Abstract
Background
Patients with biochemical recurrence (BCR) following definitive treatment for prostate cancer (PC) comprise a heterogeneous population for which standard therapy options are lacking. The purpose of this trial is to evaluate the feasibility, toxicity, and efficacy of early multimodality systemic therapy in men with BCR.
Methods
Eligible patients had an increasing prostate-specific antigen (PSA), PSA doubling time ≤10 months and no evidence of metastases following radical prostatectomy (RP) and/or radiation therapy (RT) for localized disease. Treatment consisted of docetaxel 75 mg/m2 every 3 weeks for 4 cycles, bevacizumab 15 mg/kg every 3 weeks for 8 cycles, and androgen deprivation therapy (ADT) for 18 months. The primary endpoint is the proportion of patients free from PSA progression one year following therapy completion.
Results
Forty-one patients are included in the analysis. At one year following completion of ADT, 45% (n=13/29) and 29% (n=5/17) of patients with a testosterone ≥100 ng/dL and testosterone ≥ 240 ng/dL had a PSA < 0.2 ng/mL. The median follow-up is 27.5 months (inter-quartile range: 21.8, 38.1). Eight (20%) patients are free from PSA progression, 19 (46%) did not restart ADT, and 34 (83%) are free from metastasis. Sixteen (39%) patients experienced grade 3 and five (12%) experienced grade 4 toxicities.
Conclusion
Multimodality systemic therapy with docetaxel, bevacizumab, and ADT is feasible and produces PSA responses in men with BCR. Long term follow-up is needed to determine the proportion of patients with a durable PSA response who are able to avoid restarting of prostate cancer therapy.
Keywords: Androgen deprivation therapy, Bevacizumab, Biochemical recurrence, Docetaxel, Prostate cancer
Introduction
In 2014, approximately 233,000 men were diagnosed with prostate cancer (PC) in the United States (US).1 Among those undergoing definitive local therapy, 20–80% of the individuals, depending upon initial risk factors, will experience a rising prostate-specific antigen (PSA) following local therapy without evidence of metastatic disease, termed biochemical recurrence (BCR).2
The clinical course of patients with BCR is highly variable given heterogeneous tumor biology. In 1999, Pound and colleagues were the first to describe the natural history of BCR after radical prostatectomy (RP) in patients receiving no androgen deprivation therapy (ADT) prior to the development of metastatic disease.3 In this cohort, the median time from BCR to metastases was 8 years and median time from metastasis to death was 5 years. Additionally, PSA doubling time (PSADT) and Gleason score were predictive of metastasis development.4 These data highlight that some patients with favorable disease require minimal therapy, while those with high-risk disease require more aggressive treatment options.
The standard treatment for patients with BCR has not been established given the lack of data derived from randomized controlled trials. Though salvage radiotherapy and rarely salvage RP can result in favorable outcomes in a proportion of men, in many cases salvage local therapy is not curative, likely due to the presence of occult metastatic disease at the time of BCR.5 Compounding the treatment decisions in this population is the lack of reliable imaging to discriminate between local and systemic recurrence.
The most commonly prescribed treatment for men with BCR is ADT, and its use for these patients has increased substantially over the past several decades.6 Based on results from the CAPSURE database, 59% and 93% of men who received primary RP or radiotherapy, respectively, were treated with ADT at BCR.6 Currently no clear consensus exists for the optimal timing of ADT. Initiating ADT at early relapse and with low PSAs is associated with favorable but usually transient PSA responses, however the impact of early versus later initiation of ADT on survival and quality of life remains uncertain.
Adjuvant chemotherapy has demonstrated clear benefits in terms of reduced risk of recurrence and improved survival for multiple tumors including breast, colorectal, and lung cancer.7 Recently, early treatment with docetaxel and ADT compared to ADT alone for men with castration-sensitive metastatic PC was shown to significantly improve OS (52.7 versus 42.3 months, p=0006).8 The magnitude of the survival benefit was the largest documented of any advanced PC study, raising the question of whether earlier treatment with chemotherapy for men with BCR could also be efficacious. In the context of a phase II trial, we evaluated the efficacy and toxicity of four cycles of docetaxel, estramustine, and 18 months of ADT in 62 men with BCR after local therapy.9 At one year following the end of therapy, 97% of patients had a testosterone ≥100 ng/dL and 36% had an undetectable PSA. At a median follow-up of 8.6 years, 24% of men had an undetectable or non-progressive PSA and recovered testosterone, supporting the hypothesis that a proportion of men with BCR may have long term remissions after intense but finite multimodal systemic therapy.10
Angiogenesis is known to play a central role in PC tumor growth and metastasis.11 In preclinical studies, vascular endothelial growth factor (VEGF) inhibition in combination with ADT produced significant inhibition of tumor growth when compared with either ADT or VEGF inhibition alone.11 Furthermore, increasing levels of VEGF in patients with metastatic disease correlates with disease progression. Though bevacizumab, a humanized monoclonal antibody to VEGF, has demonstrated efficacy in several tumor types including colorectal and lung cancer, it failed to improve survival in randomized trials in metastatic castration-resistant prostate cancer (CRPC).12 In the Cancer and Leukemia Group B (CALGB) 90401 trial, the addition of bevacizumab to docetaxel in metastatic CRPC improved progression-free survival (PFS) and objective response rate, but did not improve overall survival (OS).12 At the time CALGB 90401 was accruing patients, we designed a neoadjuvant trial and BCR trial (reported here) exploring the use of bevacizumab in early stages of high-risk PC. In our neoadjuvant, phase II, multicenter trial we evaluated the efficacy of docetaxel with bevacizumab in 41 men with high-risk PC undergoing RP.13 Twelve patients (29%) achieved a >50% reduction in tumor volume as measured by magnetic resonance imaging (MRI) and 9 patients (22%) achieved a 50% post-treatment decline in PSA, though no patient achieved a complete pathologic response. The role of chemotherapy and antiangiogenic approaches in the context of prostatectomy and radiation are actively being explored in randomized clinical trials
The benefit of early multimodality systemic therapy with docetaxel, anti-angiogenic therapy, and ADT in high-risk men with BCR after local therapy has not been explored. In this phase II study, we investigated the efficacy and toxicity of docetaxel, bevacizumab, and ADT in men who experienced a BCR after local therapy for PC.
Methods
Patients
This trial is a single arm, phase II study in men with BCR after definitive local therapy for PC (NCT00658697). In total, 42 patients enrolled between June 2008 and December 2010. One patient withdrew before starting treatment and was excluded from the analysis. Data retrieval was in September 2014.
Before enrollment, all patients gave written informed consent. Patients were enrolled at three institutions: Dana-Farber Cancer Institute (DFCI, Boston, MA), Beth Israel Deaconess Medical Center (BIDMC, Boston, MA), and University of Maryland Medical Center (UMMC, Baltimore, MD). Eligible patients had histologically documented prostatic adenocarcinoma, which was clinically or pathologically localized (no greater than the American Joint Committee on Cancer (AJCC) category T1-3N1M0), treated with RP, radiotherapy, or both. Eligible patients had a PSA recurrence with PSADT ≤10 months (based on a minimum of three PSA values at least two weeks apart). There was no minimum PSA requirement at entry for RP patients. For patients treated with primary radiotherapy, PSA was required to be ≥2.0 ng/mL. The use of prior neoadjuvant or adjuvant ADT was allowed if duration was ≤ eight months and testosterone was within 55 ng/dL of the institutional lower limit of normal (lower limit of normal range: DFCI 240 ng/dL, BIDMC 280 ng/dL, UMMC 72 ng/dL). Prior chemotherapy was not allowed.
Patients were required to have no evidence of disease on bone scan, chest radiograph or computed tomography (CT), and CT or MRI of the abdomen and pelvis. An Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 was required. Required laboratory values included neutrophils ≥1,500mm3, hemoglobin ≥8gm/dL, platelets ≥100,000/uL, normal bilirubin, normal alkaline phosphatase, and hepatic aminotransferases ≤1.5 × the upper limit of normal. Patients were not eligible if they had a second malignancy within five years, active treatment with corticosteroids, active infection, neuropathy requiring medical therapy, inadequately controlled hypertension (systolic blood pressure >150 mmHg and/or diastolic blood pressure >100 mmHg), prior hypertensive crisis or hypertensive encephalopathy, New York Heart Association ≥ grade II congestive heart failure, myocardial infarction or unstable angina within 12 months of enrollment, stroke or transient ischemic attack, central nervous system disease, significant vascular disease, evidence of a bleeding diathesis or coagulopathy, bleeding or thrombosis requiring medical intervention within 12 months of enrollment, abdominal fistula/perforation/ abscess within six months of enrollment, non-healing wound, or proteinuria (urine protein-to-creatinine ratio ≥1.0 or 24-hour urine protein >1.0 gm).
Treatment
Starting on day one, patients received docetaxel 75 mg/m2 intravenously every three weeks for four cycles, bevacizumab 15 mg/kg intravenously every three weeks for eight cycles, and a luteinizing-hormone-releasing hormone (LHRH) agonist for 18 months. All patients received premedication with dexamethasone before docetaxel. Bicalutamide 50 mg orally per day was started following completion of docetaxel (at three months) and continued for a total of 15 months. PSA was measured every three weeks during docetaxel and/or bevacizumab treatment, every three months during ADT, and then every three months until PSA progression. Serum testosterone was assessed every three months at the completion of ADT until testosterone was in the institutional normal range.
Outcome assessments
The primary endpoint is the proportion of patients free from PSA progression one year following completion of ADT. PSA progression is defined as two serial increases in PSA from treatment nadir (at least two weeks apart) and a PSA ≥0.2 ng/mL in RP patients and ≥2.0 ng/mL in radiation patients (based on local lab measurements). The first date of increasing PSA at or above these thresholds is considered the progression date. Radiographic evidence of disease progression is considered progression regardless of PSA. Secondary endpoints include time to PSA progression (TTP) and PSA response at completion of docetaxel, ADT, and one year following ADT completion. TTP is defined from the start of chemotherapy until date of progression and in the absence of progression, the TTP is censored at last follow-up. A complete response is defined by two alternative definitions: PSA <0.2 ng/mL or ≤0.01 ng/mL (based on local lab measurements and assay lower limits). PSA partial response is defined as a PSA decrease ≥50% from baseline. Other PSA kinetics including median time to nadir, median nadir and proportion of patients achieving a 90% decrease in PSA are described. PSA response, in reference to testosterone, is also described. Additionally, we evaluate the proportion of patients who restarted ADT, developed radiologic evidence of metastatic disease and died. Testosterone recovery following completion of ADT is recorded. Body-mass index (BMI) at baseline and during treatment is recorded. Toxicity is assessed using Common Terminology Criteria for Adverse Events version 3.0.
Statistical analysis
This study is designed to differentiate a ≥60% rate of patients free from PSA progression one year following completion of ADT from a ≤41% rate, which was observed in our prior phase II trial of early docetaxel and ADT for men with BCR after definitive local therapy for PC.9 A sample size of 42 patients provides 80% power to detect this difference with two-sided α=0.05.
Patient characteristics are summarized as number (and percentage) or median (interquartile range (IQR)), corresponding to categorical and continuous variables, respectively. The distribution of TTP, time from off treatment to re-initiation of ADT, as well as time from off treatment to development of metastasis are estimated using the Kaplan-Meir method, and the probability of patients free from PSA progression at one year following ADT completion is estimated with 95% confidence intervals (CI). The proportion of patients with a PSA response at pre-specified time-points (docetaxel completion, ADT completion, one year after ADT completion) are summarized with 95% CI. When applicable, comparisons between groups are conducted using the log-rank test and a p-value <0.05 (two-sided) is considered statistically significant. The statistical analysis is undertaken using SAS version 9.2 (SAS institute, Cary, NC) and R 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Forty-one patients are included in the analysis among them, 36 had a RP, of whom 29 received salvage RT and five had RT as definitive local therapy (Table 1). The median age of the RP patients is 58 compared to 60 years for radiation patients. Nineteen patients (46%) had T3 disease (defined pathologically for RP patients and clinically for RT patients). Twenty-seven percent (n=11) of all patients had a Gleason score of 8–10. The majority of patients did not receive prior ADT (n=28, 68%). Patients who only received RT had a higher PSA at enrollment (10.5 versus 4.5 ng/mL) and shorter PSADT (3.9 versus 4.6 months) compared with RP patients.
Table 1.
Baseline patient characteristics.
| Characteristic | RT Only (n=5) N(%) or Median(IQR) |
RP +/− RT (n=36) N (%) or Median(IQR) |
Total (n=41) N(%) or Median(IQR) |
|---|---|---|---|
| At Diagnosis | |||
| Age (years) | 60(57–61) | 58(54–62) | 58(55–62) |
| Race | |||
| White | 5(100%) | 30(83%) | 35(85%) |
| Others | 0(0%) | 6(17%) | 6(15%) |
| PSA (ng/mL) | 5.0(4.8–7.1) | 6.7(4.9–9.6) | 6.7(4.8–9.5) |
| Gleason score | |||
| ≤6 | 1(20%) | 5(14%) | 6(15%) |
| 7 | 3(60%) | 21(58%) | 24(59%) |
| 8–10 | 1(20%) | 10(28%) | 11(27%) |
| AJCC Tumor Category | |||
| T1 | 3(60%) | 0(0%) | 3(7%) |
| T2 | 2(40%) | 17(47%) | 19(46%) |
| T3 | 0(0%) | 19(53%) | 19(46%) |
| AJCC Lymph Node Category | |||
| N0 | 0(0%) | 27(75%) | 27(66%) |
| N1 | 0(0%) | 2(6%) | 2(5%) |
| Nx | 5(100%) | 7(20%) | 12(29%) |
| At Enrollment | |||
| Years from diagnosis | 2.5(2.5–2.6) | 3.9(2.5–7.4) | 3.6(2.5–6.7) |
| Prior ADT | 3(60%) | 1(28%) | 13(32%) |
| PSA (ng/mL) | 10.5(3.3–12.8) | 4.5(1.8–8.0) | 4.6(2.0–10.5) |
| PSADT (months) | 3.9(3.5–5.0) | 4.6(3.1–7.8) | 4.4(3.1–7.7) |
| Testosterone (ng/dL) | 485.0(284.0–517.0) | 299.5(251.2–375.5) | 300.0(255.0–394.0) |
| BMI (kg/m2) | 29.5(28.9–30.3) | 27.9(26.7–30.6) | 28.4(27.0–30.6) |
PSA response
The median follow-up time from the start of therapy is 27.5 months (IQR 21.8–38.1 months). The median time from treatment start to PSA nadir is 5.5 months (IQR 3.4–8.5 months) and median PSA nadir is 0.0 mg/dL for the entire cohort (Table 2). At one year following completion of ADT, median PSA is 0.31 ng/mL and 44% (n=16) of patients have a PSA < 0.2 ng/mL. An undetectable PSA (PSA ≤ 0.01 ng/mL) is observed in nine men (25%) at this time. At one year following completion of ADT, 45% (n=13/29) and 29% (n=5/17) of patients with a testosterone ≥ 100 ng/dL and testosterone ≥240 ng/dL, respectively, have a PSA <0.2 ng/mL.
Table 2.
PSA responses.
| PSA (ng/mL) Median (Q1–Q3) |
PSA <0.2 (ng/mL) N(%) (95% CI) |
PSA ≤0.01 (ng/mL) N(%) (95% CI) |
PSA decrease ≥50% from baseline N(%) (95% CI) |
PSA decrease ≥90% from baseline N(%) (95% CI) |
|
|---|---|---|---|---|---|
| At completion of docetaxel (n=38) | 0.08 (0.03–0.20) |
28(74%) (57–87) |
6(16%) (6–31) |
38(100%) (91–100) |
34(90%) (75–97) |
| At completion of ADT (n=37) | 0.01 (0.00–0.02) |
37(100%) (91–100) |
24(65%) (48–80) |
37(100%) (91–100) |
36(97%) (86–100) |
| 1 year after completion of ADT | |||||
| Overall (n=36) | 0.31 (0.02–0.88) |
16(44%) (28–62) |
9(25%) (12–42) |
31(86%) (71–95) |
17(47%) (30–65) |
| Testosterone ≥100 ng/dL (n=29) | 0.30 (0.03–0.70) |
13(45%) (26–64) |
6(21%) (8–40) |
25(86%) (68–96) |
13(45%) (26–64) |
| Testosterone ≥240 ng/dL (n=17) | 0.39 (0.08–0.80) |
5(29%) (10–56) |
2(12%) (2–36) |
15(88%) (64–99) |
5(29%) (10–56) |
TTP and patient outcomes
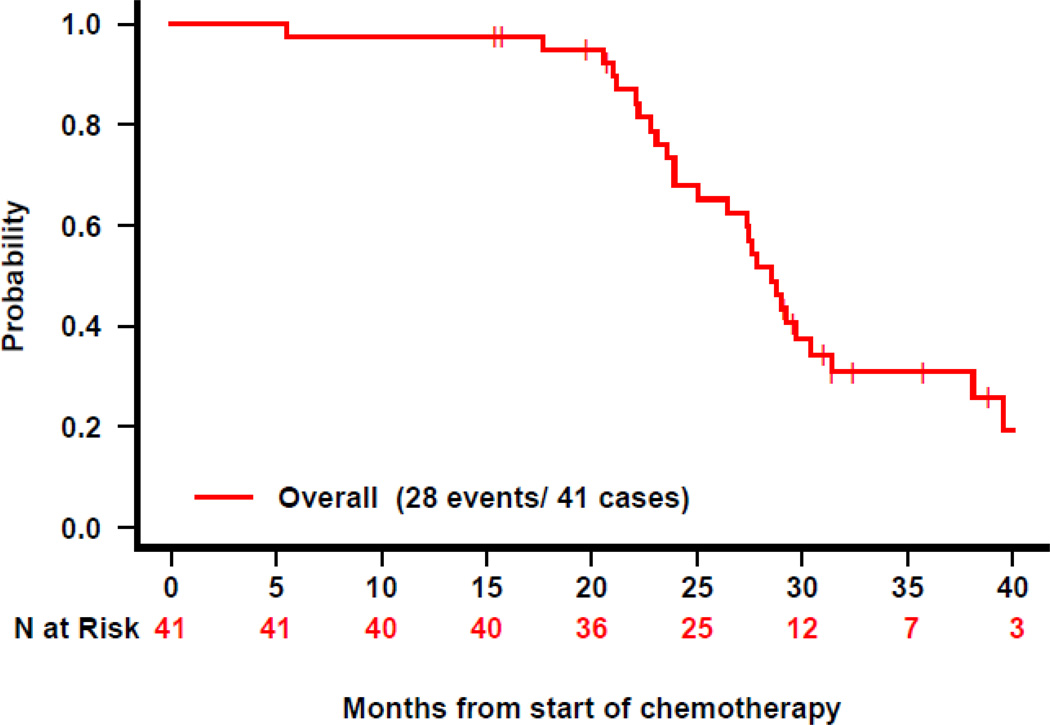
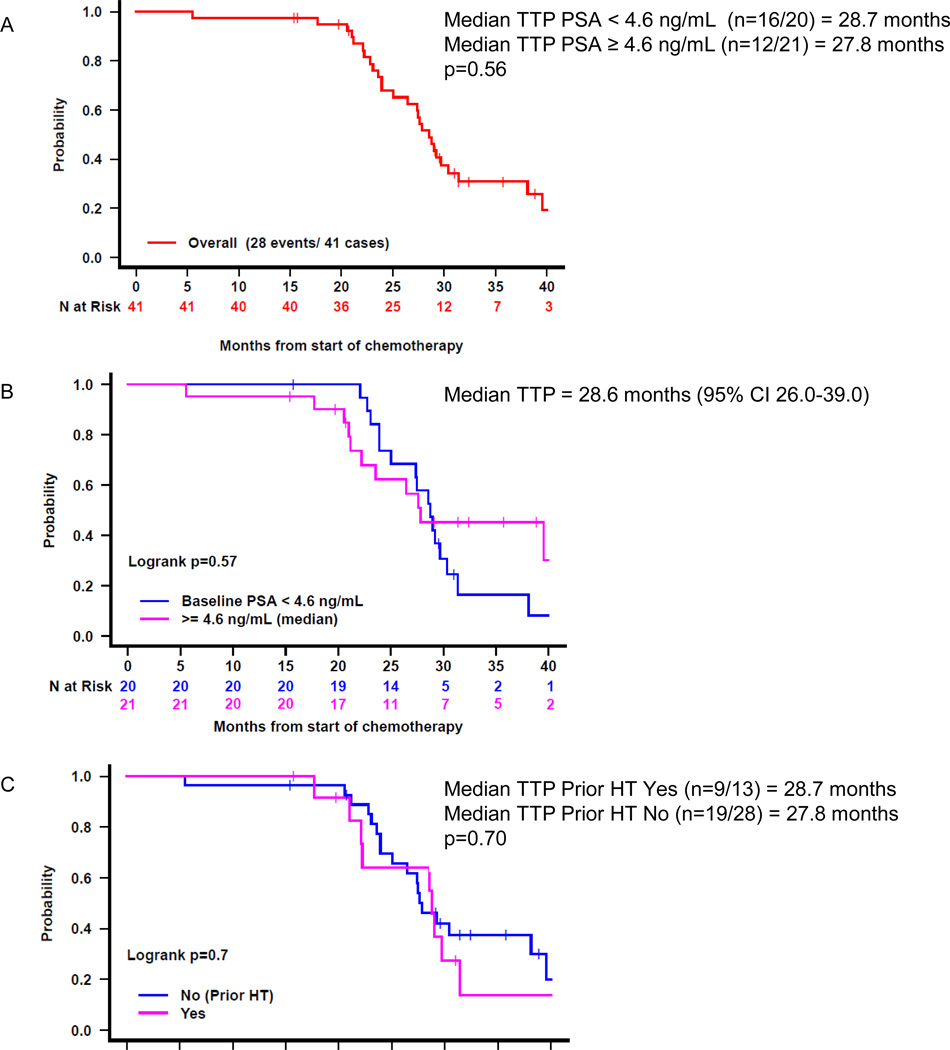
At one year following completion of ADT, the estimated probability of patients remaining free from PSA progression is 98% (95% CI 93–100). Two patients have PSA progression, both of whom had testosterone levels < 100 ng/dL. Overall, 33 patients (80%) have PSA progression with a median TTP of 27.5 months (95% CI 26–38) (Figure 1). TTP did not significantly correlate with age, race, Gleason score, local therapy type, prior ADT, baseline PSA, PSADT, or testosterone level at enrollment (Table 3).
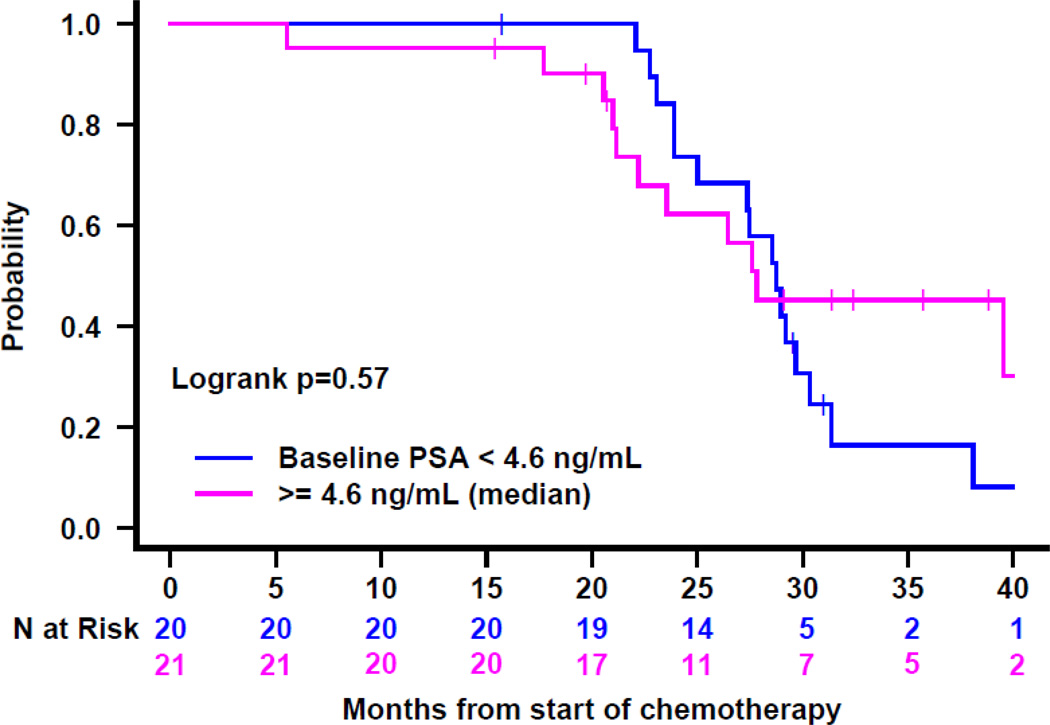
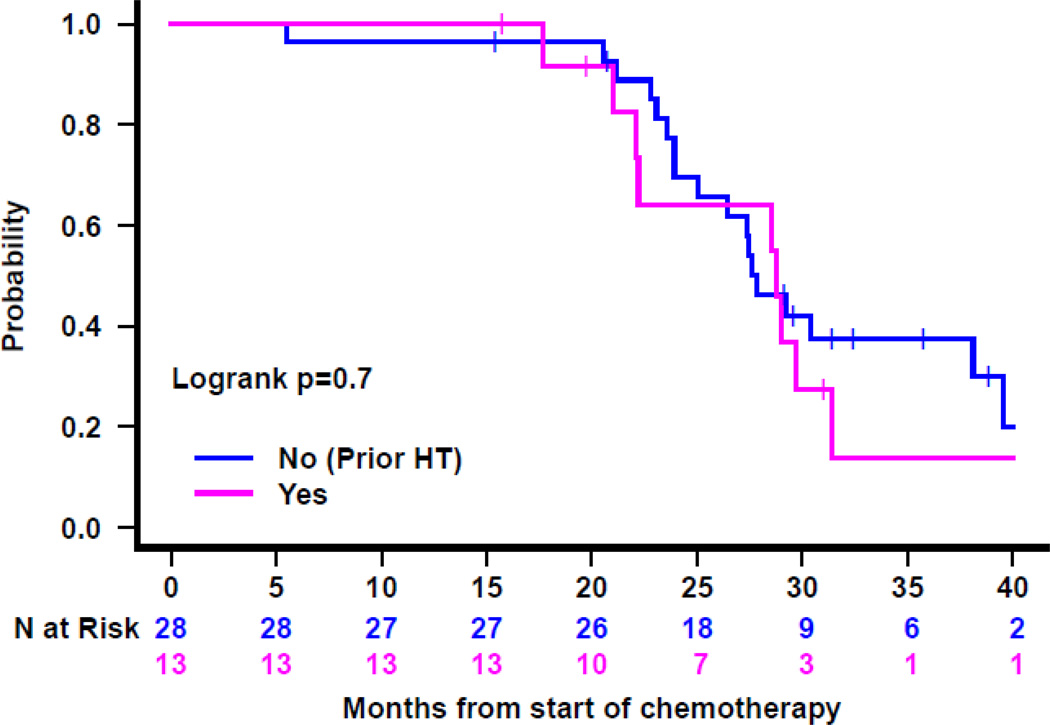
Figure 1.
Time to PSA progression after treatment start: A) for the entire cohort, B) by baseline PSA, and C) by receipt of prior ADT. HT=hormone therapy.
Table 3.
Univariate associations of patient characteristics with time to PSA progression.
| Progression Number (%) | Median Time to Progression (months) |
Log-Rank P-value | |
|---|---|---|---|
| Median age at diagnosis | |||
| <58 years (n=19) | 16(84%) | 28 | 0.99 |
| ≥58 years (n=22) | 17(77%) | 28 | |
| Race | |||
| White (n=35) | 28(80%) | 28 | 0.11 |
| Others (n=6) | 5(83%) | 26 | |
| Gleason score | |||
| ≤6 (n=6) | 4(67%) | 45 | 0.14 |
| 7 (n=24) | 20(83%) | 28 | |
| 8–10 (n=11) | 9(82%) | 26 | |
| Local therapy | |||
| RT only (n=5) | 5(100%) | 28 | 0.67 |
| RP +/− RT (n=36) | 28(78%) | 28 | |
| Prior ADT | |||
| No (n=28) | 22(79%) | 28 | 0.64 |
| Yes (n=13) | 11(85%) | 28 | |
| At Enrollment | |||
| Median age at baseline | |||
| <63 years (n=20) | 17(85%) | 28 | 0.30 |
| ≥63 years (n=21) | 16(76%) | 32 | |
| PSA | |||
| <4.6ng/mL (n=20) | 18(90%) | 28 | 0.30 |
| ≥4.6ng/mL (n=21) | 15(71%) | 28 | |
| PSADT | |||
| <4.4 months (n=20) | 14(70%) | 28 | 0.38 |
| ≥4.4 months (n=21) | 19(90%) | 29 | |
| Testosterone | |||
| <240ng/dL (n=8) | 5(62%) | 24 | 0.76 |
| ≥240ng/dL (n=33) | 28(85%) | 28 | |
Overall, 22 patients (54%) reinitiated ADT for disease progression at a median of 20.0 months (95% CI 14-not reached) from ADT completion. Seven patients (17%) developed metastatic PC at a median of 44.6 months (95% CI 27-not reached) from therapy discontinuation. Sites of metastasis included lymph nodes (n=5), bone (n=2), liver (n=2), lungs (n=1), bladder (n=1) and prostate bed (n=1). Two patients (5%) died, one secondary to progressive PC and the other secondary to cardiac amyloidosis. The cardiac amyloidosis was deemed unrelated to study treatment.
Testosterone recovery
Overall, 92% (n=36 with assessable data) of patients have a testosterone ≥100 ng/dL at an estimated median time to the testosterone recovery of 9.1 months (95% CI 8–11) following completion of ADT and 61% (n=22/36) had a testosterone ≥ 240 ng/dL at an estimated median time to recovery of 11.0 months (95% CI 9–15) following ADT completion. At one year following completion of ADT, 83% (n=35 with assessable data) have a testosterone ≥100 ng/dL and 49% (n=35) of patients have a testosterone within the normal range (≥ 240 ng/dL) (Table 4).
Table 4.
Testosterone recovery.
| Median Testosterone (ng/dL) (Q1–Q3) |
Testosterone ≥100 ng/dL N (%) (95% CI) |
Testosterone ≥240 ng/dL N (%) (95% CI) |
|
|---|---|---|---|
| Baseline (n=41)* | 300.0 (255.0–394.0) |
41(100%) (91–100) |
33(81%) (65–91) |
| At completion of docetaxel (n=36)* | 10.5 (7.0–14.3) |
0(0%) (0–10) |
0(0%) (0–10) |
| At completion of ADT (n=35)* | 11.0 (7.5–27.5) |
5(14%) (5–30) |
3(9%) (2–23) |
| 1 year after completion of ADT (n=35)* | 239.0 (145.0–345.0) |
29(83%) (66–93) |
17(49%) (31–66) |
n reflects patients for which testosterone data was available at the specified time point.
Body mass index
At docetaxel completion, median BMI is 28.3 kg/m2 (IQR 27.2–31.1), consistent with baseline BMI (28.4 kg/m2, IQR 27.0–30.6). At ADT completion, BMI increases to 29.4 kg/m2 (IQR 27.2–31.7). BMI data is not available one year after ADT completion.
Toxicity
Among the 41 patients in the analysis, 16 (39%) experienced grade 3 and 5 (12%) experienced grade 4 treatment-related adverse events. Eleven patients experienced grade 3 or 4 toxicities related to bevacizumab and 14 experienced grade 3 or 4 toxicities related to docetaxel. The most common grade ≥3 treatment related toxicities were neutropenia (n=11), febrile neutropenia (n=5) and hypertension (n=4) (Table 5). Nine patients (22%) stopped or discontinued treatment due to toxicities. One patient experienced grade 4 leukoencephalopathy approximately one month following bevacizumab. This event was suspected to be related to bevacizumab and symptoms resolved completely with supportive care.
Table 5.
All grade 3 and 4 toxicities reported per patient.
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Allergic reaction | 1 | 0 |
| Anemia | 0 | 1 |
| Bowel perforation | 1 | 0 |
| Dyspnea | 1 | 0 |
| Febrile neutropenia | 5 | 0 |
| Hand-foot syndrome | 1 | 0 |
| Hyperglycemia | 1 | 0 |
| Hypertension | 4 | 0 |
| Hyponatremia | 1 | 0 |
| Hypophosphatemia | 1 | 0 |
| Ileus | 1 | 0 |
| Infection | 3 | 0 |
| Injection site reaction | 1 | 0 |
| Left ventricular systolic dysfunction | 1 | 0 |
| Leukopenia | 2 | 0 |
| Leukoencephalopathy | 0 | 1 |
| Gastrointestinal bleed | 2 | 0 |
| Neutropenia | 8 | 3 |
| Proteinuria | 1 | 0 |
| Seizure | 0 | 1 |
| Sinus bradycardia | 1 | 0 |
| Suicidal ideation | 0 | 1 |
| Syncope | 1 | 0 |
| Thrombotic microangiopathy | 1 | 0 |
Discussion
In this study, we demonstrate that administration of docetaxel, bevacizumab and ADT is feasible in men with BCR after definitive local therapy for PC. This is the first study to investigate multimodality systemic therapy with chemotherapy, anti-angiogenic therapy and ADT in men with BCR. This study was composed of men at high risk for metastasis given PSADT ≤10 months. We demonstrate that at a median follow-up of 27.5 months, 20% of men were free from PSA progression.
Clinical trials investigating combination therapy options in PC patients with BCR are limited. We previously reported on the efficacy and toxicity of docetaxel, estramustine, and ADT in 62 men with BCR after local therapy and demonstrated that a proportion of patients receiving this therapy remained in a durable PSA remission at long-term follow up, suggesting that this approach may have cured these individuals.9 In our prior study, we report a median TTP of 35 months compared to 27.5 months in this study, likely related to the to the fact that patients on this study were higher risk with a requirement for PSADT of <10 months (Table 6). Another study investigating docetaxel followed by gonadal suppression and bicalutamide plus finasteride in 39 patients with BCR with or without measurable disease (18% with metastatic disease) demonstrated that at a median follow up of 18.9 months, 15% of patients had a PSA ≤0.1 ng/mL.14 Whether bevacizumab added significant activity to combination of chemotherapy and ADT remains unanswered given the small size and non-comparative design of this trial.
Table 6.
Outcome comparisons of prior study of docetaxel + estramustine + ADT to the current study.
| Docetaxel + Estramustine + ADT9 (n=62) |
Docetaxel + Estramustine + ADT10 (n=62) |
Docetaxel + Bevacizumab + ADT (n=41) |
|
|---|---|---|---|
| Median follow-up | 37 months | 8.6 years | 27.5 months |
| PSA progression (%) | 53% | 77% | 80% |
| PSA complete response at one year following ADT completion (%) | 36% | 52%1 | 44% |
| TTP (months) | 33.8 | 35 | 28 |
11% had an undetectable PSA at long term follow-up.
TAX-3501 was a randomized, phase III, trial in high-risk patients with PC treated with RP comparing 18 months of adjuvant ADT with or without docetaxel administered immediately at RP or deferred until BCR.15 The deferred arm would have been a comparator for our study; however, TAX-3501 was terminated prematurely given enrollment and industry support challenges (target accrual 2,172). At a median follow up of 3.4 years, of the 228 patients randomized, 18% developed either PSA or radiographic progression (22%, n=24, in the immediate-treatment arm). Additionally, TAX-3503, a randomized, phase III trial of ADT with or without docetaxel for men with BCR after definitive therapy for PC, was also terminated early.
Given inadequate phase III trials to guide clinical decision making for men with BCR, phase II studies, such as this study, although not definitive, are informative. Furthermore, this data is highly relevant given the results of the CHAARTED (Chemohormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in PC) trial, which demonstrated a significant improvement in OS with early docetaxel and ADT compared to ADT alone for men with castration-sensitive, metastatic PC.8 The OS benefit in the CHAARTED trial was more pronounced for patients with high-volume disease (visceral metastases and/or ≥ four bone metastases) (49.2 versus 32.2 months, p=0.0012), while the low-volume metastasis cohort needs longer follow-up to ascertain benefit. This trial raises the question of the role of early chemotherapy for men with BCR without metastasis.
We demonstrate that at a median follow up of 27.5 months, 46% (n=19) of men were ADT-free and of patients with assessable testosterone levels, 92% had a testosterone ≥100 ng/dL. Additionally, 34 patients (82.9%) remained free from metastasis during follow-up and there was only one (2.4%) PC related death. Though longer follow-up is warranted, therapy is feasible and associated with significant PSA response rates. It is anticipated that with longer follow-up, additional patients in our cohort will experience PSA progression; however, it is likely that a subset of men will remain in remission from this treatment, similar to the trials discussed above.9,10,14 Testosterone recovery in our study was consistent with previously reported rates with intermittent ADT.16
In our previous chemohormonal trial, we demonstrated that baseline PSA and prior hormonal therapy were significant predictors of TTP.9 In this study we did not demonstrate significant associations with patient characteristics and outcomes. Possible explanations for the lack of predictive factors are the homogenous characteristics in this trial (all had high-risk disease with a short PSADT) and small cohort.
In this study, myelosuppression and neutropenic fever, likely related to docetaxel, were similar to that described in CALGB 90401, a phase III trial of docetaxel with or without bevacizumab in men with metastatic CRPC.12 The rates of grade 3 or 4 neutropenia and febrile neutropenia in CALGB 90401 were 30% and 7%, respectively, in the bevacizumab-containing arm. Additionally, the rates of grade 3 or 4 hypertension, likely related to bevacizumab, were similar to those reported in the CALGB trial (7%).
Since the initiation of our study, new treatment options for the management of metastatic CRPC have emerged including five new therapeutic agents: abiraterone, enzalutamide, cabazitaxel, sipuleucel-T, and alpharadin. The approval of these agents provides a rich opportunity to explore sequential and combinatorial approaches and early administration for hormone-sensitive PC. Given the lack of OS benefit of docetaxel and bevacizumab in patients with metastatic CRPC, it is unlikely that bevacizumab-containing regimens will be further evaluated in patients with earlier disease stages. Furthermore, other VEGF-targeted agents including sunitinib, aflibercept, and cabozantinib have failed to demonstrate a benefit in OS in patients with metastatic CRPC. Though preclinical data implicate the VEGF pathway in the pathogenesis of PC, the utility of VEGF-targeted agents in PC, especially in the context of next generation androgen receptor-directed therapies, remains to be determined. Ongoing clinical trials are evaluating the impact of chemotherapy, including cabazitaxel (NCT01650285), hormonal therapies, and immunotherapies in patients with BCR.
Conclusions
In this study, we demonstrate that multimodality systemic therapy with docetaxel, bevacizumab, and ADT was feasible and produced PSA responses in men with BCR following definitive PC therapy. Given toxicity associated with therapy, predictive biomarkers are necessary to identify patients likely to benefit from this type of aggressive approach. We believe that intensive multimodal systemic treatment of a limited duration may benefit a subset of patients with high-risk BCR. Long-term follow up is needed to determine the proportion of patients who have a durable PSA response and are able to remain off further PC therapy.
Acknowledgments
Funding Sources: This trial was funded by Genentech. MET acknowledges research support from the Fairweather Family Fund and Uribe Family Fund at the DFCI.
Financial Disclosures: RM receives research funding from Pfizer and Bayer. MET receives research funding from Genentech. AH received research funding from Amgen.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol. 2012 Sep;62(3):382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999 May 5;281(17):1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012 Jan;109(1):32–39. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paller CJ, Antonarakis ES, Eisenberger MA, Carducci MA. Management of patients with biochemical recurrence after local therapy for prostate cancer. Hematol Oncol Clin North Am. 2013 Dec;27(6):1205–1219. doi: 10.1016/j.hoc.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008 Jan 15;112(2):307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 7.Kirkwood JM, Tarhini A, Sparano JA, et al. Comparative clinical benefits of systemic adjuvant therapy for paradigm solid tumors. Cancer Treat Rev. 2013 Feb;39(1):27–43. doi: 10.1016/j.ctrv.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeney C, Chen Y-H, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. ASCO Meeting Abstracts. 2014 Jun 11;32(18_suppl):LBA2. 2014. [Google Scholar]
- 9.Taplin ME, Xie W, Bubley GJ, et al. Docetaxel, estramustine, and 15-month androgen deprivation for men with prostate-specific antigen progression after definitive local therapy for prostate cancer. J Clin Oncol. 2006 Dec 1;24(34):5408–5413. doi: 10.1200/JCO.2006.06.6589. [DOI] [PubMed] [Google Scholar]
- 10.Nakabayashi M, Xie W, Buckle G, et al. Long-term follow-up of a phase II trial of chemotherapy plus hormone therapy for biochemical relapse after definitive local therapy for prostate cancer. Urology. 2013 Mar;81(3):611–616. doi: 10.1016/j.urology.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson B, Gulding K, Conaway M, Wedge SR, Theodorescu D. Combination antiangiogenic and androgen deprivation therapy for prostate cancer: a promising therapeutic approach. Clin Cancer Res. 2004 Dec 15;10(24):8728–8734. doi: 10.1158/1078-0432.CCR-04-0902. [DOI] [PubMed] [Google Scholar]
- 12.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012 May 1;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross RW, Galsky MD, Febbo P, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: a Prostate Cancer Clinical Trials Consortium trial. Cancer. 2012 Oct 1;118(19):4777–4784. doi: 10.1002/cncr.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain A, Dawson N, Amin P, et al. Docetaxel followed by hormone therapy in men experiencing increasing prostate-specific antigen after primary local treatments for prostate cancer. J Clin Oncol. 2005 Apr 20;23(12):2789–2796. doi: 10.1200/JCO.2005.07.152. [DOI] [PubMed] [Google Scholar]
- 15.Schweizer MT, Huang P, Kattan MW, et al. Adjuvant leuprolide with or without docetaxel in patients with high-risk prostate cancer after radical prostatectomy (TAX-3501): important lessons for future trials. Cancer. 2013 Oct 15;119(20):3610–3618. doi: 10.1002/cncr.28270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crook JM, O'Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012 Sep 6;367(10):895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]