Abstract
Purpose
The renin-angiotensin system may play a role in carcinogenesis. The purpose of this study was to evaluate the impact of angiotensin system inhibitors (ASIs) on outcomes in metastatic renal cell carcinoma (mRCC) patients treated in the targeted therapy era.
Methods
We conducted a pooled analysis of mRCC patients treated on phase II and III clinical trials. Statistical analyses were performed using Cox regression adjusted for several risk factors and the Kaplan-Meier method.
Results
4,736 patients were included, of whom 1,487 received ASIs and 783 received other anti-hypertensive agents. Overall, ASI users demonstrated improved overall survival (OS) compared to users of other anti-hypertensive agents (adjusted HR 0.838, p=0.0105, 26.68 versus 18.07 months) and individuals receiving no anti-hypertensive therapy (adjusted HR 0.810, p=0.0026, 26.68 versus 16.72 months). When stratified by therapy type, a benefit in OS was demonstrated in ASI users compared to non-users in individuals receiving vascular endothelial growth factor targeted therapy (adjusted HR 0.737, p<0.0001, 31.12 versus 21.94 months) but not temsirolimus or interferon-alpha. An in vitro cell viability assay demonstrated that sunitinib in combination with an ASI significantly decreased RCC cell viability compared to control at physiologically relevant doses. This effect was not observed with either agent alone or with other non-ASI anti-hypertensives or temsirolimus.
Conclusions
In the largest analysis to date, we demonstrate that ASI use improved survival in mRCC patients treated in the targeted therapy era. Further studies are warranted to investigate the mechanism underlying this interaction and verify our observations to inform clinical practice.
Keywords: Angiotensin system inhibitors, ACE inhibitors, Angiotensin receptor blockers, Hypertension, Renal cell carcinoma
Introduction
Tumor angiogenesis is an established mechanism of metastatic renal cell carcinoma (mRCC) growth and progression. Critical to this pathway is vascular endothelial growth factor (VEGF), as demonstrated by RCC susceptibility to VEGF blockade with several approved targeted agents. Hypertension is a common condition which affects one of every three American adults.(1) It is also commonly seen in patients with mRCC treated with VEGF-targeted therapy. Angiotensin system inhibitors (ASIs) are broadly utilized by millions of Americans to treat hypertension, congestive heart failure, and other common medical conditions. ASIs include two major classes of agents: angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). ACEIs decrease the production of angiotensin II generated from the conversion of antiogensin I to angiotensin II by ACE.(2) ARBs block the action of one of two well-described subtypes of angiotensin II receptors.(2) Given that angiotensin II can activate both types of receptors, ACEIs diminish activity at both receptors, whereas ARBs diminish only type I-receptor mediated effects.
Increasing evidence suggests that angiotensin II, an important regulator of blood pressure and cardiovascular homeostasis, plays a role in various pathologic processes including VEGF-dependent angiogenesis.(3, 4) Preclinical studies have shown that angiotensin II, which mediates its biological effects via binding to angiotensin II type 1 and type 2 receptors, regulates the expression of VEGF and the VEGF receptor.(3) Physiologically, both angiotensin II receptors are widely expressed in the kidney.(5) They localize to the renal cortex and are expressed by proximal tubular cells, which comprise the cell of origin of both clear cell and papillary RCC.(6) The most direct evidence that angiotensin II signaling regulates tumor angiogenesis comes from xenograft studies which demonstrate that angiotensin II receptor knockout mice have reduced angiogenesis and tumor growth rates compared with wild-type mice.(7) Additionally, studies of human clear-cell RCC have demonstrated that angiotensin II receptor expression strongly correlates with tumor aggressiveness and decreased survival.(8) Lever and colleagues reported the first clinical evidence that long-term angiotensin II blockade may be protective against cancer.(9) Since that time several retrospective studies have investigated the association between ASIs and cancer progression and survival.(10)
Despite increasing evidence to suggest that the renin-angiotensin system may play a role in carcinogenesis and ASIs may be associated with improved outcomes in cancer patients, there are limited studies investigating the role of ASIs in patients with mRCC treated with targeted therapy. Furthermore, the large number of individuals suffering from hypertension and mRCC presents an opportunity to explore combinatorial treatment regimens. In this analysis, we utilized a large clinical trials database to evaluate the role of ASIs on survival in patients with mRCC treated with a broad range of therapies in the modern era. Additionally, we explored the effects of a broad spectrum of anti-hypertensive agents with or without sunitinib or temsirolimus on RCC cell viability in vitro.
Patients and Methods
Study design
We conducted a pooled retrospective analysis of patients with mRCC treated on phase II and phase III clinical trials sponsored by Pfizer (Table 1).(11-22) We identified 4,736 patients treated for mRCC between January 2003 and June 2013. Patients who received at least one dose of study treatment were included in the analysis. Patients with missing concomitant medication information were excluded from the analysis. In total, 720 patients were excluded from
Table 1.
Phase II and phase III studies included in analysis.
| Clinical Trial Identifier |
Phase | Number of patients enrolled |
Number of patients excluded from multivariate analysis |
|---|---|---|---|
| NCT00267748 | II | 289 | 79 |
| NCT00077974 | II | 106 | 27 |
| NCT00137423 | II | 107 | 31 |
| NCT00054886 | II | 63 | 13 |
| NCT00338884 | II | 119 | 40 |
| NCT00835978 | II | 213 | 43 |
| NCT00065468 | III | 616 | 94 |
| NCT00678392 | III | 714 | 73 |
| NCT00083889 | III | 735 | 68 |
| NCT00474786 | III | 501 | 83 |
| NCT00631371 | III | 784 | 108 |
| NCT00920816 | III | 409 | 61 |
Baseline demographic, clinical, and laboratory data were collected. Data regarding ASI use were collected. ASIs included ACEIs and ARB. Users of ASIs or other anti-hypertensive agents were defined as patients treated with an ASI or other anti-hypertensive agent at baseline or within 30 days of study treatment initiation. The decision to start an anti-hypertensive agent prior to the initiation of study treatment and choice of agent was at the discretion of the treating physician. Each clinical trial had predefined parameters for initiating anti-hypertensive therapy should treatment-associated hypertension develop, however the choice of agent was at the discretion of the treating physician. Patient follow-up consisted of imaging assessment every 4-12 weeks until disease progression or withdrawal. Treatment-associated toxicities were defined and evaluated according to the Common Terminology Criteria for Adverse Events, Version 3.0.
Treatment outcomes
Survival data were collected for all patients. Overall survival (OS) was defined as the time from initiation of therapy to death from any cause. Progression-free survival (PFS) was defined as the time from initiation of therapy to date of progression or death from any cause, whichever came first. For the evaluation of response, the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 was applied.(23) Measurements were performed prospectively in all trials by clinical investigators.
Statistical analysis
The primary outcome was to assess OS in patients treated with ASIs compared to patients receiving other anti-hypertensive agents or those receiving no anti-hypertensive therapy. PFS, objective response rates (ORR) and toxicity were secondary endpoints. OS and PFS were calculated using the Kaplan-Meier method. Median OS and PFS along with 95% confidence intervals (CI) were reported. OS and PFS were assessed using multivariate Cox regression analysis, adjusted for age, sex, race, previously described Memorial Sloan-Kettering Cancer Center (MSKCC) risk factors (low performance status, elevated lactic dehydrogenase, elevated corrected calcium, low hemoglobin, absence of prior nephrectomy), presence of baseline hypertension and the development of treatment-associated hypertension.(24) Given that treatment-associated hypertension is a time-dependent covariable, we conducted a six-month landmark analysis in addition to the primary analysis. All p-values are two sided. All hazard ratios (HR) presented are adjusted for the above variables and used ASI users as the test group; HR < 1 indicates ASI users had a favorable outcome versus the comparator group. Subgroup efficacy analyses were performed in the following groups: 1) users of anti-hypertensive therapy, 2) users of anti-hypertensive therapy receiving VEGF-targeted therapy, 3) patients who developed treatment-associated hypertension receiving VEGF-targeted therapy, and 4) stratified by mRCC therapy type (VEGF-targeted, mammalian target of rapamycin (mTOR)-targeted and interferon-alpha (IFN-α) therapy groups).
Cell viability assay
Human Von Hippel–Lindau (VHL)-negative RCC cell lines (A-498 and 769-P) were cultured in Dulbecco’s Modified Eagle Medium (supplemented with 10% fetal bovine serum). An equal number of cells (4 × 103) were seeded into 96-well flat-bottomed plates and incubated with various doses (0.001 to 1000 μM) of anti-hypertensive agents (captopril, lisinopril, losartan, amlodipine, and propranolol) with or without sunitinib at various doses (0.001 to 1000 μM), sunitinib alone, or dimethyl sulfoxide (DMSO) for 72 hours. Similar experiments were conducted with temsirolimus at various doses (0.001 to 1000 μM). Drug concentrations were based on physiologically relevant maximum concentrations (Cmax) in humans (captopril 1153.38 μM, lisinopril 277.20 μM, losartan 445.88 μM, amlodipine 15.16 μM, propranolol 87.90 μM, sunitinib 52.58 μM, temsirolimus 0.56 μM).(25-28). Cell viability was quantified using the CellTiter 96® AQueous One Solution Cell Proliferation assay (Promega) according to manufacturers’ recommendations. Percent cell viability was calculated by normalizing the absorbance to DMSO. Difference between groups were calculated using a one-way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test to correct for multiple testing. P-values < 0.05 were considered statistically significant.
Results
Patient and disease characteristics
Of the 4,736 patients included in the analysis, the majority were less than 65 years of age, male, with good performance status, and clear-cell histology (Table 2). ASI users were similar in age to users of other anti-hypertensive agents, however both groups were older than patients not on anti-hypertensive therapy. Most patients underwent prior nephrectomy (70.2%) and a minority (33.2%) received prior systemic therapy. The presence of baseline lung, bone and liver metastases in the overall cohort was 76.6%, 27.5%, and 26.1%, respectively, and rates were similar between groups. Baseline hypertension was present in nearly half the cohort (47.9%) of whom 83.1% were on anti-hypertensive therapy at baseline.
Table 2.
Baseline patient and disease characteristics.
| Characteristic n (%) |
Anti-Hypertensive Agent(s) Users | Anti-Hypertensive Agent(s) Non-User n=2466 |
Total Cohort n=4736 |
|
|---|---|---|---|---|
| ASI Users n=1487 |
Other n=783 |
|||
| Age at initiation of therapy < 65 years ≥ 65 years |
871 (58.6%) 616 (41.4%) |
447 (57.1%) 336 (42.9%) |
1940 (78.7%) 526 (21.3%) |
3258 (68.8%) 1478 (31.2%) |
| Sex Male Female |
1025 (68.9%) 462 (31.1%) |
523 (66.8%) 260 (33.2%) |
1815 (73.6%) 651 (26.4%) |
3258 (68.8%) 1478 (31.2%) |
| Race Caucasian Other |
1192 (80.2%) 295 (19.2%) |
583 (74.5%) 200 (25.5%) |
1889 (76.6%) 577 (23.4%) |
3664 (77.4%) 1072 (22.6%) |
| ECOG Performance Status 0 1 2 Unknown |
891 (59.9%) 574 (38.6%) 16 (1.1%) 6 (0.4%) |
384 (49.0%) 386 (49.3%) 8 (1.0%) 5 (0.6%) |
1220 (49.5%) 1198 (48.6%) 36 (1.5%) 12 (0.5%) |
2495 (52.7%) 2158 (45.6%) 60 (1.3%) 23 (0.5%) |
| Pathology Clear cell Non-clear cell Unknown |
1356 (91.2%) 95 (6.4%) 36 (2.4%) |
702 (89.7%) 55 (7.0%) 26 (3.3%) |
2177 (88.3%) 187 (7.6%) 102 (4.1%) |
4235 (89.4%) 337 (7.1%) 164 (3.5%) |
| Baseline metastatic site Lung Bone Liver |
1145 (77.0%) 363 (24.4%) 350 (23.5%) |
597 (76.2%) 206 (26.3%) 210 (26.8%) |
1887 (76.5%) 732 (29.7%) 678 (27.5%) |
3629 (76.6%) 1301 (27.5%) 1238 (26.1%) |
| Previous nephrectomy Yes No Unknown |
1096 (73.7%) 351 (23.6%) 40 (2.7%) |
581 (74.2%) 168 (21.5%) 34 (4.3%) |
1648 (66.8%) 688 (27.9%) 130 (5.3%) |
3325 (70.2%) 1207 (25.5%) 204 (4.3%) |
| Prior type of therapy Any prior therapy Cytokine therapy Targeted therapy |
529 (35.6%) 200 (13.4%) 220 (14.8%) |
274 (35.0%) 111 (14.2%) 124 (15.8%) |
770 (31.2%) 360 (14.6%) 229 (9.3%) |
1573 (33.2%) 671 (14.2%) 573 (21.1%) |
| Medical Conditions Hypertension Diabetes mellitus |
1250 (84.1%) 347 (23.3%) |
634 (81.0%) 116 (14.8%) |
383 (15.5%) 197 (8.0%) |
2267 (47.9%) 660 (13.9%) |
ECOG = Eastern Cooperative Oncology Group.
Treatment exposure
Patients received treatment with sunitinib (n=1,059), sorafenib (n=772), axitinib (n=896), temsirolimus (n=457), temsirolimus + IFN-α (n=208), bevacizumab + temsirolimus (n=393), bevacizumab + IFN-α (n=391), or IFN-α (n=560), of whom 3,044 (64%) received first-line therapy. 992 patients were treated with ACEIs, 703 were treated with ARBs, and 208 were treated with the combination of ACEIs and ARBs. In total, 1,487 patients received ASI therapy and 783 were treated with other anti-hypertensive agents including beta-blockers, calcium-channel blockers, diuretics, and other agents. The most frequently utilized ASIs were enalapril (n=211), lisinopril (n=204), valsartan (n=121), and ramipril (n=102). 1,191 patients were on an ASI at baseline and 296 patients were started on an ASI within 30 days of starting study treatment.
Impact of ASIs on survival
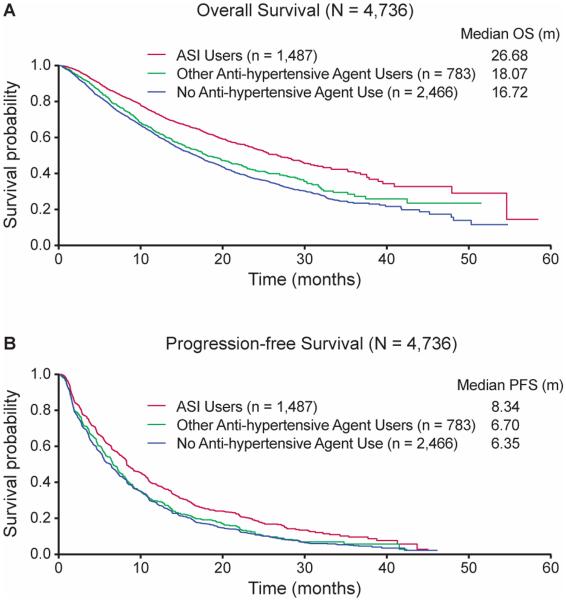
For the overall cohort, OS was significantly longer in ASI users compared to users of other anti-hypertensive agents (unadjusted HR 0.711, 95% CI 0.625-0.809; adjusted HR 0.838, 95% CI 0.731-0.960, p=0.0105, medians of 26.68 versus 18.07 months) and individuals receiving no anti-hypertensive therapy (unadjusted HR 0.630, 95% CI 0.572-0.694; adjusted HR 0.810, 95% CI 0.707-0.929, p=0.0026, medians of 26.68 versus 16.72 months,) (Table 3, Figure 1). Similarly, PFS was significantly longer in ASI users compared to users of other anti-hypertensive agents (unadjusted HR 0.786, 95% CI 0.704-0.876; adjusted HR 0.877, 95% CI 0.782-0.984, p=0.0261, medians of 8.34 versus 6.70 months) and there was a non-statistically significant improvement in PFS between ASI users compared to individuals receiving no anti-hypertensive therapy (unadjusted HR 0.747, 95% CI 0.689-0.810; adjusted HR 0.955, 95% CI 0.855-1.068, p=0.4226, medians of 8.34 versus 6.35 months). In multivariate analysis, lack of ASI use (adjusted HR 1.214, 95% CI 1.083-1.361), failure to develop treatment-associated hypertension (adjusted HR 1.976, 95% CI 1.805-2.165, p<0.0001), and individual MSKCC risk factors were independent predictors of worse OS; however age, sex, race and baseline hypertension were not predictive of OS. A 6-month landmark analysis demonstrated similar results; lack of ASI use (adjusted HR 1.305, 95% CI 1.138-1.496, p<0.0001), failure to develop treatment-associated hypertension (adjusted HR1.272, 95% CI 1.140-1.419, p<0.0001), and individual MSKCC risk factors remained statistically significant.
Table 3.
Impact of ASI use on OS and PFS.
| OS | PFS | ||||||
|---|---|---|---|---|---|---|---|
| N | Median (months) |
P-Value | HRa
(95% CI) |
Median (months) |
P-Value | HRa
(95% CI) |
|
| Overall Cohort (n=4736) | |||||||
| ASI users Other anti- hypertensive therapy usersb Anti-hypertensive therapy non-users |
1487 783 2466 |
26.68 18.07 16.72 |
Test | 8.34 6.70 6.35 |
Test | ||
|
0.0105
0.0026 |
0.838 (0.731-0.960) 0.810 (0.707-0.929) |
0.0261
0.4226 |
0.877 (0.782-0.984) 0.955 (0.855-1.068) |
||||
| Overall Cohort by Type of Therapy (n=4736) | |||||||
| VEGF Therapy (n=3511) ASI users ASI non-users |
1192 2319 |
31.12 20.21 |
<0.0001 |
0.737 (0.640-0.848) |
10.15 8.09 |
0.0869 |
0.907 (0.812-1.014) |
| mTOR Therapy (n=665) ASI users ASI non-users |
151 514 |
12.66 10.16 |
0.2975 |
0.867 (0.664-1.134) |
5.46 4.05 |
0.5650 |
0.929 (0.725-1.192) |
| IFN-α Therapy (n=560) ASI users ASI non-users |
144 416 |
15.54 14.82 |
0.2027 |
1.218 (0.899-1.647) |
3.84 3.68 |
0.9084 |
0.984 (0.747-1.297) |
| Patients Receiving VEGF-Targeted and Anti-Hypertensive Therapy (n=1769) | |||||||
| ASI users Other anti- hypertensive therapy usersb |
1192 577 |
31.12 21.94 |
0.0003 | 0.725 (0.609-0.861) | 10.15 7.82 |
0.0121 | 0.835 (0.726-0.962) |
| Patients Receiving VEGF-Targeted Therapy who Developed Treatment-Associated Hypertension (n=2466) | |||||||
| ASI users ASI non-users |
982 1484 |
33.19 24.64 |
0.0004 | 0.742 (0.629-0.875) | 10.92 9.05 |
0.0498 | 0.883 (0.779-1.000) |
HR of ASI users to comparator group from multivariate analysis, adjusted for age, sex, race, Memorial Sloan-Kettering Cancer Center (MSKCC) risk factors, presence of baseline hypertension and the development of treatment-associated hypertension.
Other anti-hypertensive agents include beta-blockers, calcium-channel blockers, diuretics, and other agents.
Bolded p-values are statistically significant.
ASI = angiotensin system inhibitors, CI = confidence interval, HR = hazard ratio, IMDC = International mRCC Database Consortium, IFN-α = interferon alpha, mTOR = mammalian target of rapamycin, OS = overall survival, PFS = progression-free survival, VEGF = vascular endothelial growth factor.
Figure 1.
Kaplan–Meier estimates of A) OS for the overall cohort, and B) PFS for the overall cohort stratified by ASI users, other anti-hypertensive agent(s) users, versus no anti-hypertensive agent use.
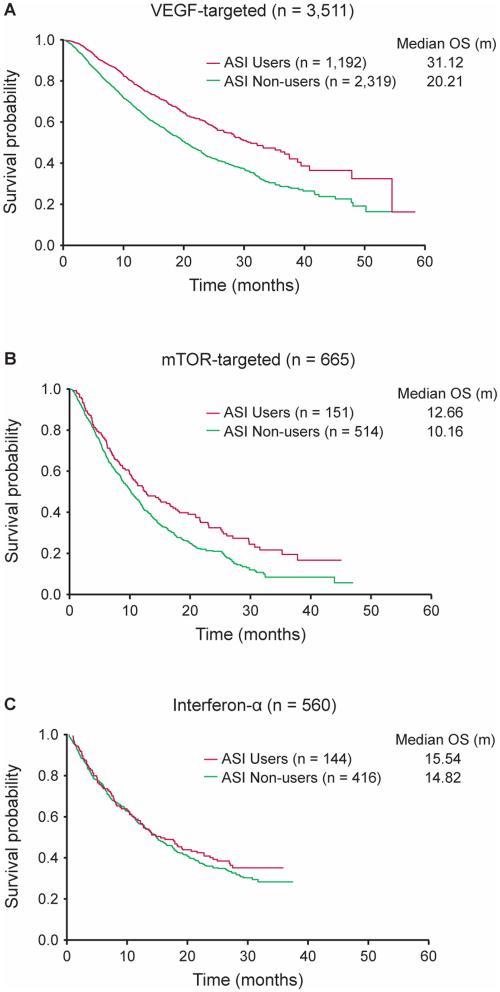
When stratified by mRCC therapy type, OS was significantly longer in ASI users compared to non-users in patients receiving VEGF-targeted therapy only (n=3,511) (adjusted HR 0.737, 95% CI 0.640-0.848, p<0.0001, medians of 31.12 versus 20.21 months), and there was no statistically significant difference in OS between ASI users and non-users in those receiving mTOR-targeted (n=665) or IFN-α therapy (n=560) (Figure 2).
Figure 2.
Kaplan–Meier estimates of A) OS for patients receiving VEGF-targeted therapy, B) OS for patients receiving mTOR-targeted therapy, and C) OS for patients receiving IFN-a therapy stratified by ASI users versus non-users.
To investigate the impact of anti-hypertensive therapy and treatment-associated hypertension in patients specifically receiving VEGF-targeted therapy, we conducted additional subgroup analyses in these patient populations. In patients receiving VEGF-targeted therapy and anti-hypertensive therapy (n=1,769), ASI use was associated with a statistically significant improvement in OS (adjusted HR 0.725, 95% CI 0.609-0.861, p=0.0003, medians of 31.12 versus 21.94 months) and PFS (adjusted HR 0.835, 95% CI 0.726-0.962, p=0.0121, medians of 10.15 versus 7.82 months). In patients receiving VEGF-targeted therapy who developed treatment-associated hypertension (n=2,466), a similar benefit in OS and PFS was observed (OS adjusted HR 0.742, 95% CI 0.629-0.875, p=0.0004, medians of OS 33.19 versus 24.64 months,; PFS adjusted HR 0.883, 95% CI 0.779-1.000, p=0.0498, medians of PFS 10.92 versus 9.05 months,). Survival was the longest in patients who received VEGF-targeted therapy, developed treatment-associated hypertension, and were treated with an ASI (n=982) (median OS 33.19 months).
Impact of ASIs on response
Objective response rates (ORR) were similar in ASI users compared to non-users in the overall cohort (28.31% versus 22.74%, p=0.7484). Similar results were seen and in the following subgroups: anti-hypertensive agent users (28.31% versus 22.35%, p=1.000), anti-hypertensive agent users receiving VEGF-targeted therapy (32.29% versus 26.32%, p=1.000), and patients receiving VEGF-targeted therapy who developed treatment-associated hypertension (35.64% versus 33.96%, p=0.9002).
Adverse events
2,690 patients (62.5%) in the overall cohort developed treatment-associated hypertension. Of the 3,511 patients treated with VEGF-targeted therapy, 2,466 (70.2%) developed treatment-associated hypertension. For the overall cohort, the frequency of grade 3 or higher toxicities was similar between ASI users and non-users, except hypertension which was more common in the ASI users (17.82% versus 4.77%) (Table 4). For the total cohort, the frequency of cardiac and thromboembolic events was also similar between groups. In patients receiving VEGF-targeted therapy, hypertension was more frequent in ASI users, while other adverse events were similar between the groups. In patients receiving mTOR-targeted therapy, hyperglycemia was more frequent in ASI users.
Table 4.
Adverse events.
| ASI Users (n=1487) | ASI Non-Users (n=3249) | Total (n=4736) | |
|---|---|---|---|
| Total Cohort | |||
| Selected adverse events (any grade) | |||
| Angioedema | 2 (0.13%) | 2 (0.06%) | 4 (0.0.84%) |
| Arterial thrombosis | 0 (0%) | 0 (0%) | 0 (0%) |
| Cardiac ischemia/infarction | 19 (1.28%) | 22 (0.68%) | 41 (0.87%) |
| Cerebrovascular event | 55 (3.70%) | 86 (2.65%) | 141 (2.98%) |
| Cough | 349 (23.47%) | 681 (20.96%) | 1030 (21.74%) |
| Hyperkalemia | 86 (5.78%) | 138 (4.25%) | 224 (4.73%) |
| Hypertensive crisis | 8 (0.54%) | 8 (0.25%) | 16 (0.34%) |
| Renal Insufficiency | 67 (4.51%) | 71 (2.19%) | 138 (2.91%) |
| Most frequent grade 3-5 adverse events (observed in >3% of patients) | |||
| Fatigue | 155 (10.42%) | 309 (9.51%) | 464 (9.80%) |
| Hypertension | 265 (17.82%) | 155 (4.77%) | 420 (8.87%) |
| Anemia | 84 (5.65%) | 279 (8.59%) | 363 (7.66%) |
| Asthenia | 85 (5.72%) | 186 (5.72%) | 271 (5.72%) |
| Hand-foot syndrome | 90 (6.05%) | 177 (5.45%) | 267 (5.64%) |
| Diarrhea | 109 (7.33%) | 147 (4.52%) | 256 (5.41%) |
| Dyspnea | 56 (3.77%) | 137 (4.22%) | 193 (4.08%) |
| Neutropenia | 49 (3.30%) | 137 (4.22%) | 186 (3.93%) |
| Disease progression | 36 (2.42%) | 149 (4.59%) | 185 (3.91%) |
| Proteinuria | 65 (4.37%) | 94 (2.89%) | 159 (3.36%) |
| Decreased appetite | 42 (2.82%) | 99 (3.05%) | 141 (2.98%) |
| Patients receiving VEGF-targeted therapy | |||
| Most frequent grade 3-5 adverse events (observed in >3% of patients) | |||
| Hypertension | 261 (21.89%) | 150 (6.47%) | 411 (11.70%) |
| Fatigue | 104 (8.72%) | 187 (8.06%) | 291 (8.29%) |
| Hand-foot syndrome | 90 (7.55%) | 174 (7.50%) | 264 (7.52%) |
| Diarrhea | 103 (8.64%) | 126 (5.43%) | 229 (6.52%) |
| Asthenia | 64 (5.37%) | 133 (5.74%) | 197 (5.61%) |
| Anemia | 48 (4.03%) | 123 (5.30%) | 171 (4.87%) |
| Disease progression | 33 (2.77%) | 133 (5.74%) | 166 (4.73%) |
| Proteinuria | 64 (5.37%) | 93 (4.01%) | 157 (4.47%) |
| Neutropenia | 41 (3.44%) | 90 (3.88%) | 131 (3.73%) |
| Dyspnea | 29 (2.43%) | 84 (3.62%) | 113 (3.22%) |
| Hyponatremia | 43 (3.61%) | 65 (2.80%) | 108 (3.08%) |
| Patients receiving mTOR-targeted therapy | |||
| Most frequent grade 3-5 adverse events (observed in >3% of patients) | |||
| Anemia | 33 (21.85%) | 129 (25.09%) | 162 (24.35%) |
| Fatigue | 20 (13.24%) | 63 (12.25%) | 83 (12.48%) |
| Hyperglycemia | 19 (12.58%) | 34 (6.61%) | 53 (7.97%) |
| Dyspnea | 15 (9.93%) | 37 (7.20%) | 52 (7.82%) |
| Asthenia | 5 (3.31%) | 29 (5.64%) | 34 (5.11%) |
| Lymphopenia | 3 (1.97%) | 31 (6.03%) | 34 (5.11%) |
| Hypophosphatemia | 7 (4.64%) | 26 (5.06%) | 33 (4.96%) |
| Neutropenia | 4 (2.65%) | 28 (5.45% | 32 (4.81%) |
| Hypertriglyceridemia | 8 (5.30%) | 21 (4.09%) | 29 (4.36%) |
| Decreased appetite | 6 (3.97%) | 20 (3.89%) | 26 (3.91%) |
| Abdominal pain | 2 (1.32%) | 23 (4.47%) | 25 (3.76%) |
| Pneumonia | 3 (1.99%) | 21 (4.09%) | 24 (3.61%) |
| Alkaline phosphatase increase | 6 (3.97%) | 17 (3.31%) | 23 (3.46%) |
| Dehydration | 2 (1.32%) | 21 (4.09%) | 23 (3.46%) |
| Thrombocytopenia | 9 (5.96%) | 14 (2.72%) | 23 (3.46%) |
| Stomatitis | 5 (3.31%) | 16 (3.11%) | 21 (3.16%) |
| Diarrhea | 5 (3.31%) | 15 (2.92%) | 20 (3.01%) |
ASI = angiotensin system inhibitors.
Cell viability assay
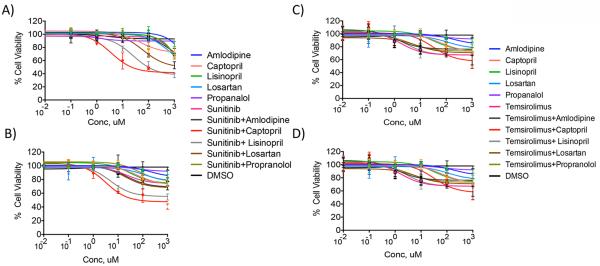
Cell viability assays were conducted in 769-P and A-498 RCC cells which mimic clear cell RCC and have previously demonstrated expression of angiotensin II receptor, type I.(29, 30) Physiologic concentrations of sunitinib are near 10 μM. The combination of sunitinib with captopril decreased cell viability to 59.3%, 48.1%, and 36.8% at 10, 100 and 1000 μM concentrations in A-498 RCC cells (Figure 3A). The combination of sunitinib with lisinopril decreased cell viability to 78.2%, 52.7%, and 37.2% at 10, 100 and 1000 μM concentrations. Additionally, the combination of sunitinib with losartan at 100 μM and above concentrations significantly decreased A-498 RCC cell viability (68.2% and 52.8% at 100 and 1000 μM). A statistically significant decrease in cell viability was not detected with either agent alone at concentrations below 1000 μM compared to DMSO.
Figure 3.
Cell viability assays of various anti-hypertensive agents with or without sunitinib at differing concentrations on A-498 (A) and 769-P (B) RCC cells and various anti-hypertensive agents with or without temsirolimus at differing concentrations on A-498 (C) and 769-P (D) RCC cells. The combination of sunitinib with captopril or lisinopril at 10 μM and above concentrations significantly decreased A-498 and 769-P RCC cell viability when compared to DMSO.
A similar trend was observed in 769-P RCC cells (Figure 3B). The combination of sunitinib with captopril or lisinopril at 10 μM and above concentrations significantly decreased 769-P RCC cell viability compared to DMSO. The combination of sunitinib with captopril decreased cell viability to 61.4%, 56.8%, and 40.6% at 10, 100 and 1000 μM concentrations in 769-P cells. The combination of sunitinib with lisinopril decreased cell viability to 66.8%, 59.8%, and 54.5% at 10, 100 and 1000 μM concentrations in 769-P cells. A statistically significant decrease in cell viability was not detected with either agent alone at 10 and 100 μM concentrations compared to DMSO. No decrease in cell viability was observed when either cell line was incubated with amlodipine or propranolol alone or in combination with sunitinib at concentrations below 1000 μM compared to DMSO.
Similar studies were conducted with temsirolimus in 769-P and A-498 RCC cells (Figure 3C and 3D). Physiologic concentrations of temsirolimus are near 0.10 μM. In both cell lines, the combination of temsirolimus with captopril significantly decreased cell viability only at higher dose concentrations compared to DMSO (769-P RCC cells: 70.8% and 56.9% at 100 and 1000 μM; A-498 RCC cells: 63.5% and 57.8% at 100 and 1000 μM). Temsirolimus combined with lisinopril or losaratan did not decreased cell viability at concentrations below 1000 μM compared to DMSO (769-P RCC cells: 62.3% at 1000 μM lisinopril, 62.6% at 1000 μM losartan; A-498 RCC cells: 67.8% at 1000 μM lisinopril, 66.5% at 1000 μM losartan).
Discussion
This is the largest analysis to date evaluating the impact of ASIs on outcomes, not just in mRCC, but in any type of cancer. To conduct our analysis, we used a large and robust clinical trials database containing over 4,700 patients treated with a broad range of systemic therapies in the modern era. The database contains prospectively collected information, providing a valuable resource for the evaluation of patient characteristics and outcomes.
In our analysis, we demonstrate that ASI users had an improved survival compared to ASI non-users in patients with mRCC. There have been limited studies investigating the role of ASIs in patients with mRCC. Keizman and colleagues were the first to demonstrate the clinical benefit of ASIs in patients with mRCC treated with VEGF-targeted therapy.(31) In a small retrospective study of 127 patients with mRCC treated with sunitinib, ASI users had an improved PFS (HR 0.537, p=0.0055, medians of 13 versus 6 months) and a non-statistically significant improvement in OS (HR 0.688, p=0.21, medians of 30 versus 23 months) compared to ASI non-users.
We hypothesize that the improved survival may be related to the ability of ASIs to synergize with anti-angiogenics to inhibit tumor cell proliferation and angiogenesis.(32) Angiotensin II via binding to the angiotensin II receptors in tumor cells can activate tumor cell proliferation via the PI3K/AKT pathway and epithelial growth factor receptor trans-activation.(33, 34) Additionally, angiotensin II can increase expression and production of VEGF, which is hypothesized to be trigged by stabilization of hypoxia inducible factor, and promoting angiogenesis.(35)
To investigate the interaction between ASIs and mRCC therapy, we conducted a subset analysis evaluating the impact of ASIs in patients receiving VEGF-targeted, mTOR-targeted, or IFN-α therapy. In this analysis, we demonstrated that ASI use was associated with a significant improvement in OS for patients receiving VEGF-targeted therapy only. We postulate that this is likely related to a synergistic interaction between ASIs and VEGF-targeted therapy given that these agents are thought, at least in part, to augment a similar pathway. Several studies have evaluated the role of ASIs alone in RCC mouse models.(36-38) Araujo and colleagues evaluated the losartan, captopril or the combination in vivo and demonstrated decreased tumor proliferation with renin-angiotensin system blockade.(36) Other studies demonstrated that captopril resulted in significant dose-related reduction in tumor development and candesartan dramatically prevented the development of metastatic lung nodules and inhibited neovascularization and VEGF expression in a RCC mouse xenograft model.(37, 38) Preclinical studies involving other cancer types have demonstrated similar effects with ASI treatment.(4) Recently, Verhoest and colleagues reported that combination therapy with sunitinib and telmisartan, an ARB, resulted in slower tumor growth, increased tumor necrosis, decreased central microvascular density, and decreased circulating VEGF levels in a murine xenograft model of RCC.(30)
In this study, we demonstrate that ASIs when combined with sunitinib significantly decrease cell viability of RCC cells in vitro, and effect which was not seen with temsirolimus or with either agent alone, suggesting synergy between agents. Though prior studies have demonstrated that sunitinib at physiologic concentrations acts primarily on tumor endothelium rather than tumor cells to inhibit RCC growth, we demonstrate that at physiologic concentrations of the combination of sunitinib and captopril or lisinopril cell viability is decreased.(39) Though not statistically significant, ORR were higher in ASI users compared to non-users, and thus further preclinical investigations are necessary to explore the interactions of both agents on tumor cells and the tumor microenvironment.
To distinguish from prior preclinical work, in this study we utilized two distinct RCC cells lines and evaluated a broad spectrum of anti-hypertensives including ASIs, beta blockers, and calcium-channel blockers. Further studies are warranted to investigate the effect of combination therapy on angiogenesis and cell signaling. This type of work will help illuminate the potential mechanisms underlying our clinical observations
Hypertension is commonly associated with VEGF blockade, as demonstrated by recent studies in mRCC documenting an incidence of 30-40%. It is postulated that treatment-associated hypertension develops secondary to decreased nitric oxide and increased endothelin-1 production resulting in vasoconstriction, decreased endothelial cell viability, and vessel rarefaction.(40) Growing evidence suggests that treatment-associated hypertension is a clinical biomarker of efficacy in patients receiving angiogenesis-targeted therapy.(41, 42) Rini and colleagues conducted a retrospective analysis of 544 patients with mRCC treated with sunitinib which demonstrated that the development of treatment-associated hypertension was associated with significantly improved PFS (p<0.001, medians of 12.5 versus 2.5 months,) and OS (p<0.001, medians of 30.9 versus 7.2 months), while adverse events related to hypertension were similar between the two groups.(41) In our analysis, we confirm that the OS for patients who develop treatment-associated hypertension was higher compared to the overall cohort (33.19 versus 26.64 months for ASI users who developed treatment-associated hypertension on VEGF-targeted therapy compared to ASI users in the overall cohort). To account for treatment-associated hypertension as a potential confounder, we conducted a separate analysis in patients receiving VEGF-targeted therapy who developed treatment-associated hypertension (n=2,466). Despite the development of treatment-associated hypertension, we demonstrated that there was an OS benefit specifically attributable to ASI use in these patients, which was not attributable to other anti-hypertensive agents or the sole effect of developing hypertension.
Though this is the largest database using prospectively collected clinical trials information to assess the impact of ASIs in cancer patients in general and in mRCC patients specifically, there are several limitations. All patients in the database were enrolled in clinical trials, which could lead to decreased generalizability of the study findings, given that patients with severe comorbidities, unstable medical conditions, or poor performance status are typically excluded from therapeutic clinical trials. Additionally, data collection was not specifically designed to examine ASI use. Furthermore, there was significant variability with regard to choice of ASI and type of mRCC therapy received. Indications for ASI treatment and modifications were at the discretion of the treating physician and were not collected in the database. In addition, data was lacking regarding dosing, schedule and duration of ASI use, though we presume on-label usage of these commonly utilized agents. Finally, though patients receiving a broad range of targeted therapies were included in the analysis, not every approved agent to treat mRCC was represented. Given these limitations, specific recommendations about ASI use in patients with mRCC receiving targeted therapy cannot be made based on the results of this analysis.
In conclusion, we demonstrate that ASI use improved survival outcomes in patients with mRCC treated in the era of targeted therapy. Though hypertension is a known on-target effect and a demonstrated clinical biomarker of efficacy to VEGF-targeted therapy, we show that even in cancer patients who develop treatment-associated hypertension secondary to VEGF blockade, ASI users experience an OS and PFS benefit compared to ASI non-users. We do not believe a randomized prospective clinical trial of a VEGF inhibitor with and without an ASI is easy to conduct in this population. Though our study was based on a pooled analysis of prospectively collected data, further studies are warranted to verify our observations and inform the clinical practice of ASI use in combination with VEGF targeted agents in patients with mRCC. Furthermore, we showed that ASIs have a synergistic effect with sunitinib on cell viability and additional preclinical studies are required to investigate the mechanisms underlying this interaction.
Translational Relevance.
Advances in the understanding of the molecular biology of renal cell carcinoma (RCC) have identified angiogenesis as a key factor in the pathogenesis of the disease. A major component of the angiogenic process in RCC is vascular endothelial growth factor (VEGF). Growing evidence suggests that angiotensin II, via activation of the angiotensin II receptor, plays a critical role in VEGF-dependent angiogenesis and key steps in carcinogenesis including cell proliferation and migration. Angiotensin II blockade by broadly utilized angiotensin system inhibitors may be a potential therapeutic strategy for patients with metastatic RCC. Elucidation of the impact of angiotensin system inhibitors on survival outcomes in patients with RCC is therefore highly relevant to optimizing the current treatment paradigm for patients with metastatic RCC.
Acknowledgements
The authors thank the patients and investigators who participated in the clinical trials used for this analysis.
Funding: This study was funded by Pfizer, Inc. Additionally, this research was funded in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE, and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at Dana-Farber Cancer Institute for Toni K. Choueiri.
Footnotes
Disclosures: Toni K. Choueiri has received research funding from Pfizer and has an advisory role at Aveo, Pfizer, Novartis, GlaxoSmithKline, Genentech, and Bayer and Onyx. Rana R. McKay has received research funding from Pfizer and Bayer. He is not involved in any Speaker Bureau. Venkata S. Sabbisetti is a consultant to Millipore. Gustavo E. Rodriguez, Xun Lin and Ronit Simantov are employees at Pfizer. The remaining authors have no disclosures.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodfriend TL, Elliott ME, Catt KJ. Angiotensin receptors and their antagonists. N Engl J Med. 1996;334(25):1649–54. doi: 10.1056/NEJM199606203342507. [DOI] [PubMed] [Google Scholar]
- 3.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16(7):293–9. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: a review. J Hum Hypertens. 2009;23(10):623–35. doi: 10.1038/jhh.2009.21. [DOI] [PubMed] [Google Scholar]
- 5.Norwood VF, Craig MR, Harris JM, Gomez RA. Differential expression of angiotensin II receptors during early renal morphogenesis. Am J Physiol. 1997;272:R662–8. doi: 10.1152/ajpregu.1997.272.2.R662. 2 Pt 2. [DOI] [PubMed] [Google Scholar]
- 6.Weerackody RP, Chatterjee PK, Mistry SK, McLaren J, Hawksworth GM, McLay JS. Selective antagonism of the AT1 receptor inhibits the effect of angiotensin II on DNA and protein synthesis of rat proximal tubular cells. Exp Nephrol. 1997;5(3):253–62. [PubMed] [Google Scholar]
- 7.Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112(1):67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolley-Hitze T, Jouan F, Martin B, Mottier S, Edeline J, Moranne O, et al. Angiotensin-2 receptors (AT1-R and AT2-R), new prognostic factors for renal clear-cell carcinoma? Br J Cancer. 2010;103(11):1698–705. doi: 10.1038/sj.bjc.6605866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352(9123):179–84. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 10.Mc Menamin UC, Murray LJ, Cantwell MM, Hughes CM. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control. 2012;23(2):221–30. doi: 10.1007/s10552-011-9881-x. [DOI] [PubMed] [Google Scholar]
- 11.Barrios CH, Hernandez-Barajas D, Brown MP, Lee SH, Fein L, Liu JH, et al. Phase II trial of continuous once-daily dosing of sunitinib as first-line treatment in patients with metastatic renal cell carcinoma. Cancer. 2012;118(5):1252–9. doi: 10.1002/cncr.26440. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(25):4068–75. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 13.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 14.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(8):760–7. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. The Lancet Oncology. 2013;14(13):1287–94. doi: 10.1016/S1470-2045(13)70465-0. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30(12):1371–7. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014;32(8):752–9. doi: 10.1200/JCO.2013.50.5305. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 22.Rini BI, Melichar B, Ueda T, Grunwald V, Fishman MN, Arranz JA, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. The Lancet Oncology. 2013;14(12):1233–42. doi: 10.1016/S1470-2045(13)70464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(8):2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 25.Bendtsen F, Henriksen JH. Comparative pharmacokinetics and pharmacodynamics of lisinopril and enalapril, alone and in combination with propranolol. J Hum Hypertens. 1989;3(Suppl 1):139–45. [PubMed] [Google Scholar]
- 26.Carvalho M, Oliveira CH, Mendes GD, Sucupira M, Moraes ME, De Nucci G. Amlodipine bioequivalence study: quantification by liquid chromatography coupled to tandem mass spectrometry. Biopharm Drug Dispos. 2001;22(9):383–90. doi: 10.1002/bdd.282. [DOI] [PubMed] [Google Scholar]
- 27.Kim A, Balis FM, Widemann BC. Sorafenib and sunitinib. Oncologist. 2009;14(8):800–5. doi: 10.1634/theoncologist.2009-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onoyama K, Hirakata H, Iseki K, Fujimi S, Omae T, Kobayashi M, et al. Blood concentration and urinary excretion of captopril (SQ 14,225) in patients with chronic renal failure. Hypertension. 1981;3(4):456–9. doi: 10.1161/01.hyp.3.4.456. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko G, Miyajima A, Kosaka T, Mizuno R, Kikuchi E, Oya M. mTOR pathway regulates the expression of angiotensin 2 type 1 receptor in renal cell carcinoma (abstract 310) The Journal of Urology. 2013;189(4):e126. [Google Scholar]
- 30.Verhoest G, Dolley-Hitze T, Jouan F, Belaud-Rotureau MA, Oger E, Lavenu A, et al. Sunitinib combined with angiotensin-2 type-1 receptor antagonists induces more necrosis: a murine xenograft model of renal cell carcinoma. BioMed research international. 2014;2014:901371. doi: 10.1155/2014/901371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, et al. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer. 2011;47(13):1955–61. doi: 10.1016/j.ejca.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg H, Nielsen D, Jensen BV, Eriksen J, Skovsgaard T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncol. 2004;43(2):142–52. doi: 10.1080/02841860310022346. [DOI] [PubMed] [Google Scholar]
- 33.Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, Fujinami K, et al. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Molecular cancer therapeutics. 2003;2(11):1139–47. [PubMed] [Google Scholar]
- 34.Zhao Y, Chen X, Cai L, Yang Y, Sui G, Fu S. Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF-7 breast cancer cells survival via PI3-kinase/Akt pathway. J Cell Physiol. 2010;225(1):168–73. doi: 10.1002/jcp.22209. [DOI] [PubMed] [Google Scholar]
- 35.Kosaka T, Miyajima A, Shirotake S, Kikuchi E, Hasegawa M, Mikami S, et al. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2010;70(2):162–9. doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 36.Araujo W, Naves M, Ravanini J, Teixeira V. Renin angiotensin system (RAS) blockade attenuates growth and metastasis formation of renal cell carcinoma in mice. Ann Oncol. 2014;25(suppl_4):iv53–iv7. doi: 10.1016/j.urolonc.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Hii SI, Nicol DL, Gotley DC, Thompson LC, Green MK, Jonsson JR. Captopril inhibits tumour growth in a xenograft model of human renal cell carcinoma. Br J Cancer. 1998;77(6):880–3. doi: 10.1038/bjc.1998.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyajima A, Kosaka T, Asano T, Seta K, Kawai T, Hayakawa M. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62(15):4176–9. [PubMed] [Google Scholar]
- 39.Huang Y, Wang Y, Li Y, Guo K, He Y. Role of sorafenib and sunitinib in the induction of expressions of NKG2D ligands in nasopharyngeal carcinoma with high expression of ABCG2. J Cancer Res Clin Oncol. 2011;137(5):829–37. doi: 10.1007/s00432-010-0944-2. [DOI] [PubMed] [Google Scholar]
- 40.Bair SM, Choueiri TK, Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23(4):104–13. doi: 10.1016/j.tcm.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rini BI, Schiller JH, Fruehauf JP, Cohen EE, Tarazi JC, Rosbrook B, et al. Diastolic blood pressure as a biomarker of axitinib efficacy in solid tumors. Clin Cancer Res. 2011;17(11):3841–9. doi: 10.1158/1078-0432.CCR-10-2806. [DOI] [PubMed] [Google Scholar]