Abstract
National liver transplant volume has declined since 2006, in part due to worsening donor organ quality. Trends that degrade organ quality are expected to continue over the next two decades. We used the United Network for Organ Sharing (UNOS) database to inform a 20-year discrete event simulation estimating liver transplant volume from 2010 to 2030. Data to inform the model were obtained from deceased organ donors between 2000 and 2009. If donor liver utilization practices remain constant, utilization will fall from 78% to 44% by 2030, resulting in 2230 fewer liver transplants. If transplant centers increase their risk tolerance for marginal grafts, utilization would decrease to 48%. Institution of “opt-out” organ donation policies to increase the donor pool would still result in 1380-1866 fewer transplants. Ex-vivo perfusion techniques that increase the use of marginal donor livers may stabilize liver transplant volume. Otherwise, the number of liver transplants in the US will decrease substantially over the next 15 years.
Conclusions
The transplant community will need to accept inferior grafts and potentially worse post-transplant outcomes and/or develop new strategies for increasing organ donation and utilization in order to maintain the number of liver transplants at the current level.
Keywords: Diabetes, Obesity, Organ Donor, Donation after Cardiac Death, Forecast
Introduction
Liver transplantation (LT) is the only effective therapy for end stage liver disease. One year post-LT survival is above 85%(1) compared to ∼50% one year survival for those with Child-Pugh C cirrhosis.(2) Unfortunately, LT as a cure for end stage liver disease is limited by a shortage of organ donors. Efforts to increase the donor pool have included adding donation after cardiac death (DCD) to standard donation after brain death (DBD).(3) Additionally, older donors and donors with fatty livers have been utilized in recent years.(4) Initial enthusiam for these additional donors has waned because the suboptimal quality of the livers has translated into inferior transplant outcomes in many cases.(5-9) Thus, these efforts to increase the total donor pool have been thwarted by higher discard rates for less desirable grafts .(10) Such discarding of liver grafts, often from older donors with nonalcoholic fatty liver disease (NAFLD) and DCD donors, is becoming more common as the population ages and obesity and diabetes become more prevalent.(11, 12) In addition, US national policies have encouraged the option of DCD, which now comprises an increasing portion of the donor pool.(13)
The worsening quality of donated livers and increasing discard may be negatively impacting LT utilization on a national level. Between 2004 and 2010, there was a 40% increase in the proportion of donated livers that were discarded.(10) Historically, there was steady growth of deceased donor LT in the US, from fewer than 2,000 performed in 1988 to over 6,300 in 2006.(1) However, with rising discard rates, this figure has since plateaued; only 6,203 LTs were performed in 2013.(1) Continued increases in the prevalence of NAFLD, along with increasing reliance on DCD, may result in further increases in the discard rate and further declines in national LT volume in the future. Quantification of these anticipated trends is important for researchers and policymakers to identify and implement interventions to improve organ utilization.
Statistical forecasting models have traditionally been used to predict trends. However, when trends are based on causal relationships, it is not practical to build a single causal model accounting for all relationships among dynamic factors. Furthermore, when input factors are random variables (e.g. total number of organ donors), a probabilistic description of them is required. Standard modeling strategies such as Markov models and Monte-Carlo simulation, commonly used in the medical literature, lack the flexibility to adjust to changing inputs over time.(14) Discrete event simulation (DES) is an analytical tool typically used by systems engineers for evaluation of complex stochastic systems. Due to its ability to evaluate individuals moving through a model while concurrently considering demographic and health attributes, it is increasingly being used to assess health and economic outcomes in clinical settings, including end stage liver disease(15), organ allocation(16), colonoscopy performance(17), diabetes care(18), and rheumatologic disease(19) among others. Furthermore, DES allows for dynamic and probabilistic changes in external factors, such as phased policy changes, that may impact individuals differentially. Because the factors that influence donor liver discard change over time, DES can allow for more accurate estimates of future organ availability. We therefore used DES to simulate the future of adult deceased donor LT in the US and considered several scenarios that might impact liver utilization.
Methods
Overview
We used the United Network for Organ Sharing (UNOS) database to build a DES model to forecast liver donor characteristics, liver discard, and LT volume over a twenty year period. The model was informed by data from 2000 to 2009 to create predictions from 2010 to 2030 using Arena version 15 (Rockwell Automation, Milwaukee, WI). Available data from 2010 and 2011 were not included in model derivation, but were instead reserved for validation. Because of systematic differences between the organ donor population and the general population, other population-based data sources were evaluated, but not used. Full details of the DES model construction have been reported previously.(20)
Outcome
The primary outcome of the simulation was the proportion of donated livers used for transplantation annually from 2010 to 2030.
Inclusion/Exclusion Criteria
We used the UNOS database to derive the input data for the DES model. We included all deceased adult (age ≥ 18) solid organ donors who had at least one organ (liver, heart, intestine, kidney, lung, or pancreas) transplanted into a recipient between 2000 and 2009. This group was chosen because it lacks an absolute contraindication to organ donation in general (e.g. HIV), and therefore liver discard would likely be specifically due to poor liver quality. Split liver donations and those with extreme body mass index (BMI) were also excluded as previously described. (10, 20)
DES Model Construction
Important variables of interest were donor age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic), obesity (BMI ≥ 30 kg/m2), diabetes, alcohol use (≥ 2 drinks per day), alanine transaminase (ALT), bilirubin, cause of death, and DCD. These variables were selected due to their impact on donor liver discard. (10, 20) The independent odds ratios of discard based on these factors are shown in Figure 1. The DES donor generator produced a simulated donor starting with gender and, based on this, would assign an age and race. From these demographics, obesity, diabetes, alcohol use, and stroke would be assigned using the relationships discovered among the variables adjusted for the passage of time (e.g. the likelihood of diabetes as a function of age changes with each passing year and is dependent on obesity). Mild-to-moderate elevation of ALT in the absence of other liver disease may reflect NAFLD (21) whereas more severe elevations are likely due to acute donor illness. ALT was therefore assigned using nested logistic regression, including NAFLD risk factors of age, obesity, and diabetes for elevations up to 200 U/L, with higher ALT elevations modeled on age (with and without diabetes) adjusted for time.(20) Total bilirubin and DCD were not found to be dependent on other factors and thus assigned independently based on historic time dependent trends. The rationale and modeling for the donor generator has been previously described.(20) The relationships among variables and flow of the simulated variable dependencies are shown in Figure 1. Based on the characteristics of the simulated donor, the probability of organ discard was calculated. The simulator performed 15 replications of 100,000 donors in order to create an annual forecast for years 2010-2030. As a result of the number of donors and replications, the 95% confidence intervals for all of the estimates were within +/- 0.01% of the mean values shown, and were therefore suppressed in the results. (20)
Figure 1.
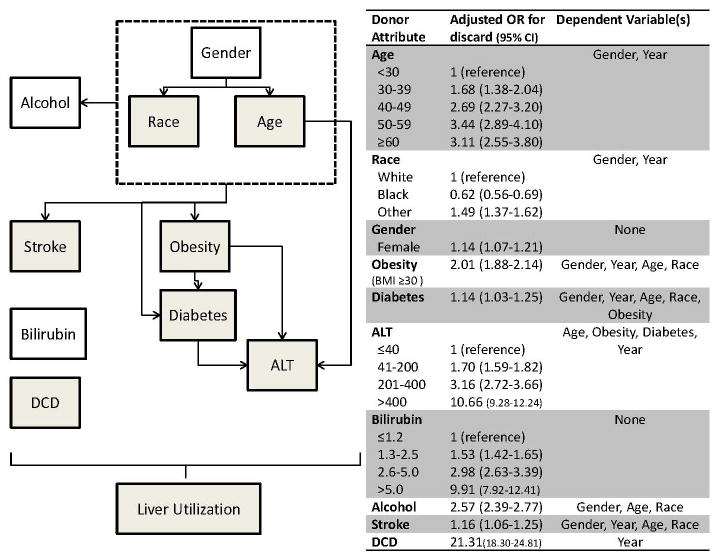
DES simulation framework. Dependent variable relationships, represented by lines, modeled via logistic regressions using UNOS data. Shaded variables are also dependent on the passage of time. 100,000 potential donors simulated with 15 replications for each scenario. Odds ratios adapted from Orman, et al10, from prior work in UNOS database and used to inform model.
Validation
Data were gathered from 2000 to 2009 to inform the DES model with available data from 2010 and 2011 withheld. Once the model was constructed, validity was assessed by comparing model derived data to actual historical data (Table 1). Overall, the estimation error was at or below 2% of the historical data for all variables.
Table 1. DES Model Validation: Actual and Predicted Donor Characteristics: 2010.
| Characteristic | 2010 (Actual) |
2010 (Predicted) |
|---|---|---|
| Age | ||
| < 30, % | 24.6 | 23.7 |
| 30-39, % | 15.9 | 14.4 |
| 40-49, % | 21.7 | 22.6 |
| 50-59, % | 22.6 | 23.6 |
| ≥ 60, % | 15.2 | 15.7 |
| Sex, % male | 58.9 | 58.7 |
| Race/ethnicity | ||
| White, % | 69.2 | 68.1 |
| Black, % | 16.8 | 17.0 |
| Hispanic, % | 14.1 | 15.0 |
| Obese, % | 30.7 | 31.2 |
| Alcohol use, % | 17.9 | 18.8 |
| Diabetes, % | 12.0 | 13.8 |
| Stroke, % | 40.8 | 43.0 |
| DCD, % | 11.4 | 12.5 |
| Bilirubin, mg/dL | ||
| ≤ 1.2, % | 76.2 | 73.7 |
| 1.3-2.5, % | 18.4 | 21.0 |
| 2.6-5.0, % | 4.1 | 4.1 |
| > 5.0, % | 1.4 | 1.3 |
| ALT, U/L | ||
| ≤ 40, % | 56.1 | 55.5 |
| 41-200, % | 36.4 | 36.5 |
| 201-400, % | 4.0 | 3.8 |
| > 400, % | 3.5 | 4.2 |
| Liver discard, % | 20.5 | 22.1 |
The DES model was created by UNOS data from 2000-2009. Available data from 2010 were withheld from model construction and used for validation.
Baseline Model: Donor Characteristics 2010-2030
The baseline model assumes that the previous population trends of obesity and diabetes in organ donors will continue unchanged in the future, and that the use of DCD will continue to increase as it has in previous years. This model provides the baseline data for donor liver use over a 20 year period and has been reported previously in a description of the simulation methodology.(20) We estimate the proportion of donors aged ≥ 50 will increase from 39.2% in 2010 to 43.7% in 2030 (Table 2). The prevalence of diabetes among organ donors is projected to increase from 13.8% to 45.7%, and the prevalence of obesity (BMI ≥ 30 kg/m2) will increase from 31.2% to 58.2%. Donors with an abnormal ALT (> 40 U/L) will increase from 44.5% to 60.9%. Overall, liver utilization will fall from 77.9% in 2010 to 43.6% in 2030, if current utilization practices remain constant. Therefore, for a static donor population of 6500, the current trends of DCD procurement, the aging donor population, and increases in diabetes and obesity among donors will result in 2,230 fewer LTs per year by 2030. These data are summarized in Table 3. Figure 2 illustrates the forecasted number of liver transplants performed in 2030 based on a donor population that grows with the census population with the other assumptions of the baseline scenario.
Table 2. Selected attributes of liver donor population 2010-2030.
| Male | Age >=50 yrs. | White | Black | Hispanic | Alcohol Use | Obese (BMI>30 kg/m2) | Diabetic | DCD | Abnormal ALT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | % | |||||||||
| 2010 | 58.8 | 39.2 | 68.2 | 16.9 | 14.9 | 18.8 | 31.2 | 13.8 | 12.5 | 44.5 |
| 2015 | 58.8 | 40.3 | 63.8 | 19.8 | 16.5 | 18.9 | 38.0 | 19.7 | 18.4 | 48.7 |
| 2020 | 58.7 | 41.3 | 59.4 | 22.5 | 18.1 | 18.9 | 45.0 | 27.2 | 24.3 | 52.8 |
| 2025 | 58.7 | 42.6 | 55.0 | 25.3 | 19.7 | 19.0 | 51.8 | 36.1 | 30.2 | 56.9 |
| 2030 | 58.7 | 43.7 | 50.7 | 28.0 | 21.3 | 19.0 | 58.2 | 45.7 | 36.1 | 60.9 |
Table 3. Proportion of liver grafts suitable for transplantation and forecasted number for adult orthotopic liver transplants performed based on current organ donation trends.
| Scenario | 2010 | 2015 | 2020 | 2025 | 2030 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| utilization rate (%) |
transplants | utilization rate (%) |
transplants | utilization rate (%) |
transplants | utilization rate (%) |
transplants | utilization rate (%) |
transplants | ||
| Baseline Model | 77.9 | 5064 | 70.1 | 4557 | 61.6 | 4004 | 52.6 | 3419 | 43.6 | 2834 | |
| Increased aggressiveness | 1 | 77.9 | 5064 | 70.2 | 4560 | 64.0 | 4162 | 57.9 | 3761 | 48.0 | 3117 |
| Changes in organ procurement policy | 2a | 77.9 | 5064 | 70.1 | 4557 | 62.1 | 4037 | 54.2 | 3523 | 45.3 | 2945 |
| 2b | 77.9 | 5064 | 70.2 | 4563 | 65.5 | 4258 | 64.4 | 4186 | 57.0 | 3705 | |
| Ex vivo perfusion | 3(20%-90% reduction in unused grafts) | 82.3-97.8 | 5351-6356 | 76.1-96.9 | 4945-6306 | 69.3-96.2 | 4503-6250 | 62.1-95.7 | 4035-6220 | 54.9-95.2 | 3567-6191 |
This table shows the proportion of liver grafts appropriate for adult whole liver orthotopic transplant by five year intervals from 2010 through 2030 among donors who were able to successfully donate at least one organ. Scenario changes are inserted into the model starting in 2015 with a ten year diffusion of innovation curve. Number of total donors set at 6500 based on data from 2006-2010.
In scenario 1, organ utilization is increased by 10% from 2015 to 2025 using a diffusion curve
In scenario 2a, 5-20% of DCD donors are converted to DBD donors using triangular distribution, in 2b, 90% of DCD donors are converted to DBD donors
In scenario 3, ex vivo perfusion of all grafts is examined. A range of relative reduction in non-use was considered from 20-90%.
Figure 2.
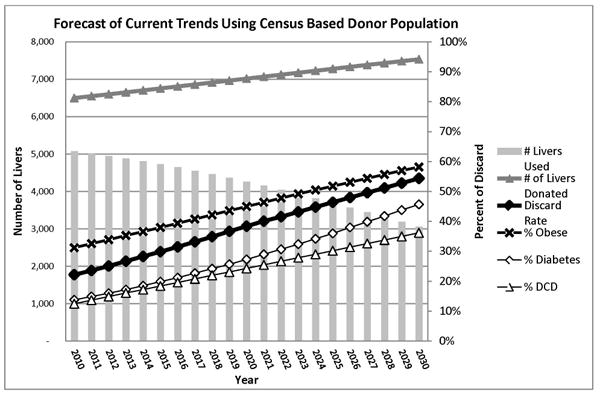
Using a population based estimate, the total number of donors will increase by 2030, but the corresponding increases in DCD, donor obesity and diabetes will increase the discard rate and reduce the total number of adult whole liver transplants over the next 20 years
Scenario Analyses
In addition to the previously published baseline model, we considered plausible scenarios that might alter these trends or change the risk of liver discard.
Scenario 1 acknowledges the potential for greater programmatic risk tolerance and organ utilization as a direct response to shrinking volumes. Some regions utilize more expanded criteria donors and have non-use rates below the national mean.(10, 22) This scenario increases the “aggressiveness” to which all centers and regions pursue marginal grafts and increases utilization by 10%. This increased utilization is phased into clinical practice in 2015 according to an S-shaped diffusion of innovation curve.(23)
Scenario 2 considered an organ procurement policy change that muted the detrimental impact of DCD on LT volume. This scenario assumes that a portion of DCD donors would have progressed to brain death if given more time, and that such a conversion of DBD to DCD explains in part the recent rapid increase in DCD. Although controversial, this possibility is supported by data demonstrating concurrent decreases in DBD with increasing DCD.(24, 25) In scenario 2, we assumed an implementation of policies discouraging DCD, with an associated “reverse” conversion of DCD to DBD. In 2a, we estimated this conversion to occur for between 5 to 20% of donors, using a triangular distribution. A second iteration of this scenario (2b) was run with the assumption of a profound shift in policy, converting 90% of DCD to DBD, virtually eliminating DCD as a procurement strategy.
Scenario 3 considers a disruptive technological change in regard to ex vivo reperfusion (i.e. “pumping”) of donor organs that might mitigate the non-use of marginal grafts from older donors and donors with diabetes, obesity or DCD. This technology was applied to all donated grafts where more marginal grafts would receive a proportionately greater benefit in utilization than grafts that had a greater probabilistic chance of being accepted and utilized. As in the other scenarios, this change was introduced in 2015 using a diffusion of innovation curve. Relative reduction in graft discard was calculated across a range from 20-90% and applied to the model.
Scenario 4 adjusted the total population of donors. Static increases of 1-4% per annum were investigated as well as specific strategies for increasing donation such as opt-in versus opt-out policies for organ donation. For the use of opt-out donation policies, a range for the change in total number of additional donors was derived from the literature(26-30) and incorporated into the simulation as a onetime increase in donors using an innovation diffusion curve.(23) Risks for organ discard remained static in this simulation.
The scenario analyses made changes independently; changes in one scenario were set to the baseline for other scenarios. This allowed for examination of the effects of single changes. Combination of scenarios 1-3 were not performed to avoid double counting donors and falsely adjusting liver discard.
Results
Scenario 1: Increasing risk tolerance and organ utilization
We performed a simulation where organ utilization increased by 10% from 2015 to 2025 according to a diffusion curve. In 2025, after these behavioral changes had completely taken effect, organ utilization was 58%, but fell to 48% by 2030 due to ongoing worsening of donor comorbidities. This scenario demonstrated a 4.4% increase in overall liver graft utilization over the baseline scenario by 2030.
Scenario 2: Policy to Reduce DCD
In scenario 2, we simulated the impact of a procurement policy change in 2015 that discouraged DCD and encouraged DBD donation, assuming that a proportion of DCD donors can progress to brain death. Initially, we performed a conservative simulation that converted 5 to 20% of DCD donors to DBD (specified using a triangular distribution). This intervention resulted in the conversion of 237 DCD livers to DBD (assuming a static donor population of 6500) and increased utilization by 1.7% to 45.3% by 2030. A more dramatic assumption where 90% of DCD donors are converted to DBD increased utilization by 13.4% to 57.0% by 2030, the highest level seen in any simulation.
Scenario 3: Disruptive technology change with ex vivo perfusion of all grafts
Scenario 3 examines ex vivo perfusion of all grafts with greater proportionate benefit to the most marginal grafts which are more likely to be those that come from older, diabetic donors and DCD. A range of relative reduction in non-use was considered from 20-90%. With such a technology, implemented in 2015 with a stable donor pool, utilization rates would increase from the baseline scenario to 82-98% yielding a liver transplant volume of 5,351 – 6,356. Applied over time, “pumping” of livers would result in a utilization rate between 55-95% in 2030 for a volume of 3567-6191 transplants. A comparison of all utilization scenarios is shown in Table 3.
Scenario 4: Expanding the donor population
Increasing the donor pool by 1% annually would increase the number of potential donors from 6,500 to 7,931 by 2030. A 3% annual increase would result in 11,740 donors by 2030. A 4% per annum increase would yield 14,242. Matching the growth of the donor pool to population growth data from the US census yields 7,536 donors by 2030. With 43.6% utilization in 2030, a 3-4% per annum increase would allow the total number of liver transplants to remain stable. Organ donation “opt-out” policies assume as a default that people are organ donors and require active participation to opt out of donation. Such policies have been shown to increase the number of donors by from 2.7 per million population to 30%, depending on the study population.(26-30) Extrapolating from the US population, such a policy would increase the absolute number of organ donors somewhere between 834 and 1950 donors. In a more optimistic assumption of a 90% increase in the donor pool due to opt-out policy, transplant volume would remain relatively stable. The effects of various strategies to increase the total donor pool are summarized in Table 4.
Table 4. Expanding the total organ donor population.
| Strategy/Assumption | 2010 | 2015 | 2020 | 2025 | 2030 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donors | Transplants | Donors | Transplants | Donors | Transplants | Donors | Transplants | Donors | Transplants | ||
| Static Donor population Growth | 1% annual growth | 6,500 | 5064 | 6,832 | 4789 | 7,180 | 4423 | 7,546 | 4241 | 7,931 | 3458 |
| 2% annual growth | 6,500 | 5064 | 7,177 | 5031 | 7,923 | 4881 | 8,748 | 4916 | 9,659 | 4211 | |
| 3% annual growth | 6,500 | 5064 | 7,535 | 5282 | 8,735 | 5381 | 10,127 | 5691 | 11,740 | 5119 | |
| 4% annual growth | 6,500 | 5064 | 7,908 | 5543 | 9,622 | 5927 | 11,706 | 6579 | 14,242 | 6210 | |
| Donor population linked to Census | Census projection | 6,500 | 5064 | 6,756 | 4736 | 7,019 | 4324 | 7,282 | 4092 | 7,536 | 3286 |
| International opt out donation strategy estimations | Abadie et al(30) | 6,500 | 5064 | 6,516 | 4568 | 7,272 | 4480 | 8,450 | 4749 | 8,450 | 3684 |
| Neto et al(33) | 6,500 | 5064 | 6,511 | 4564 | 7,040 | 4337 | 7,865 | 4420 | 7,865 | 3429 | |
| Healy et al(32) | 6,500 | 5064 | 6,507 | 4561 | 6,830 | 4207 | 7,334 | 4122 | 7,334 | 3198 | |
| Gimbel et al(31) | 6,500 | 5064 | 6,515 | 4567 | 7,250 | 4466 | 8,396 | 4719 | 8,396 | 3661 | |
| Universal opt out strategy | 90% of eligible population donate | 6,500 | 5064 | 6,547 | 4589 | 8,815 | 5430 | 12,350 | 6941 | 12,350 | 5385 |
Table 4 summarizes the total number of organ donors who successfully donate at least one organ pending changes in various donation strategies.
Total liver transplants performed calculated based on utilization rates from baseline assumptions in scenario 1.
In order to keep pace with the current number of liver transplants performed, there needs to be approximately 3% per year growth in the donor population or a universal opt in organ donor strategy
Discussion
According to these simulations, the number of deceased donor liver transplants (LT) done will decrease considerably through 2030 due to worsening donor liver quality and consequent increasing organ discard. In 2010, 5,064 out of 6,500 potential livers were transplanted for a utilization rate of 78%. Increases in donor age, obesity, diabetes and donation after cardiac death (DCD) will combine to decrease utilization to 44% by 2030. Without a disruptive technological change or a significant increase in the total number of organ donors, the number of liver transplants performed in the United States may fall substantially.
In addition to this baseline simulation, we examined several scenarios that incorporated changing behaviors, procurement policies and donor health attributes. While all adjustments to the model increased utilization, only two increased the utilization rate by more than 5% over the 2030 baseline model: (1) converting 90% of DCD donors to standard DBD and (2) an innovative technological change with ex vivo perfusion that allows pumping of all grafts. These adjustments improved 2030 utilization projections, but total utilization will still remain lower than current levels unless the most optimistic projections come to fruition. Increase in overall donation could mitigate the effect of lower utilization, but the increase in donation would have to be 3-4% per year which is unrealistic based on current donation trends. Increasing the “aggressiveness” of centers when utilizing marginal donors yields only a marginal increase in the overall number of liver transplants performed at an untold cost of worsening post-transplant outcomes.
DCD has been emphasized as a strategy to increase the organ donor pool as a part of the Organ Donation Breakthrough Collaborative.(13) As experience with DCD grafts has grown, the LT community has seen inferior outcomes with DCD grafts(9) and consequently a higher discard rate of these livers.(10) The deleterious impact of DCD may not be so evident in other fields of organ transplantation, especially kidney transplant,(31-33) thus setting up a potential conflict between increasing the total number of donor kidneys and maintaining the quality of donor livers. Many in the liver transplant community have been concerned that DCD has not added to the total donor pool, but has instead eroded into the donation after brain death (DBD) population resulting in no net gain of organ donors.(10) Since 2006, the total number of donated livers has remained stagnant, while the proportion of DCD donors has steadily increased from ∼1% to 12.5% in 2010.(10) Similar trends have been described in Europe.(34) If these trends continue, our projections estimate the proportion of DCD donors could reach 36% by 2030. The possibility of DCD displacing DBD is further supported by a multinational study that found that countries with higher DCD rates have lower DBD rates.(25)
Increasing the rate of organ, tissue and eye donation has long been the goal of multiple organizations including the U.S. Department of Health Resources and Service Administration's Workplace Partnership for Life campaign, the Donate Life America alliance and the American Hospital Association. Unfortunately, these organizations would need to significantly increase donations to meet simulated demand. Relying on living donor liver transplantation is unlikely to completely fill the void created by older, obese or diabetic cadaveric donors. Thresholds for steatosis are much lower among living donor grafts(35) and the epidemics of diabetes and obesity will impact this population as well. Even now, only a minority of liver failure patients has an appropriate living donor who qualifies for right lobe donation.(36) The ability to identify suitable living donors depends on multiple factors beyond donor comorbidities and procurement strategies, including transplant center, family dynamics, social factors and donor consent that are beyond the scope of the current simulation.
Major technological advances, such as the advent of an ex-vivo perfusion technique that is widely accessible enough to allow back table pumping of all grafts, might change this otherwise dreary forecast. An innovative technology that mitigates the risk of non-use among older, diabetic and obese donors as well as DCD may be the only avenue for sustaining the current level of liver transplantation. This technology, and even the concept of organ assessment and repair centers, is under development currently and had shown benefit in markers of cell death and regeneration, but has yet to fully make the leap from bench to bedside. (37-40) While not simulated, an additional benefit of ex vivo graft perfusion could also include expanded cold ischemia time and a wider geographic range for graft sharing.
Three dimensional bio-printing is another potentially disruptive technology that could eliminate the need for organ donors entirely. Bio-printing builds functional biological tissue over a biological scaffold and holds the potential to meet the need for organ transplantation(41). This technology is in its infancy, however, and the ability of bio-printing to solve the organ donor crisis when even extracorporeal liver support systems have yet to be realized remains controversial.
The strengths of our simulation include a rich data source, the UNOS donor database, on which to base our simulation. The UNOS donor data, particularly for age, BMI, DCD, diabetes and hypertension, are probably quite accurate because they are collected over a relative brief period of time by professional procuring teams who understand how critical such data are for proper organ disposition. Withholding data from 2010 and 2011 in construction of the DES allowed model validation. We found our model estimated all variables to 2% or closer to actual known values. Compared to other forecasts of future liver diseases, such as HCV (42), our estimates appear to be more accurate likely due to the DES methodology that allows for more complex modeling over prolonged time horizons.(14)
With any long term forecast of future disease, there can always be unanticipated developments that alter predictions. New technologies, changes in medical care, or substantial shifts in transplant and/or organ procurement policy may render these simulations less accurate. We have tried to account for these events through our various scenarios but we could not model on all possible changes in demographics and policy that could occur simultaneously. Furthermore, we were unable to model combinations of scenarios (e.g. ex-vivo perfusion and opt-out donation together). Such combinations of technological advances with policy changes in response to this potential crisis may actually be more likely than individual changes and would be more likely to preserve transplant volumes. Nevertheless, even if our models' estimates are too pessimistic by 50%, these data are alarming as the total number of liver transplants will still fall significantly below current levels while the burden of end stage liver disease (ESLD) from HCV and NAFLD is only expected to increase over this time period.(42, 43)
The liver transplant community and general public face a choice between accepting lower quality organs with the possibility of inferior post-transplant outcomes, or continuing current practices at the expense of increased mortality for patients on the waiting list. Reductions in liver transplant volume will result in increased numbers of patients waiting for transplant, longer waitlist times and higher Model of End-Stage Liver Disease scores at the time of transplant. Each of these events will likely lead to an increase in complications of ESLD, longer post transplant hospitalizations, and overall increased healthcare costs.
The complications of ESLD are expensive, and as patients wait longer for transplant, these episodes will become more common. In 2008 dollars, complicated variceal bleeding hospitalization mean costs were $23,207 with a mean length of stay of 15 days.(44) Over a five year period from 2005 to 2009, inpatient charges for hepatic encephalopathy rose from $46,663 to $63,108 per case leading to a national increase in encephalopathy related inpatient spending from 4.7 billion to 7.2 billion.(45) During the same time period, HCC related inpatient charges rose from $29,466 to $31,656 per case for an overall national spending increase from 1.0 billion to 2.0 billion.(46) These complications may occur repeatedly while patients wait for a transplant.
Increased MELD at the time of transplant and increased donor comorbidities have been shown to increase transplant related costs and the combination of these two factors is synergistic.(47) Donors in the highest risk quartile of the Donor Risk Index add $12,000 to the cost of transplant and another $22,000 to post transplant costs, relative to low risk donors, pushing overall one year costs to over $200,000 in 2008 U.S. dollars. DCD donors increased costs by $21,000 over standard donation after brain death (DBD) donors.(47) These costs are directly attributable to longer post transplant hospital stays associated with increasing donor comorbidities. (48)
Our model suggests a dire forecast for the future of liver transplantation that has major implications for the increasing number of patients suffering from liver failure. National epidemics of diabetes and obesity will increase the number of patients with NAFLD related liver failure,(49) while at the same time compromising the quality of donated livers for all indications for liver transplantation. The use of new technology for organ preservation, living donation and increasing the donation rate may slow the decline but not arrest it. Accepting worse outcomes by using worse organs may be the only way to maintain organ utilization rates. Whether this can be done in a cost-effective manner based on quality of life years saved is unclear.
Acknowledgments
Grant Support: This work was supported, in part, by the National Institutes of Health, T32 DK07634, 1KL2-RR025746, 1-K12 HS019468-01, and UL1-TR000083, KL2 TR001106, Health Resources and Services Administration contract 231-00-0115, and by the National Science Foundation, CMMI-141833.
Abbreviations
- UNOS
United Network of Organ Sharing
- LT
Liver Transplantation
- NAFLD
Non Alcoholic Fatty Liver Disease
- DCD
Donation after Cardiac Death
- DBD
Donation after Brain Death
- DES
Discrete Event Simulation
- ALT
alanine transaminase
Footnotes
Disclosures: The authors have no relevant conflicts of interest.
Conflicts of Interest: The authors have no relevant conflicts to disclose
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN / SRTR 2011 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. Table 9.14b. In. [Google Scholar]
- 2.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555–562. doi: 10.1097/01.sla.0000239006.33633.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack L, Petrowsky H, Jochum W, Mullhaupt B, Weber M, Clavien PA. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940–946. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 946-948. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielli M, Moisan F, Vidal M, Duarte I, Jimenez M, Izquierdo G, Dominguez P, et al. Steatotic livers. Can we use them in OLTX? Outcome data from a prospective baseline liver biopsy study. Ann Hepatol. 2012;11:891–898. [PubMed] [Google Scholar]
- 6.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, Stewart G, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 7.Leithead JA, Tariciotti L, Gunson B, Holt A, Isaac J, Mirza DF, Bramhall S, et al. Donation After Cardiac Death Liver Transplant Recipients Have an Increased Frequency of Acute Kidney Injury. Am J Transplant. 2012 doi: 10.1111/j.1600-6143.2011.03894.x. [DOI] [PubMed] [Google Scholar]
- 8.Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 2010;10:2512–2519. doi: 10.1111/j.1600-6143.2010.03293.x. [DOI] [PubMed] [Google Scholar]
- 9.Jay C, Ladner D, Wang E, Lyuksemburg V, Kang R, Chang Y, Feinglass J, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant - an analysis of the national registry. J Hepatol. 2011;55:808–813. doi: 10.1016/j.jhep.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orman ES, Barritt ASt, Wheeler SB, Hayashi PH. Declining liver utilization for transplantation in the United States and the impact of donation after cardiac death. Liver Transpl. 2013;19:59–68. doi: 10.1002/lt.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA. 2003;289:1371–1373. [PubMed] [Google Scholar]
- 12.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 13.Shafer TJ, Wagner D, Chessare J, Zampiello FA, McBride V, Perdue J. Organ donation breakthrough collaborative: increasing organ donation through system redesign. Crit Care Nurse. 2006;26:33–42. 44–38. quiz 49. [PubMed] [Google Scholar]
- 14.Karnon J. Alternative decision modelling techniques for the evaluation of health care technologies: Markov processes versus discrete event simulation. Health Econ. 2003;12:837–848. doi: 10.1002/hec.770. [DOI] [PubMed] [Google Scholar]
- 15.Alagoz O, Bryce CL, Shechter S, Schaefer A, Chang CC, Angus DC, Roberts MS. Incorporating biological natural history in simulation models: empirical estimates of the progression of end-stage liver disease. Med Decis Making. 2005;25:620–632. doi: 10.1177/0272989X05282719. [DOI] [PubMed] [Google Scholar]
- 16.Shechter SM, Bryce CL, Alagoz O, Kreke JE, Stahl JE, Schaefer AJ, Angus DC, et al. A clinically based discrete-event simulation of end-stage liver disease and the organ allocation process. Med Decis Making. 2005;25:199–209. doi: 10.1177/0272989X04268956. [DOI] [PubMed] [Google Scholar]
- 17.Berg B, Denton B, Nelson H, Balasubramanian H, Rahman A, Bailey A, Lindor K. A discrete event simulation model to evaluate operational performance of a colonoscopy suite. Med Decis Making. 2010;30:380–387. doi: 10.1177/0272989X09345890. [DOI] [PubMed] [Google Scholar]
- 18.Elgart JF, Caporale JE, Gonzalez L, Aiello E, Waschbusch M, Gagliardino JJ. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev. 2013;3:11. doi: 10.1186/2191-1991-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran-Duy A, Boonen A, van de Laar MA, Franke AC, Severens JL. A discrete event modelling framework for simulation of long-term outcomes of sequential treatment strategies for ankylosing spondylitis. Ann Rheum Dis. 2011;70:2111–2118. doi: 10.1136/annrheumdis-2011-200333. [DOI] [PubMed] [Google Scholar]
- 20.Toro-Diaz H, Mayorga ME, Barritt AS, Orman ES, Wheeler SB. Predicting Liver Transplant Capacity Using Discrete Event Simulation. Med Decis Making. 2014 doi: 10.1177/0272989X14559055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi PH, Axelrod DA, Galanko J, Salvalaggio PR, Schnitzler M. Regional differences in deceased donor liver transplantation and their implications for organ utilization and allocation. Clin Transplant. 2011;25:156–163. doi: 10.1111/j.1399-0012.2010.01214.x. [DOI] [PubMed] [Google Scholar]
- 23.Rogers EM. Diffusion of innovations. 4th. New York: Free Press: A Division of Simon and Schuster; 1995. [Google Scholar]
- 24.Sampson BG, O'Callaghan GP, Russ GR. Is donation after cardiac death reducing the brain-dead donor pool in Australia? Crit Care Resusc. 2013;15:21–27. [PubMed] [Google Scholar]
- 25.Bendorf A, Kelly PJ, Kerridge IH, McCaughan GW, Myerson B, Stewart C, Pussell BA. An international comparison of the effect of policy shifts to organ donation following cardiocirculatory death (DCD) on donation rates after brain death (DBD) and transplantation rates. PLoS One. 2013;8:e62010. doi: 10.1371/journal.pone.0062010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abadie A, Gay S. The impact of presumed consent legislation on cadaveric organ donation: a cross-country study. J Health Econ. 2006;25:599–620. doi: 10.1016/j.jhealeco.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Gimbel RW, Strosberg MA, Lehrman SE, Gefenas E, Taft F. Presumed consent and other predictors of cadaveric organ donation in Europe. Prog Transplant. 2003;13:17–23. doi: 10.1177/152692480301300104. [DOI] [PubMed] [Google Scholar]
- 28.Healy K. The Political Economy of Presumed Consent. 2005 [Google Scholar]
- 29.Neto GB, Katarina Campelo A, Nunes da Silva E. The Impact of Presumed Consent Law on Organ Donation: An Empirical Analysis from Quantile Regression for Longitudinal Data. 2007 [Google Scholar]
- 30.Rithalia A, McDaid C, Suekarran S, Myers L, Sowden A. Impact of presumed consent for organ donation on donation rates: a systematic review. BMJ. 2009;338:a3162. doi: 10.1136/bmj.a3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SK, Kim SJ. Does expanded criteria donor status modify the outcomes of kidney transplantation from donors after cardiac death? Am J Transplant. 2013;13:329–336. doi: 10.1111/j.1600-6143.2012.04311.x. [DOI] [PubMed] [Google Scholar]
- 32.Nagaraja P, Roberts GW, Stephens M, Horvath S, Fialova J, Chavez R, Asderakis A, et al. Influence of delayed graft function and acute rejection on outcomes after kidney transplantation from donors after cardiac death. Transplantation. 2012;94:1218–1223. doi: 10.1097/TP.0b013e3182708e30. [DOI] [PubMed] [Google Scholar]
- 33.de Vries EE, Hoogland PE, Wind J, Snoeijs MG, van Heurn EL. Transplantation of kidneys from paediatric DCD donors: a comparison with DBD donors. Nephrol Dial Transplant. 2013;28:220–226. doi: 10.1093/ndt/gfs464. [DOI] [PubMed] [Google Scholar]
- 34.Cohen B, Smits JM, Haase B, Persijn G, Vanrenterghem Y, Frei U. Expanding the donor pool to increase renal transplantation. Nephrol Dial Transplant. 2005;20:34–41. doi: 10.1093/ndt/gfh506. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Ashworth A, Behnke M, Cotterell A, Posner M, Fisher RA. Donor selection for adult-to-adult living donor liver transplantation: well begun is half done. Transplantation. 2013;95:501–506. doi: 10.1097/TP.0b013e318274aba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trotter JF, Wisniewski KA, Terrault NA, Everhart JE, Kinkhabwala M, Weinrieb RM, Fair JH, et al. Outcomes of donor evaluation in adult-to-adult living donor liver transplantation. Hepatology. 2007;46:1476–1484. doi: 10.1002/hep.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knaak JM, Spetzler VN, Goldaracena N, Boehnert MU, Bazerbachi F, Louis KS, Adeyi OA, et al. Subnormothermic ex vivo liver perfusion reduces endothelial cell and bile duct injury after donation after cardiac death pig liver transplantation. Liver Transpl. 2014;20:1296–1305. doi: 10.1002/lt.23986. [DOI] [PubMed] [Google Scholar]
- 38.Knaak JM, Spetzler VN, Goldaracena N, Louis KS, Selzner N, Selzner M. Technique of subnormothermic ex vivo liver perfusion for the storage, assessment, and repair of marginal liver grafts. J Vis Exp. 2014:e51419. doi: 10.3791/51419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitson BA, Black SM. Organ assessment and repair centers: The future of transplantation is near. World J Transplant. 2014;4:40–42. doi: 10.5500/wjt.v4.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, Ratner LE, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161–169. doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 41.Yoo SS. 3D-printed biological organs: medical potential and patenting opportunity. Expert Opin Ther Pat. 2015:1–5. doi: 10.1517/13543776.2015.1019466. [DOI] [PubMed] [Google Scholar]
- 42.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521, e511–516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 43.Charlton M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: Molehill or mountain? Hepatology. 2008;47:1431–1433. doi: 10.1002/hep.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viviane A, Alan BN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11:1–3. doi: 10.1111/j.1524-4733.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- 45.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10:1034–1041. e1031. doi: 10.1016/j.cgh.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Otgonsuren M, Venkatesan C, Afendy M, Erario M, Younossi ZM. The inpatient economic and mortality impact of hepatocellular carcinoma from 2005 to 2009: analysis of the US nationwide inpatient sample. Liver Int. 2013;33:1281–1286. doi: 10.1111/liv.12201. [DOI] [PubMed] [Google Scholar]
- 47.Salvalaggio PR, Dzebisashvili N, MacLeod KE, Lentine KL, Gheorghian A, Schnitzler MA, Hohmann S, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transpl. 2011;17:233–242. doi: 10.1002/lt.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Axelrod DA, Schnitzler M, Salvalaggio PR, Swindle J, Abecassis MM. The economic impact of the utilization of liver allografts with high donor risk index. Am J Transplant. 2007;7:990–997. doi: 10.1111/j.1600-6143.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 49.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]


