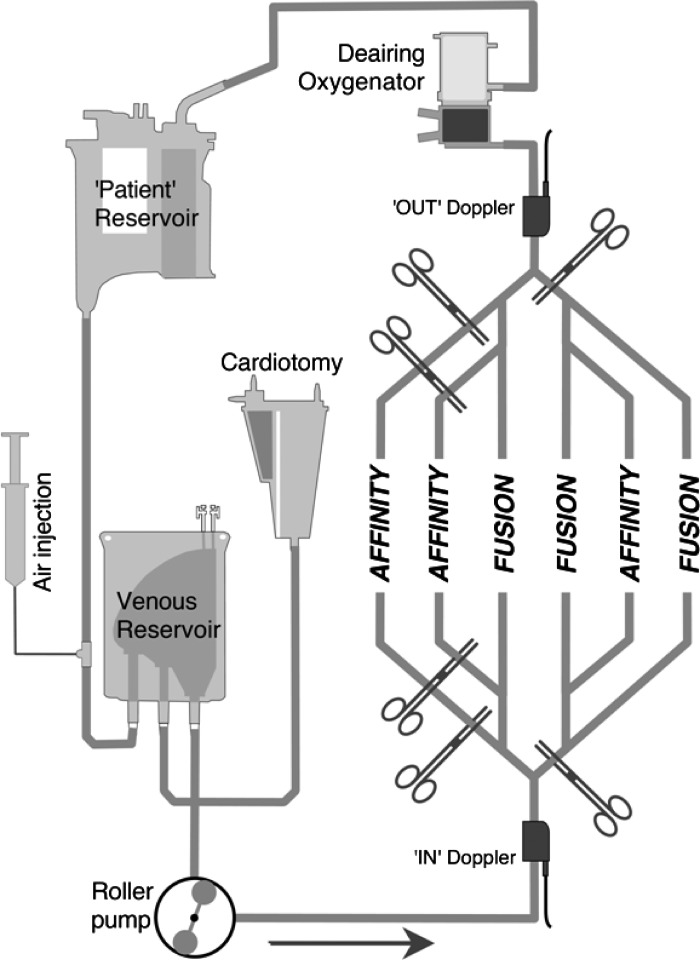
A recirculating test circuit was constructed using heparin-coated tubing (Carmeda® Medtronic Inc.); all components were new (Figure 1). The patient was simulated by a hardshell venous reservoir (Affinity Fusion CVR; Medtronic Inc.). This “patient” reservoir was filled to the 1000-mL level to facilitate the deairing of the recirculating prime. To provide constant siphonage drainage pressure, the “patient” reservoir fluid level was kept at 100 cm above the outlet of the circuit venous reservoir; this was accomplished by adjusting a variable clamp positioned on the “venous line” connecting the “patient” reservoir to the circuit venous reservoir. Emulating our institutions clinical ECC, the circuit venous reservoir was a collapsible bag (CBMVR 1600; Medtronic Inc.) attached and open to a separate cardiotomy (Intersept CB1351; Medtronic Inc.). This venous reservoir bag was tested in a fully compressed (minimal volume) position by closing the reservoir holder cage onto its back plate. A total of 1500 mL of prime filled the reservoir– cardiotomy system alone.
Figure 1.
Diagram of the in vitro circuit. See text for details. Affinity, Affinity NT oxygenator with Affinity arterial filter; Fusion, Fusion oxygenator.
The prime that drained into the circuit venous reservoir was pumped out into the test oxygenator–arterial filter system by an appropriately calibrated roller pump that was set to be just occlusive (Sarns 8000; Terumo Corporation, Tokyo, Japan). Oxygen at 2 L per minute flowed to the test oxygenator’s gas inlet port. The next component the prime entered was another membrane oxygenator (Affinity NT CB511; Medtronic Inc.). This “deairing” oxygenator helped remove microbubbles that had been introduced into the circuit, a process that was facilitated by connecting the oxygenator’s gas inlet port to suction (−200 mmHg) while sealing all its other gas openings (6). Circuit pressure was controlled by adjusting a variable clamp on the inlet to the deairing oxygenator. From the “deairing” oxygenator, the prime was returned back to the “patient” reservoir.
Test Oxygenators–Arterial Filter Systems
Two oxygenator–arterial filter systems were assessed for their air handling performance: the Fusion, with an integrated arterial filter of 25-μm, and the Affinity system, with an attached 38-μm arterial filter (Affinity CB351; Medtronic Inc.; Table 1). In the Affinity system, the arterial filter was connected to its oxygenator by 75 cm of 3/8-inch tubing, which included a filter bypass loop. Both the oxygenator and arterial filter in the Affinity system were heparin-coated (Carmeda® Medtronic Inc.); the Fusion had a hydrophilic polymer coating (Balance® Medtronic Inc.).
Table 1.
Oxygenator and arterial filter specifications.
| Membrane Oxygenators |
Arterial Filter |
||
|---|---|---|---|
| Affinity Fusion | Affinity NT CB511 | Affinity CB351 | |
| Recommended blood flow rate | 1–7 L/min | 1–7 L/min | Up to 7 L/min |
| Static priming volume | 260 mL | 270 mL | 205–219 mL |
| Filtration | 25 μm (depth design) | 38 μm (screen design) | |
| Surface gas transfer fibers | 2.5 m2 | 2.5 m2 | |
| Oxygenator material | Microporous polypropylene hollow fibers | Microporous polypropylene hollow fibers | |
| Coating | Balance® | Carmeda® | Carmeda® |
| Heat exchanger design | Capillary | Bellows | |
| Heat exchanger material | Polyethylene Terephthalate | Stainless steel | |
| Study’s nomenclature | “Fusion” | “Affinity system” | |
Three copies of each oxygenator–arterial filter model were used. All six oxygenator–arterial filter systems were connected in parallel; appropriate clamping directed the blood flow only through the oxygenator–arterial filter system being tested.
The purge line on the Affinity arterial filter was kept open as were the purge lines on both oxygenator models; all purge lines returned prime to the cardiotomy. Clinically, our institution routinely continuously purges the Affinity NT oxygenator’s Luer lock access port while intermittently purges the arterial filter.
Prime
The prime consisted of human red blood cells (RBCs) suspended in colloid and crystalloid. After denitrogenation by carbon dioxide flushing for at least 5 minutes, the circuit was primed with Plasma-Lyte 148 (Baxter Healthcare, Old Toongabbie, Australia). Then 3000 mL of 4% albumin was added followed by nine units of expired donated packed RBCs that were previously washed using a cell salvage machine (Dideco Electa; Sorin Group Italia, Mirandola, Italy). The prime was hemofiltered (Sorin SH14 hemoconcentrator; Sorin Group Italia) through a temporary circuit interposed between the “deairing” oxygenator access port and the “patient” reservoir venous inlet Luer lock port. Hemofiltering was performed to yield approximately 5000 mL of prime with an hematocrit of approximately 21% and an albumin content of 3%. A heater–cooler unit (HCU-30; Maquet Australia Pty. Ltd., Murarrie, Australia) attached to the in situ “deairing” oxygenator kept this prime at 31–32°C.
Air Challenge
The experiment was designed to challenge each oxygenator-arterial filter system with microbubbles (<500 μm diameter). The air injection site was in the venous line entering the venous reservoir; some of the injected air would be expected to be removed by the soft-shell reservoir. The air injection site was a Luer lock connector with an injection port pierced by a 38-mm, 25-gauge hypodermic needle. Using a syringe pump as a holder, a 60-mL syringe with 60 mL of air was manually compressed down to 30 mL against a closed three-way tap directly attached to the syringe outlet (i.e., this 30 mL of air was held at 2 atm). With the syringe holder now keeping the syringe plunger depressed, opening the three-way tap allowed 30 mL of air to be rapidly discharged.
Microbubble Detection
A GAMPT BC100 pulsed ultrasound Doppler system (GAMPT mbH, Merseburg, Germany) was used to detect microbubbles. The GAMPT Doppler was configured for microbubble detection in the 10- to 500-μm range. The program is unable to measure the volume of bubbles larger than 500 μm; so, if any bubble of >500 μm diameter occurred, a default 65.45 nL with a count of one was recorded (i.e., one bubble of 500 μm diameter). In addition to a factory calibration, during measurement, the instrument automatically self-calibrated so that parameters such as tubing material, blood concentration, and flow speed have no influence (7).
A transluminal 3/8-inch internal diameter microbubble detection probe was attached to the tubing approximately 80 cm distal to the outflow of the test oxygenator (this corresponds to approximately 5 cm after the Affinity arterial filter). The detection probe was connected to the bubble counter BC100 and in turn connected to a laptop computer running BCView version 3.4.4 data acquisition software (GAMPT mbH). To maximize the reproducibility of all readings, the same probe was used, thereby avoiding issues of subtle differences in probe sensitivities. Ultrasonic gel was used to couple the probe to the tubing to exclude any air. The probe was reapplied for every trial.
A second microbubble detection probe was positioned immediately proximal to the test oxygenator (after the roller pump) to record inlet air entry for comparison with outlet air transmission. The study was designed to minimize the occurrence of >500-mm bubbles, thereby allowing for more accurate and precise inflow bubble measurements.
Immediately before bolusing, a 60-second baseline measurement was recorded once the background bubble count had dropped to –3 counts/sec). A measurement was made for 180 seconds immediately on bolusing. Thus, a total of four 60-second measurement intervals was created.
Test Procedure
The test oxygenator–arterial filters systems were randomly subjected to two pump flows (3 L/min; 5 L/min). Both flow rates were chosen to represent typical adult pump flows. Variabilities of approximately 2.5% are given for flows (e.g., 5000 ± 125 mL/min) and 5% for reservoir levels (e.g., 1000 ± 50 mL).
Three copies of the two oxygenator–arterial filter systems at both flow rates while air bolusing or during baseline yielded a total of 24 scenarios. These 24 scenarios (tests) were randomly sequenced and performed in one trial; three trials were run for a total of 72 tests.
Arterial line pressures were kept at approximately 80 mmHg at 3 L/min pump flow and 110 mmHg at 5 L/min pump flow.
Statistical Analysis
Microbubble counts, volumes, and sizes in tables are presented asmedians and interquartile ranges. Microbubble size was derived from the median bubble size transmitted during each test. A null hypothesis of no difference in median microbubble count, volume, or size transmitted during air bolus between the two oxygenator–arterial filter systems or within each oxygenator–arterial filter system—at varying flows or times—was rejected if p < .05.
To compare the differences between the two oxygenator–arterial filter systems’ microbubble transmission (count, volume, and size) during air bolus at the two flow rates, nonparametric comparisons (using Mann-Whitney U tests for two groups) were computed. Effect sizes of significant differences were also calculated. Effect sizes (r) of significant differences were calculated with r = .1 as small, r = .3 as medium, and r = .5 as having a large effect (8). Non-parametric Wilcoxon signed-rank tests were used to compare each of the oxygenator–arterial filter system’s [within] difference in microbubble transmission during air bolus (count, volume, and size) at varying flow rates. Additionally, effect sizes of significant differences were calculated (9). To compare each of the oxygenator–arterial filter system’s [within] difference in microbubble transmission during baseline and air bolus (count and volume) and during air bolus only (size), the nonparametric Friedman test was used. Post hoc analysis with Wilcoxon signedrank tests was conducted with a Bonferroni correction applied. Additionally, effect sizes of significant differences were calculated.
The percentage of the bubble count of >500 μm in diameter is identified in the tables.
Statistical analyses were performed using StatView (StatView; Abacus Concepts, Berkeley, CA) and Microsoft Excel (Microsoft Inc., Redmond, WA).
RESULTS
Microbubble Transmission [count]
Between Oxygenator–Arterial Filter Systems:
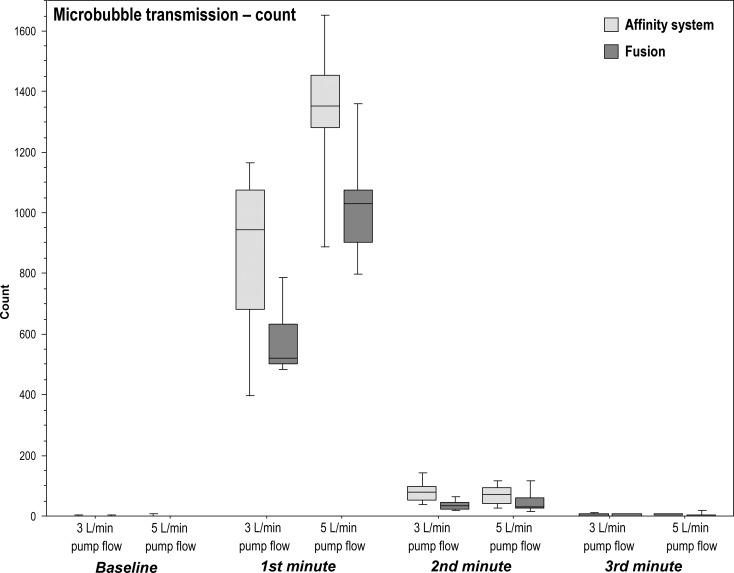
Total microbubbles transmitted by the Fusion during microbubble challenge was significantly less than the Affinity system at both pump flow rates of 3 L/min (p = .019; r = .59) and 5 L/min (p = .024; r = .54) and more specifically during the first minute (3 L/min: p = .012, r = .59; 5 L/min: p = .024, r = .54), second minute at 3 L/min (p = .006, r = .64) and third minute at 5 L/min (p = .036, r = .51; Table 2; Figure 2).
Table 2.
Microbubble transmission [count]: median microbubbles count in the outflow of the two oxygenator–arterial filter systems during baseline and microbubble challenge with pump flows of 3 L/min or 5 L/min.
| 3 L/min |
5 L/min |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | First Minute | Second Minute | Third Minute | Total | Baseline | First Minute | Second Minute | Third Minute | Total | |
| Affinity system | 0 | 945†§ | 77*§ | 2.0 | 1030† | 0 | 1352†§ | 72§ | 5† | 1458†‡ |
| (1.0) | (392.8) | (46) | (4.3) | (441.8) | (1.3) | (174.5) | (51.3) | (3) | (164.8) | |
| Fusion | 0 | 519§ | 32§ | 1.0 | 542 | 0 | 1028§ | 30§ | 2 | 1047‡ |
| (1.3) | (131) | (20.3) | (5.8) | (143.8) | (.3) | (171) | (32.8) | (3.3) | (196.5) | |
Data are medians and (interquartile range).
p < .01, Affinity system versus Fusion.
p < .05, Affinity system versus Fusion.
p < .01, Same oxygenator–arterial filter system 3 L/min versus 5 L/min.
p< .05 versus baseline.
Figure 2.
Boxplots of microbubble transmission (count) in the outflow of the two oxygenator–arterial filter systems during baseline and microbubble challenge with pump flows of 3 L/min and 5 L/min.
Within Each Oxygenator–Arterial Filter Systemat 3 L/min versus 5 L/min Pump Flows:
Consistently, the total microbubble count transmitted during microbubble challenge at 5 L/min pump flow was significantly greater than at a pump flow rate of 3 L/min in both oxygenator–arterial filter systems (both scenarios: p = .008; r = .64; Table 2).
Within Each Oxygenator–Arterial Filter System during Baseline, First, Second, and Third Minutes:
Among both oxygenator–arterial filter systems, the number of transmitted microbubbles significantly altered over the four time periods (baseline, first, second, and third minutes) at both pump flow rates (all four scenarios: p < .0001). Post hoc analysis identified significant differences between all time comparisons (p < .05; r = .64) with the notable exceptions that by the third minute after the bolus of air, bubbles transmitted had dropped to baseline levels at both 3 L/min and 5 L/min (Table 2; Figure 2).
Microbubble Transmission [volume]
Between Oxygenator-Arterial Filter Systems:
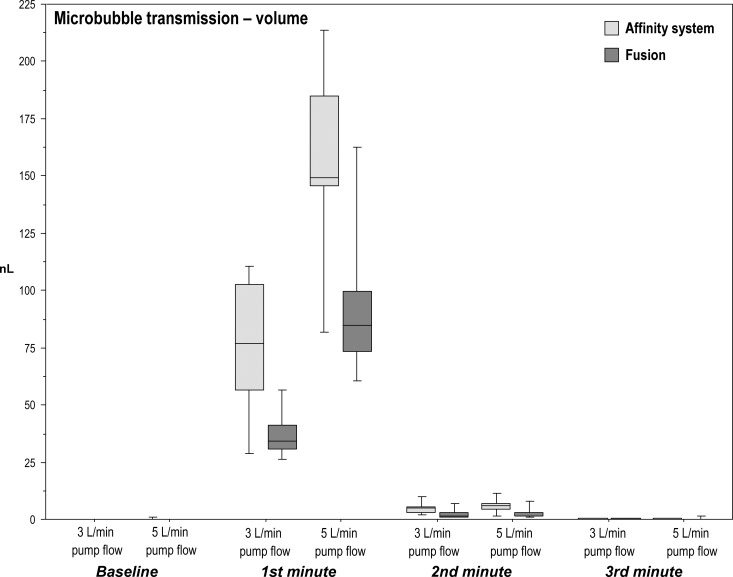
Total microbubble air volume transmitted by the Fusion during the microbubble challenge was significantly less than the Affinity system at both pump flow rates of 3 L/min (p = .019; r = .54) and 5 L/min (p = .024; r = .54) and more specifically during the first minute (3 L/min: p = .009, r = .61; 5 L/min: p = .024, r = .54) and second minute (3 L/min: p = .015, r = .52; 5 L/min: p = .038, r = .49). Notably, no significant difference in transmitted air volume occurred at the third minute between the Fusion and Affinity system at either flow rates (Table 3; Figure 3).
Table 3.
Microbubble transmission [volume]: median microbubbles volume (nL/min) in the outflow of the two oxygenator–arterial filter systems during baseline and microbubble challenge with pump flows of 3 L/min or 5 L/min.
| 3 L/min |
5 L/min |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | First Minute | Second Minute | Third Minute | Total | Baseline | First Minute | Second Minute | Third Minute | Total | |
| Affinity system | 0 | 76.8†§ | 4.79*§ | .08 | 81.6† | 0 | 149*§ | 6.17*§ | .15 | 157*‡ |
| (.01) | (46.4) | (2.67) | (.49) | (49.1) | (.15) | (39.3) | (2.61) | (.17) | (41.7) | |
| Fusion | 0 | 34.0§ | 1.37§ | .009 | 35.4 | 0 | 84.8§ | 1.62§ | .038 | 86.3‡ |
| (.05) | (10.6) | (1.76) | (.27) | (8.9) | (.01) | (26.2) | (1.34) | (.18) | (25.1) | |
Data are medians and (interquartile range).
p < .05, Affinity system versus Fusion.
p < .01, Affinity system versus Fusion.
p < .01, Same oxygenator–arterial filter system 3 L/min versus 5 L/min.
p < .05 versus baseline.
Figure 3.
Boxplots of microbubble transmission (volume nL) in the outflow of the two oxygenator–arterial filter systems during baseline and microbubble challenge with pump flows of 3 L/min and 5 L/min.
Within Each Oxygenator-Arterial Filter System at 3 L/min versus 5 L/min Pump Flows:
Consistently, the total microbubble air volume transmitted during the microbubble challenge at 5 L/min pump flow was significantly greater than at a pump flow rate of 3 L/min in both oxygenator–arterial filter systems (both scenarios: p = .008; r = .64; Table 3).
Within Each Oxygenator–Arterial Filter System during Baseline, First, Second, and Third Minutes:
Among both oxygenator–arterial filter systems, transmitted microbubble air volume significantly altered over the four time periods (baseline, first, second, and third minutes) at both pump flow rates (all four scenarios: p < .0001). Post hoc analysis identified significant differences among all time comparisons (p < .05; r = .64) with the notable exceptions of between baseline and the third minute during 3 L/min and 5 L/min; by the third minute after the bolus of air, air transmitted had dropped to baseline levels (Table 3; Figure 3).
Trapped Percent—Between and within Oxygenator–Arterial Filter Systems:
At both pump flow rates, the Fusion trapped a greater percentage of inflowing air than the Affinity system (3 L/min: p = .038, r = .49; 5 L/min: p = .015, r = .57).
The trapped percent of introduced air significantly dropped at the higher pump flow rate for both oxygenator–arterial filter systems (both scenarios: p = .008; r = .64).
Of note was the increased inlet air volume associated with the Affinity system running at 3 L/min despite the similar air introduction strategy (p = .019; r = .54) (see Table 4).
Table 4.
Microbubble transmission [volume]: median trapped percentage of microbubbles volume in the outflow of the two oxygenator–arterial filter systems during microbubble challenge and pump flows of 3 L/min or 5 L/min over 3 minutes.
| 3 L/min |
5 L/min |
|||||
|---|---|---|---|---|---|---|
| Inlet (nL) | Outlet (nL) | Trap (%) | Inlet (nL) | Outlet (nL) | Trap (%) | |
| Affinity system | 6228§* | 81.6† | 98.6%* | 5991‖ | 157* | 97.5%*‡ |
| (2307) | (49) | (.62) | (2413) | (41.7) | (.69) | |
| Fusion | 4277 | 35.4 | 99.3% | 6574¶ | 86.3 | 98.6%‡ |
| (618) | (8.9) | (.28) | (2077) | (25.1) | (.51) | |
Data are medians and (interquartile range).
p < .05, Affinity system versus Fusion.
p < .01, Affinity system versus Fusion.
p < .01, Same oxygenator-arterial filter system 3 L/min versus 5 L/min.
.003% bubbles >500 μm diameter.
.07% bubbles >500 μm diameter.
.03% bubbles >500 μm diameter.
Microbubble Transmission [size]
Between Oxygenator–Arterial Filter Systems:
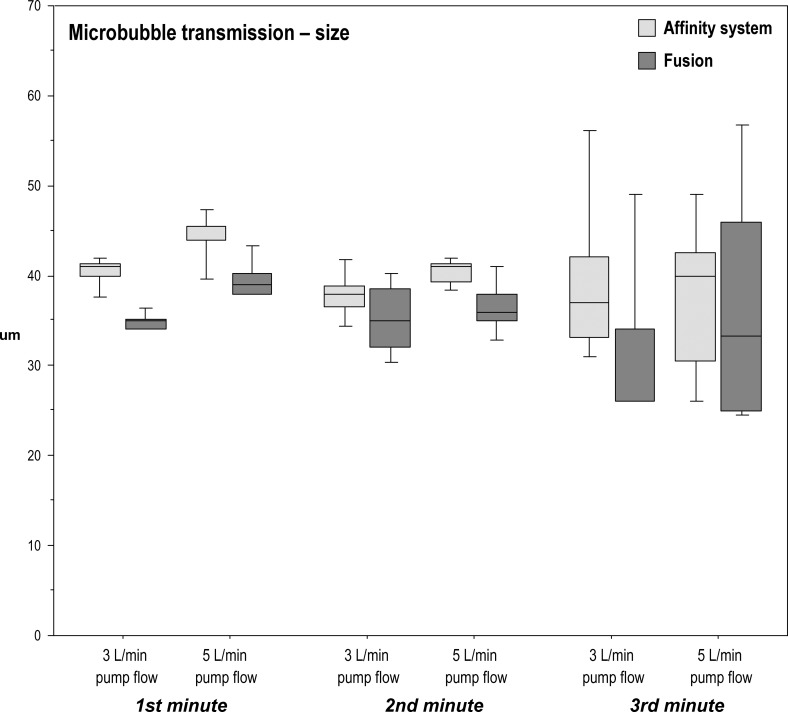
Median microbubble size transmitted by the Fusion was smaller than that of the Affinity system during the first minute at pump flow rates of 3 L/min (p = .0004; r = .82) and during the first and second minutes at 5 L/min (p = .02; r = .54; p = .008; r = .61). No statistical differences were seen during the other measured time intervals (Table 5; Figure 4).
Table 5.
Microbubble transmission [size]: median microbubbles size (μm) in the outflow of the two oxygenator–arterial filter systems during microbubble challenge and pump flows of 3 L/min or 5 L/min.
| 3 L/min |
5 L/min |
|||||
|---|---|---|---|---|---|---|
| First Minute | Second Minute | Third Minute | First Minute | Second Minute | Third Minute | |
| Affinity system | 41.0‡ | 38.0 | 37.0 | 44.0* | 41.0† | 40.0 |
| (1.25) | (2.38) | (9.0) | (1.63) | (1.88) | (12.0) | |
| Fusion | 35.0 | 35.0 | 26.0 | 39.0 | 36.0 | 33.3 |
| (1.13) | (6.38) | (8.0) | (2.25) | (3.0) | (21.0) | |
Data are medians and (interquartile range).
p < .05, Affinity system versus Fusion.
p < .01, Affinity system versus Fusion.
p < .001, Affinity system versus Fusion.
Figure 4.
Boxplots of microbubble transmission (size μm) in the outflow of the two oxygenator–arterial filter systems during microbubble challenge and pump flows of 3 L/min and 5 L/min.
Within Each Oxygenator-Arterial Filter System during the First, Second, and Third Minutes:
Median microbubble size transmitted significantly dropped over the three time periods within the Affinity system at 5 L/min (p = .048); however, the conservative post hoc analysis failed to identify a significant difference in size between the measured minutes. No significant effect of the time periods on median bubble size was noted with the Fusion at both flows or the Affinity system at 3 L/min. Note that the rising interquartile range of bubble size seen during the second and third minutes reflects lower bubble counts (Table 5; Figure 4).
DISCUSSION
The results of this study show that the Medtronic Fusion oxygenator with an integrated arterial filter does not transmit more introduced air than the Medtronic Affinity NT oxygenator with a separate Affinity arterial filter over a range of pump flow rates suggesting that it is as safe in terms of air handling.
More specifically, this study shows that during air bolusing into the ECC, both oxygenator–arterial filter systems transmit microbubbles. However, the Fusion oxygenator with an integrated arterial filter tends to transmit fewer bubbles, less bubble volume, and smaller bubbles than the Affinity NT oxygenator with a separate arterial filter.
Introducing air into the inlet of the oxygenator was associated with an increase in bubble count and volume transmitted by both oxygenator–arterial filter systems. Although these oxygenator–arterial filter systems are designed to remove ECC air, we confirm with other investigators that some of this introduced air manages to elude their bubble removal mechanisms (10,11). Furthermore, both oxygenator–arterial filter systems allowed the transmission of bubbles larger than their rated filter pore size. For bubbles larger than the pore size to transit through the filter requires the bubble to distort through the pore under conditions of higher prescreen pressures or coalesce postfilter under conditions of higher pressure drops (12,13).
Microbubbles were less effectively removed by both of the oxygenator–arterial filter systems at higher pump flows. This phenomenon of more microbubbles transmitted with increased flow rates has been noted in other studies (14,15). Increasing the pump flow rate directly shortens the blood transit time within the oxygenator or arterial filter, decreasing the time for any entrained bubbles to float up and be eliminated. Higher preoxygenator pressures associated with increased pump flows may displace trapped bubbles within the oxygenator, including within the fiber bundles, heat exchanger, and housing. Additionally, by exceeding the bubble point pressure of the arterial filter micropores, bubbles may be squeezed through the screen filters. Indeed, as more bubbles larger than the pore size accumulate on the filter screen and partially obstruct fluid flow, there would be increasing prescreen pressure contributing more force to exceed the bubble point pressure. Furthermore, the elevated pressure drop across the filter would facilitate dissolved gases to come out of the solution postfilter (12,13).
Although both oxygenator–arterial filter systems transmitted microbubbles during air injection, the magnitude of the effect was generally less with the Fusion oxygenator. We suggest that the Fusion’s two-step approach to capturing bubbles was more effective. First, positioned within the inlet of the Fusion oxygenator is a bubble trap composed of a vortex, which retains and then expels any entering bubbles through a purge line into the cardiotomy. The larger and consequently more buoyant bubbles would be expected to be selectively and preferentially captured and then eliminated. Second, within the Fusion oxygenator, any recalcitrant escaping microbubbles from the bubble trap are restrained within the progressively more tightly bundled oxygenator fibers acting as a depth filter. These trapped bubbles may then be eliminated across the microporous oxygenator fiber. This mechanism entails the oxygenator fibers dissipating the captured microbubbles through the pressure differential between the blood and gas zones causing these microbubbles to enter the inner lumen of the microporous hollow fiber whereby they are eliminated through the gas outlet (16). The Affinity NT oxygenator, also composed of bundles of microporous hollow fibers, would again act as a depth filter with the same bubble-dissipating properties. However, unlike the purposeful graduated fiber bundle density in the Fusion, the Affinity NT oxygenator is of an earlier design; the bundle’s construction was probably more influenced by prioritizing gas transfer and minimizing prime volume than considering blood filtering capacity.
When challenged with microbubbles, both systems were able to drop transmitted air to baseline levels by the third minute. However, it would be instructive to compare the two systems’ performance when subjected to a gross air challenge because the Affinity system is not designed to rapidly remove bubbles in a large air-entry scenario, unlike the Fusion’s vortex bubble trap.
It was noted that there was an increased inlet air volume associated with the Affinity system running at 3 L/min (versus the Fusion) despite a similar air introduction strategy. Recirculation of bubbles through the arterial filter and oxygenator purge lines into the cardiotomy may be the explanation with rates of bubble recirculation possibly differing between the two systems. Measuring microbubble load in the line connecting the cardiotomy to the venous reservoir would elucidate this observation.
The Fusion oxygenator had a different surface coating to the Affinity NT’s. At the time of the study, Carmedacoated Fusion oxygenators were not available. Future studies would ideally compare oxygenators with similar surface coatings to exclude this variable impacting on outcomes.
The median size of transmitted bubbles during air injection of the Fusion oxygenator was generally smaller than the Affinity system regardless of flow rate. The more efficient selective retention of larger bubbles by the “dynamic” bubble trap of the Fusion may play a role; Schönburg and colleagues (17) were able to show that their dynamic bubble trap was more effective at removing larger sized bubbles. An additional contributing factor is the approximately 25-μm depth filtration ability of the Fusion oxygenator versus 38 μm in the Affinity system’s arterial filter; generally, there is better sieving with smaller filter pore size (18).
The GAMPT bubble counter may underestimate microbubble counts and overestimate bubble size, particularly at higher flow rates (19). This may be a contributing explanation for the transmission of bubbles measured larger than the rated filter pore size, because the bubble may be actually smaller. Although caution should be made when considering clinical implications, the findings are still valid because the purpose of the study was to quantitatively rank the oxygenator–arterial filter systems in terms of transmitted microbubble counts, volumes, and size.
Although our study focused on the air handling capabilities of the Fusion oxygenator, any future study also needs to assess its particulate load handling capabilities. Arterial filters have been confirmed to trap particulate material when examined under scanning electron microscopy (20) and are clinically used for this purpose. Although the relative contribution of solid versus gaseous microemboli to postoperative cognitive deficit has yet to be elucidated, solid emboli are presumed to be more persistent and damaging (2). Injecting glass microspheres of known diameter into the oxygenator inlet and counting them postfilter using a Doppler instrument could enable oxygenator–arterial filter device ranking.
There is also a need for clinical trials confirming biocompatibility, reduced priming volume, satisfactory gas exchange, and heat exchanger performance. Practical issues of perfusing a patient with no additional arterial filter such as executing oxygenator changeouts without having a separate arterial filter to capture introduced air or performing modified ultrafiltration need to be considered.
In summary, we investigated the relative air handling capabilities of two adult oxygenator–arterial filter systems: an Affinity NT oxygenator with a separate Affinity arterial filter and the Fusion oxygenator with an integrated arterial filter. These oxygenator–arterial filter systems’ relative microbubble transmission performances were examined under equal in vitro conditions of bolus air infusion while varying pump flow rates. We conclude that, under the parameters of this in vitro study, the Fusion oxygenator is as safe as the Affinity NT oxygenator with a separate Affinity arterial filter in terms of microbubble handling and could be considered as an alternative oxygenator–arterial filter system. As both oxygenator–arterial filter systems transmitted microbubbles during air introduction, particularly at the higher pump flows, it is important to develop strategies to minimize microbubble entry into the ECC.
REFERENCES
- 1.Hammon JW, Stump DA, Kon ND, et al. Risk factors and solutions for the development of neurobehavioural changes after coronary artery bypass grafting. Ann Thorac Surg. 1997;63:1613–1618. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Omar Y, Balacumaraswami L, Pigott DW, Matthews PM, Taggart DP.. Solid and gaseous cerebral microembolisation during off-pump, on-pump, and open cardiac surgery procedures. J Thorac Cardiovasc Surg. 2004;127:1759–1765. [DOI] [PubMed] [Google Scholar]
- 3.Pugsley W, Klinger L, Paschalis C, Treasure T, Harrison M, Newman S.. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–1399. [DOI] [PubMed] [Google Scholar]
- 4.Taggart DP, Bhattacharya K, Meston N, et al. Serum S-100 protein concentration after cardiac surgery: A randomized trial of arterial line filtration. Eur J Cardiothorac Surg. 1997;11:645–649. [DOI] [PubMed] [Google Scholar]
- 5.Medtronic Inc. Affinity Fusion Oxygenation System, 2013. Available at: http://www.fusionoxygenator.com. Accessed June 4, 2014.
- 6.Rudolph JL, Tilahun D, Treanor PR, et al. Use of a large bore syringe creates significantly fewer high intensity transient signals (HITS) into a cardiopulmonary bypass system than a small bore syringe. Perfusion. 2006;21:67–71. [DOI] [PubMed] [Google Scholar]
- 7.Gesellschaft für Angewandte Medizinische Physik und Technik mbH. Bubble Counter BC100: A device for the detection of micro bubbles in streaming fluids [User Manual]. Merseburg, Germany: GAMPT mbH; April 2010. [Google Scholar]
- 8.Yatani K.. Statistics for HCI Research: Mann-Whitney. Available at: http://yatani.jp/HCIstats/MannWhitney. Accessed January 28, 2014.
- 9.Yatani K.. Statistics for HCI Research: Wilcoxon Signed. Available at: http://yatani.jp/HCIstats/WilcoxonSigned. Accessed January 28, 2014.
- 10.Jones TJ, Deal DD, Vernon JC, Blackburn N, Stump DA.. Does vacuum-assisted venous drainage increase gaseous microemboli during cardiopulmonary bypass? Ann Thorac Surg. 2002;74:2132–2137. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen PF, Funder JA, Jensen MØ, Nygaard H.. Influence of venous reservoir level on microbubbles in cardiopulmonary bypass. Perfusion. 2008;23:347–353. [DOI] [PubMed] [Google Scholar]
- 12.Herbst DP, Najm HK.. Development of a new arterial-line filter design using computational fluid dynamics analysis. J Extra Corpor Technol. 2012;44:139–144. [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst DP.. The effects of pressure on gases in solution: Possible insights to improve microbubble filtration for extracorporeal circulation. J Extra Corpor Technol. 2013;45:94–106. [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Newland RF, Tully PJ, Tuble SC, Baker RA.. In vitro evaluation of gaseous microemboli handling of cardiopulmonary bypass circuits with and without integrated arterial line filters. J Extra Corpor Technol. 2011;43:107–114. [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Dogal NM, Marthis RK, Qui F, Kunselman A, Ündar A.. Evaluation of Quadrox-I and Capiox FX neonatal oxygenators with integrated arterial filters in eliminating gaseous microemboli and retaining hemodynamic properties during simulated bypass. Perfusion. 2012;27:235–243. [DOI] [PubMed] [Google Scholar]
- 16.De Somer F, Dierickx P.. Can an oxygenator design potentially contribute to air embolism in cardiopulmonary bypass? A novel method for the determination of the air removal capabilities of neonatal membrane oxygenators. Perfusion. 1998;13:157–163. [DOI] [PubMed] [Google Scholar]
- 17.Schönburg M, Urbanek P, Erhardt G, et al. Significant reduction of air microbubbles with the dynamic bubble trap during cardiopulmonary bypass. Perfusion. 2001;16:19–25. [DOI] [PubMed] [Google Scholar]
- 18.Riley JB.. Arterial line filters ranked for gaseous micro-emboli separation performance: An in vitro study. J Extra Corpor Technol. 2008;40:21–26. [PMC free article] [PubMed] [Google Scholar]
- 19.De Somer FM, Vetrano MR, Van Beeck JP, Van Nooten GJ.. Extracorporeal bubbles: A word of caution. Interact Cardiovasc Thorac Surg. 2010;10:995–1001. [DOI] [PubMed] [Google Scholar]
- 20.Kim WG, Kim KB, Yoon CJ.. Scanning electron microscopic analysis of arterial line filters used in cardiopulmonary bypass. Artif Organs. 2000;11:874–878. [DOI] [PubMed] [Google Scholar]