Abstract
Background
Understanding the mechanisms driving disease progression is fundamental to identifying new therapeutic targets for the treatment of men with metastatic castration-resistant prostate cancer (mCRPC). Due to the prevalence of bone metastases in mCRPC, obtaining sufficient tumor tissue for analysis has historically been a challenge. In this exploratory analysis, we evaluated imaging, procedural, and clinical variables associated with tumor yield on image-guided bone biopsy in men with mCRPC.
Methods
Clinical data were collected prospectively from men with mCRPC enrolled on a phase II trial with serial metastasis biopsies performed according to standard clinical protocol. Imaging was retrospectively reviewed. We evaluated the percent positive biopsy cores (PPC), calculated as the number of positive cores divided by the total number of cores collected per biopsy.
Results
Twenty-nine men had 39 bone biopsies. Seventy-seven percent of bone biopsies had at least 1 positive biopsy core. We determined that lesion size and distance from the skin to the lesion edge correlated with tumor yield on biopsy (median PPC 75% versus 42% for lesions > 8.8 cm3 versus ≤ 8.8 cm3, respectively, p=0.05; median PPC 33% versus 71% for distance ≥ 6.1 versus < 6.1 cm, respectively, p=0.02). There was a trend towards increased tumor yield in patients with increased uptake on radionuclide bone scan, higher calcium levels, and shorter duration of osteoclast-targeting therapy, though this was not statistically significant. Ten men had 14 soft tissue biopsies. All soft tissue biopsies had at least 1 positive biopsy core.
Conclusions
This exploratory analysis suggests there are imaging, procedural, and clinical variables which impact image-guided bone biopsy yield. In order to maximize harvest of prostate cancer tissue, we have incorporated a prospective analysis of the metrics described here as part of a multi-institutional project aiming to use molecular characterization of mCRPC tumors to direct individual therapy.
Keywords: Bone, Castration-resistant prostate cancer, Genomics, Metastasis biopsy, Tumor yield
Introduction
Prostate cancer is a heterogeneous disease with marked variability in patient outcomes. Though curative treatments are available, over 29,000 men per year die from prostate cancer.(1) To identify drivers of resistance and novel therapeutic targets, a better understanding of the genomic landscape of metastatic castration-resistance prostate cancer (mCRPC) is essential. Over the past decade, new laboratory techniques have accelerated the molecular characterization of prostate cancer. Currently underway is a large multi-institutional project entitled “Precision Therapy in Advanced Prostate Cancer” funded by Stand Up To Cancer (SU2C) that uses next-generation sequencing technologies for the molecular characterization of mCRPC tumors isolated from metastasis biopsies.
A key initial step in the molecular characterization of mCRPC involves isolation of high-quality nucleic acid from prostate cancer cells. However, acquiring sufficient tumor tissue for molecular characterization often represents a challenge due to the paucity of soft tissue metastases. Additionally, prostate cancer metastases to bone frequently cause a dense sclerotic reaction and biopsies are technically challenging and associated with low yield, leading to insufficient tumor tissue for sequencing analysis.(2)
Strategies to improve tumor yield on bone biopsy in mCRPC have been limited.(3-6) The largest study comes from a 26-center trial of 184 men with mCRPC which demonstrated that 25.5% of non-image guided bone marrow biopsies were positive for tumor by histology.(7) Another study of 31 men with mCRPC undergoing 54 image-guided biopsies showed that 67% of samples were positive by histology.(8)
Collecting tissue samples necessitates the development of practical methods to optimize tumor acquisition, a process which involves collaboration between radiologists, pathologists, and investigators. Given limited studies delineating strategies to maximize tumor yield on bone biopsy, we embarked on this exploratory analysis to better inform tissue collection as part of the large multi-institutional project described above. In this pilot study, we evaluate variables associated with tumor yield on image-guided bone biopsy in mCRPC.
Patients and methods
Patient population
Data from a phase II trial of abiraterone and dutasteride in men with mCRPC undergoing metastasis biopsies were used (NCT01393730). This trial enrolled 40 men with mCRPC at 3 centers: Dana-Farber Cancer Institute, Beth Israel Deaconess Medical Center, and University of Washington. The primary endpoint was to analyze mechanisms of abiraterone resistance using serial metastasis biopsies.
Patients had a mandatory baseline research biopsy of a soft tissue or bone metastasis. They were then treated with abiraterone (1,000mg daily) and prednisone (5mg daily) for two 28-day cycles, after which dutasteride (3.5mg daily) was added. Patients were continued on the three-drug regimen until study withdrawal or tumor progression (defined by Response Evaluation Criteria In Solid Tumors version 1.1 for measurable disease and Prostate Cancer Working Group-2 for non-measurable disease).(9, 10) Mandatory repeat metastasis research biopsy was obtained at progression, in patients completing at least 4 treatment cycles. At the time of this analysis, 20 men remained on treatment.
Overall, 29 men had 39 bone biopsies, (30 baseline and 9 progression), which were included in the analysis. Ten men underwent 14 soft tissue biopsies (9 baseline and 5 progression), which were not included in the analysis, though pathologic information was summarized.
Biopsy procedure
Prior to each biopsy, informed consent was obtained. Biopsy site selection was determined by an interventional radiologist in consultation with the treating physician. Sites were selected based on clinical judgment and safety. Eligible soft tissue biopsy sites included lymph nodes or visceral metastases and biopsies were performed under ultrasound or computed tomography (CT) guidance. Eligible bone biopsy sites included vertebrae, pelvis, and long bones, and biopsies were performed using CT guidance. Sites of prior radiation were excluded. Immediate cytologic evaluation was not performed. The progression biopsy site was not mandated to be the same as the baseline site. Neither the radiologist nor treating physician had knowledge of tumor yield from the baseline research biopsy when considering the progression biopsy site.
Radiologic and clinical variables
Prior to biopsy, all patients had CT and 99mTc-methylene diphosphonate bone scans. The baseline biopsy was obtained within 28 days of screening imaging and the progression biopsy was obtained within 14 days of imaging confirming progression. For this analysis, imaging was reviewed retrospectively by a radiologist blinded to the pathology results and assessed for the following biopsy lesion variables: 1) anatomic location, 2) size (length, width, and height), 3) qualitative attenuation subjectively graded as sclerotic, mixed, or lytic, 4) quantitative attenuation defined by Hounsfield units (HU) rounded to the nearest 50 HU, 5) distance from the cortical surface to the lesion edge measured in centimeters (cm) along the needle track, 6) distance from the skin to the lesion edge measured in cm along the needle track, and 7) location of sampling within the lesion (center, periphery, or both). Quantitative attenuation was determined as an average of three measurements at the biopsy site. For focal lesions, the entire lesion was measured, and for diffuse lesions, an area of approximately 2 cm2 corresponding to the biopsy site was measured. The intensity of radiotracer uptake in the lesion on bone scan was documented as no uptake or mild, moderate, or marked uptake using bladder radioactivity as a reference. Biopsy needle gauge was recorded from the procedure documentation.
Baseline demographic, clinical and laboratory data including age, months since diagnosis, performance status, location of metastases (bone only or bone plus other), prostate-specific antigen (PSA), calcium, alkaline phosphatase, hemoglobin, and platelet count were collected prospectively. Gleason score, prior therapy (prostatectomy, radiotherapy, chemotherapy), and osteoclast-targeting therapy were collected. Trial eligibility included normal marrow function with platelets ≥100,000/uL and hemoglobin ≥10 g/dL.
Pathology review
Sections from each core were stained with hematoxylin and eosin (H-E). A pathologist assessed each specimen for the presence of prostate cancer cells by light microscopy. A biopsy core was considered positive if tumor cells were morphologically identified on H-E. Specimens were not analyzed for the presence of necrosis.
Statistical analyses
Variables at the time of bone biopsy were summarized using numbers and percentages for categorical variables and medians and interquartile ranges for continuous variables. To account for the degree of tumor burden, we evaluated the percent positive biopsy cores (PPC), calculated as the number of positive cores divided by the total number of cores collected per bone biopsy, as opposed to describing a biopsy as positive or negative. We analyzed baseline and progression biopsies at separate time points given pathology from baseline biopsies was not available at the time of progression biopsy (4/9 progression biopsies were performed on the same lesion as the baseline biopsy). All continuous variables were dichotomized by the median into two groups, except for quantitative attenuation, given that this would have resulted in a greater number of patients with HU in the sclerotic range in both categories. A logistic regression model using the Williams methods with a quasi-likelihood approach for handling over-dispersion was used to assess the association of variables and positive extraction of tumor tissue from each individual core. This approach accounted for the correlation among cores taken within each biopsy. For imaging and procedural variables, analyses were conducted on baseline and progression biopsies. For clinical variables, analyses were conducted on baseline biopsies only. Soft tissue biopsies were not included in the analysis.
Results
Imaging, procedural, and pathologic characteristics
The majority of bone biopsies were collected from the pelvis (69%) (Table 1, Figure 1). Most lesions were sclerotic (59%) and had moderate uptake on bone scan (41%). Biopsies were collected predominately from both the center and periphery (44%) of each lesion and needle gauge varied (11-18 gauge). At least 3 cores were obtained in the majority of cases (80%). In total, 30 bone biopsies (77%) had at least 1 core which was positive for tumor. Of the 129 cores obtained from bone, 64 (50%) were positive for tumor. There were no immediate procedural complications.
Table 1.
Baseline and progression bone biopsy imaging, procedural, and pathologic characteristics.
| Number of Biopsies (n=39) |
Median (q1-q3) or Percent | |
|---|---|---|
| Imaging Characteristics | ||
| Lesion location Pelvis Femur Spine |
27 7 5 |
69% 18% 13% |
| Lesion size (cm3) | 39 | 8.79 (4.86-35.11) |
| Quantitative attenuation (HU) | 39 | 600 (400-800) |
| Qualitative attenuation Sclerotic Mixed Lytic |
23 14 2 |
59% 36% 5% |
| Distance from skin to lesion edge (cm) | 39 | 6.1 (4.4-7.5) |
| Distance from cortex to lesion edge (cm) | 39 | 1.5 (0-2.5) |
| Radionuclide bone scan correlate Marked uptake Moderate uptake Mild uptake No uptake |
14 16 7 2 |
36% 41% 18% 5% |
| Procedural Characteristics | ||
| Location of lesion sampling Center Periphery Both |
16 6 17 |
41% 15% 44% |
| Needle gauge 11 13 14 18 Unknown |
12 6 1 2 18 |
31% 15% 3% 5% 46% |
| Number of cores sampled per biopsy 1 2 3 4 5 |
4 4 13 12 6 |
10% 10% 33% 31% 15% |
| Pathologic Characteristics | ||
| Number of cores positive per biopsy 0 1 2 3 4 |
9 9 11 7 3 |
23% 23% 28% 18% 8% |
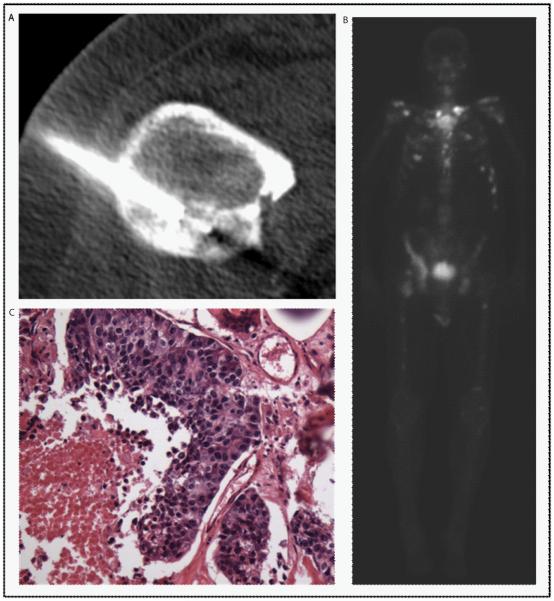
Figure 1.
CT (A), bone scan (B), and pathology (C) images from a CT-guided biopsy of a sclerotic, right lesser trochanter lesion, measuring 4.1 × 3.2 × 3.4 cm obtained at progression on abiraterone. The bone scan showed mild radiotracer uptake at the site of the lesion targeted for biopsy. The pathology was positive for tumor by light microscopy.
Baseline clinical characteristics
The median age was 66 years (Table 2). The majority of men had a Gleason score ≥8 at diagnosis (55%). 62% of men received an osteoclast-targeting agent (n=18), with all but 3 continuing treatment at the time of baseline biopsy. Of the 11 patients with available dosing information, all received treatment within 3 months of the baseline biopsy. Thirteen men received a bisphosphonate, 4 received denosumab, and 1 received both. The bisphosphonates used included zoledronic acid (n=13), alendronate (n=1), and pamidronate (n=1). The median duration of therapy was 10 months. The majority of patients had mild anemia and otherwise normal platelets, alkaline phosphatase, and calcium.
Table 2.
Baseline clinical characteristics of the men who underwent bone biopsy.
| Clinical Characteristics | Number of Men (n=29)1 |
Median (q1-q3) or Percent |
|---|---|---|
| Age (years) | 29 | 66 (63-73) |
| Months from diagnosis Unknown |
28 1 |
57 (15-144) |
| Gleason score at diagnosis ≤ 6 7 ≥ 8 |
5 8 16 |
17% 28% 55% |
| Prior therapy Radical prostatectomy Radiotherapy Chemotherapy |
12 16 6 |
41% 55% 21% |
| Osteoclast-targeting therapy Yes No |
18 11 |
62% 38% |
| Duration of osteoclast-targeting therapy (months) Unknown |
19 10 |
10 (6-33) |
| ECOG performance status 0 1 |
26 3 |
90% 10% |
| Sites of metastatic disease Bone only Bone and other2 Unknown |
21 4 4 |
72% 14% 14% |
| PSA (ng/dL) | 29 | 24.4 (8.3-40.01) |
| Hemoglobin (g/dL) | 29 | 12.9 (12.5-13.6) |
| Platelets (per uL) | 29 | 228 (185-253) |
| Alkaline phosphatase (mg/dL) | 29 | 98 (69-165) |
| Calcium (mg/dL) Unknown |
25 4 |
9.5 (9.1-9.7) |
ECOG=Eastern Cooperative Oncology Group, PSA=prostate-specific antigen.
A total of 29 men underwent 39 biopsies (30 baseline and 9 progression). Listed here are the clinical characteristics of the 29 men.
Other includes sites of metastasis other then bone such as lymph nodes, soft tissue, liver, etc.
Associations between imaging and procedural variables and tumor yield
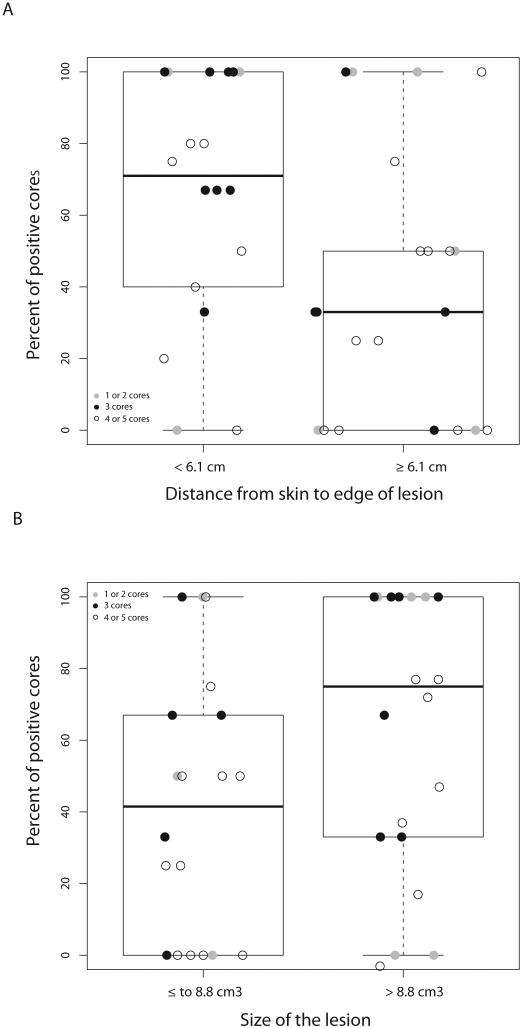
Biopsy yield was similar between groups irrespective of anatomic location, attenuation, distance from the cortex to the lesion edge, location of lesion sampling, and needle gauge (Table 3). There was a statistically significant correlation between lesion size and tumor yield (median PPC 75% versus 42% for lesions >8.8 cm3 versus ≤8.8 cm3, p=0.05) (Figure 2). Additionally, increased distance from the skin to the lesion edge was associated with decreased biopsy yield (median PPC 33% versus 71% for distance ≥6.1 versus <6.1 cm, p=0.02). There was a trend towards increased yield in patients with increased radionuclide uptake on bone scan (median PPC 67%, 50%, 33% for marked, moderate, and mild/no uptake, respectively), though this was not statistically significant (p=0.06). The number of cores collected was inversely related with tumor acquisition (median PPC 75%, 67%, and 45% for 1-2, 3, or 4-5 cores collected, respectively).
Table 3.
Associations between imaging and procedural variables on baseline and progression bone biopsies and tumor yield.
| Imaging and Procedural Variables |
Number of Biopsies1 |
Percent Positive Cores Median (q1-q3) |
Odds Ratio (95% CI) |
P- Value |
|---|---|---|---|---|
| Lesion location Pelvis Femur or spine |
27 12 |
50% (0%-100%) 42% (29%-78%) |
1.10 (0.40-3.03) Reference |
0.86 |
| Lesion size (cm3) ≤ 8.8 > 8.8 |
20 19 |
42% (0%-67%) 75% (3%-100%) |
0.39 (0.15-1.01) Reference |
0.05 |
| Quantitative attenuation (HU) ≤ 400 > 400 |
10 29 |
65% (50%-100%) 40% (0%-75%) |
1.77 (0.60-5.24) Reference |
0.30 |
| Qualitative attenuation Sclerotic Mixed or lytic |
23 16 |
50% (20%-100%) 50% (13%-78%) |
1.17 (0.45-3.04) Reference |
0.74 |
| Distance from skin to lesion edge (cm) < 6.1 ≥ 6.1 |
18 21 |
71% (40%-100%) 33% (0-50%) |
2.98 (1.16-7.64) Reference |
0.02 |
| Distance from cortex to lesion edge (cm) < 1.5 ≥ 1.5 |
19 20 |
50% (20%-80%) 50% (13-100%) |
0.92 (0.36-2.35) Reference |
0.86 |
| Radionuclide bone scan correlate Marked uptake Moderate uptake Mild or no uptake |
14 16 9 |
67% (50%-100%) 50% (0%-90%) 33% (20%-33%) |
4.54 (1.26-16.42) 1.93 (0.57-6.46) Reference |
0.06 |
| Location of lesion sampling Center Periphery or both |
16 23 |
65% (27%-100%) 50% (0%-75%) |
1.97 (0.75-5.2) Reference |
0.17 |
| Needle gauge1
11 13, 14 or 18 |
12 9 |
45% (13%-84%) 25% (0%-50%) |
1.46 (0.39-5.49) Reference |
0.58 |
There were a total of 39 baseline and progression biopsies, however needle gauge had missing information as detailed in Table 1.
Figure 2.
A. Percent positive cores (PPC) by lesion size. B. PPC by distance from the skin to the lesion edge. The box signifies the upper and lower quartiles, and the median is represented by the solid black line within the box. Outliers, defined as values above or below 1.5 × upper and lower quartiles, are represented by the dashed lines extending vertically.
Associations between clinical variables and tumor yield
Overall, tumor acquisition was similar in men irrespective of age, disease duration, Gleason score, prior therapy, performance status, or baseline metastatic sites (Table 4). In men receiving an osteoclast-targeting agent, there was a trend towards increased tumor yield with shorter duration of therapy (median PPC 78% in men receiving <10 months versus 0% in those receiving ≥10 months of treatment) (p=0.06). With regard to laboratory values, there was a trend towards increased yield with higher calcium, however, this was not statistically significant (median PPC 33% for <9.5 mg/dL versus 67% for ≥9.5 mg/dL, p=0.06). PSA, hemoglobin, platelets and alkaline phosphatase did not associate with tumor acquisition.
Table 4.
Associations between clinical variables and tumor yield on baseline bone biopsy.
| Clinical Variables | Number of Baseline Biopsies1 |
Percent Positive Cores Median (q1-q3) |
Odds Ratio (95% CI) |
P- Value |
|---|---|---|---|---|
| Age (years) ≤ 65 > 65 |
11 19 |
50% (0%-100%) 50% (25%-100%) |
0.80 (0.25-2.55) Reference |
0.70 |
| Months from diagnosis ≤ 57 > 57 |
15 15 |
50% (0%-80%) 67% (0%-100%) |
0.78 (0.26-2.36) Reference |
0.66 |
| Gleason score at diagnosis ≤ 6 7 ≥ 8 |
5 8 17 |
80% (0%-100%) 59% (42%-90%) 50% (0%-75%) |
1.45 (0.27-7.70) 1.67 (0.46-6.11) Reference |
0.72 |
| Radical prostatectomy No Yes |
19 11 |
42% (0%-100%) 67% (50%-100%) |
0.49 (0.16-1.54) Reference |
0.22 |
| Radiotherapy No Yes |
13 17 |
33% (0%-100%) 67% (33%-100%) |
0.51 (0.17-1.55) Reference |
0.24 |
| Chemotherapy No Yes |
24 6 |
45% (0%-00%) 63% (50%-100%) |
0.41 (0.10-1.67) Reference |
0.21 |
| Osteoclast-targeting therapy No Yes |
11 19 |
50% (25%-80%) 50% (0%-100%) |
0.84 (0.27-2.59) Reference |
0.75 |
| Duration of osteoclast-targeting therapy (months)1 < 10 months ≥ 10 months |
10 9 |
78% (50%-100%) 0% (0%-50%) |
4.10 (0.90-18.80) Reference |
0.06 |
| ECOG performance status 0 1 |
27 3 |
50% (0%-100%) 67% (0%-80%) |
0.77 (0.11-5.28) Reference |
0.79 |
| Sites of metastatic disease1
Bone and other Bone only |
4 22 |
65% (50%-90%) 50% (0%-100%) |
1.89 (0.34-10.55) Reference |
0.47 |
| PSA (ng/dL) ≤ 24.4 > 24.4 |
16 14 |
50% (13%-90%) 50% (25%-100%) |
0.98 (0.32-2.97) Reference |
0.97 |
| Hemoglobin (g/dL) ≤ 12.9 > 12.9 |
15 15 |
50% (25%-85%) 50% (0%-100%) |
0.93 (0.31-2.82) Reference |
0.90 |
| Platelets (per uL) ≤ 228 > 228 |
16 14 |
50% (0%-90%) 50% (25%-100%) |
0.97 (0.32-2.95) Reference |
0.92 |
| Alkaline phosphatase (mg/dL) ≤ 98 > 98 |
16 14 |
50% (13%-90%) 59% (0%-100%) |
0.89 (0.29-2.71) Reference |
0.84 |
| Calcium (mg/dL)1
< 9.5 ≥ 9.5 |
13 13 |
33% (0%-75%) 67% (50%-100%) |
0.33 (0.10-1.05) Reference |
0.06 |
ECOG=Eastern Cooperative Oncology Group, PSA=prostate-specific antigen.
A total of 29 men underwent 30 baseline biopsies, however several parameters had missing information as detailed in Table 2.
Soft tissue biopsies
Of the 14 soft tissue biopsies, 2 were of hepatic lesions, 1 was of a soft tissue lesion associated with a femoral metastasis, and the remaining were of lymph nodes. All 14 biopsies had at least 1 core which was positive for tumor. Of the 42 cores obtained, 86% (n=36) were positive for tumor.
Discussion
The need to interrogate the cellular and molecular nature of cancer is becoming increasingly important to understand emerging resistance mechanisms. Difficulties in obtaining tumor tissue from bone metastases have blunted efforts to characterize the genomic landscape in mCRPC. Given the importance of quality specimen collection, challenges associated with biopsy, and patient effort, we sought to describe variables that may predict increased tumor yield on bone biopsy. This analysis was exploratory in nature, but importantly sets the stage for future efforts to collect tissue for projects that aim to molecularly characterize mCRPC tumors to direct individual therapy.
We hypothesized that imaging variables associated with increased ease of biopsy would be associated with increased yield. In our analysis, lesion size and distance from the skin to the lesion were the two imaging variables which most strongly correlated with biopsy yield. This is consistent with prior data demonstrating that bone and soft-tissue lesions >5 cm result in increased diagnostic yield.(2) Lesion size and distance from the skin to the lesion warrant consideration when selecting the optimal bone biopsy site. We anticipated that biopsy of vertebral body lesions, which requires passage through the vertebral pedicle, would be more technically challenging resulting in decreased tumor yield. However, in our analysis, only 5 spine biopsies were performed, likely related to selection bias by the performing radiologist, limiting our ability to detect meaningful differences between groups. Based on prior studies, we predicted that sclerotic lesions would result in lower yield.(8, 11-14) Though we did not show a statistically significant correlation between attenuation and tumor yield, likely related to the small sample size of this cohort, lesions with greater attenuation (>400 HU) did have decreased PPC. In a retrospective analysis of 410 CT-guided biopsies of osseous lesions, imaging appearance was associated with biopsy accuracy (accuracy for sclerotic, lytic, or mixed lytic-sclerotic was 76%, 93%, and 92%, respectively).(13) This may be secondary to decreased cellularity associated with sclerotic lesions and increased risk of crush artifact, hampering diagnostic yield. Additionally, we hypothesized that increased radiotracer uptake on bone scan, suggestive of higher osteoblastic turnover and active disease, would be associated with increased yield. Our analysis did suggest such a trend and is the first to evaluate the value of this variable.
Furthermore, we hypothesized that peripheral lesion sampling, as opposed to central sampling, would result in increased tumor acquisition, given presumed increased cellularity at the stromal-tumor interface. However, in our limited cohort we did not observe this correlation. Of the six biopsies with only peripheral sampling, 3/15 cores contained tumor. Explanations include difficulty of peripheral targeting and inability to ascertain the exact location of sampling retrospectively. A previous study demonstrated that peripheral lesion sampling may be associated with increased yield (78% versus 52% for peripheral versus central sampling, p=0.24).(8)
Additionally, in our study, increased sampling seemed to correlate with decreased tumor yield. The exact reason for increased sampling was not specified in the procedure note; however, difficulty of biopsy could have resulted in increased biopsy attempts. Wu and colleagues previously described that the cumulative diagnostic yield for bone biopsy reached a plateau at the third specimen.(2)
With regard to soft tissue, all biopsies had at least 1 core which was positive for tumor. Though we did not review the variables associated with tumor yield for soft tissues lesions, overall pathologic yield was greater than that observed with bone. Given such, we recommend soft tissue as the preferred site of biopsy for molecular analyses, understanding that the biology of soft tissue and bone metastasis may differ and remains an unanswered question to be explored in future studies.
We hypothesized that clinical parameters associated with higher disease burden would predict increased yield, including increased disease sites, PSA, alkaline phosphatase, and calcium, and lower hemoglobin and platelets. Higher calcium seemed to correlate with increased biopsy yield; however, a correlation was not observed across the remaining clinical parameters. The lack of correlation may be, at least in part, due to our relatively small sample size and the fact that men were enrolled on a clinical trial requiring normal organ function and good performance status, limiting our ability to detect small differences between groups. In a trial of undirected bone marrow biopsies in mCRPC, lower hemoglobin and higher alkaline phosphatase and lactate dehydrogenase were associated with an increased likelihood of a positive biopsy.(7) Additionally, survival was shorter in men with a positive biopsy, suggesting these individuals had more advanced disease.
Given that men with prostate cancer are susceptible to skeletal complications secondary to treatment-associated bone loss and bone metastases, osteoclast-targeting agents are frequently utilized. Bisphosphonates, analogues of pyrophosphate, have an inhibitory effect on osteoclasts, which are responsible for bone resorption.(15) Denosumab, a monoclonal antibody against the receptor activator of nuclear factor-κβ ligand, prevents osteoclast function.(15) We hypothesized that treatment with an osteoclast-targeting agent would result in decreased tumor yield given the risk of osseous sclerosis associated with therapy. We observed that longer duration of therapy seemed to correlate with decreased yield. In a prior analysis, zoledronic acid therapy for ≥1 year resulted in a decreased number of positive bone biopsies (50%) compared to patients who did not receive therapy or received <1 year of treatment (74%) (p=0.27).(8) The direct effect of osteoclast-targeting agents in patients undergoing bone biopsy needs to be further explored.
Given the exploratory nature of our analysis, several limitations should be highlighted. Though samples were collected prospectively, imaging and procedural variables were analyzed retrospectively. Also, our sample size was relatively small, limiting our ability to identify small differences between the variables analyzed. Biopsy site selection, needle gauge, and number of cores obtained were at the discretion of the radiologist performing the biopsy, potentially resulting in selection bias. Our analysis was not comprehensive of all possible factors which can influence tumor yield. Other metrics to be considered include biopsy of “new” (within 3-6 months) versus “old” lesions, total number of needle passes, volume of tissue collected, and evidence of necrosis. Lastly, we do not report nucleic acid quality/quantity or success of molecular analysis, which are important and should be included in future analyses. Unlike diagnostic biopsies, biopsies performed for tumor molecular analysis require special handling, given that bone is not decalcified during processing and occasionally laser capture microdissection is performed to increase sample purity. These additional steps have the potential to impact the success of molecular sequencing.
As molecular characterization of metastatic tumors becomes increasingly important, there is a pressing need to establish guidelines to improve biopsy yield. In this exploratory analysis, we identified several variables associated with increased tumor yield on bone biopsy. As we embark on a large multi-institutional project that aims to molecularly characterize mCRPC tumors, we have developed the following guidelines to optimize tumor yield in our patients undergoing biopsies as part of this endeavor:
Discuss the optimal lesion for biopsy with the collaborating radiologist.
Select soft tissue over bone lesions when possible.
Avoid biopsy of a previously irradiated lesion.
Use a larger needle for bone biopsies (11 gauge preferred for bone biopsies and 18 gauge preferred for soft tissue biopsies).
For bone biopsy, select lesions with larger size, low HU, short distance from the skin to the lesion, and increased radiotracer activity on bone scan.
Minimize use of osteoclast-targeting therapy preceding bone biopsy when possible.
Larger studies are needed to develop the optimal algorithm for successful bone biopsy, specifically biopsies with the goal of downstream molecular analysis. Given such, we have incorporated a prospective analysis of the variables described here as part of a SU2C multi-institutional project. Furthermore, a critical next step is correlating these parameters with successful genome sequencing, which is the ultimate goal of these efforts as we enter the era of personalized medicine.
Acknowledgements
The clinical trial described in this manuscript was funded by Janssen. GlaxoSmithKline provided dutasteride for this trial. The cost of the tumor biopsies was funded through the Fairweather Family Fund and the Prostate Cancer Foundation Challenge Award.
Footnotes
Conflicts of Interest: Dr. Taplin has received research funding for clinical trials of abiraterone from Janssen and has an advisory role at Janssen.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248(3):962–70. doi: 10.1148/radiol.2483071742. [DOI] [PubMed] [Google Scholar]
- 3.Chua DT, Ackermann W, Veenema RJ. Bone marrow biopsy in patients with carcinoma of the prostate. J Urol. 1969;102(5):602–6. doi: 10.1016/s0022-5347(17)62209-2. [DOI] [PubMed] [Google Scholar]
- 4.Mehan DJ, Broun GO, Jr., Hoover B, Storey G. Bone marrow findings in carcinoma of the prostate. J Urol. 1966;95(2):241–4. doi: 10.1016/S0022-5347(17)63440-2. [DOI] [PubMed] [Google Scholar]
- 5.Spiers AS, Deal DR, Kasimis BS, Miller BR. Evaluation of the bones and bone marrow in patients with metastatic carcinoma of the prostate: radiologic, cytologic and cytogenetic findings. J Med. 1982;13(4):303–7. [PubMed] [Google Scholar]
- 6.Sy FA, Gursel EO, Veenema RJ. Positive random iliac bone biopsy in advanced prostatic cancer. Urology. 1973;2(2):125–7. doi: 10.1016/0090-4295(73)90243-4. [DOI] [PubMed] [Google Scholar]
- 7.Ross RW, Halabi S, Ou SS, Rajeshkumar BR, Woda BA, Vogelzang NJ, et al. Predictors of prostate cancer tissue acquisition by an undirected core bone marrow biopsy in metastatic castration-resistant prostate cancer--a Cancer and Leukemia Group B study. Clin Cancer Res. 2005;11(22):8109–13. doi: 10.1158/1078-0432.CCR-05-1250. [DOI] [PubMed] [Google Scholar]
- 8.Spritzer CE, Afonso PD, Vinson EN, Turnbull JD, Morris KK, Foye A, et al. Bone Marrow Biopsy: RNA Isolation with Expression Profiling in Men with Metastatic Castration-resistant Prostate Cancer-Factors Affecting Diagnostic Success. Radiology. 2013;269(3):816–23. doi: 10.1148/radiol.13121782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brugieres P, Revel MP, Dumas JL, Heran F, Voisin MC, Gaston A. CT-guided vertebral biopsy. A report of 89 cases. J Neuroradiol. 1991;18(4):351–9. [PubMed] [Google Scholar]
- 12.Ghelman B, Lospinuso MF, Levine DB, O'Leary PF, Burke SW. Percutaneous computed-tomography-guided biopsy of the thoracic and lumbar spine. Spine. 1991;16(7):736–9. doi: 10.1097/00007632-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Lis E, Bilsky MH, Pisinski L, Boland P, Healey JH, O'Malley B, et al. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol. 2004;25(9):1583–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Stoker DJ, Kissin CM. Percutaneous vertebral biopsy: a review of 135 cases. Clin Radiol. 1985;36(6):569–77. doi: 10.1016/s0009-9260(85)80235-x. [DOI] [PubMed] [Google Scholar]
- 15.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]