Abstract
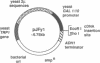
Galactose-1-phosphate uridylyltransferase (GALT) (UTP: alpha-D-hexose-1-phosphate uridylyltransferase, EC 2.7.7.10) is an essential enzyme of the Leloir pathway of galactose metabolism. Mutations in human GALT are associated with the potentially lethal disorder galactosemia, which affects 1 in 30,000-60,000 live-born infants. Although a number of base substitutions have been identified in the GALT alleles of galactosemia patients, the detailed biochemical impact of these mutations on GALT enzymatic activity remains obscure. Similarly, little is known about the sequence/structure/function relationships for wild-type human GALT. As a first step toward addressing these questions, we have developed a yeast-based expression system for the human enzyme. The wild-type human GALT coding sequence has been introduced into a strain of Saccharomyces cerevisiae that carries a disruption of the GALT-encoding GAL7 gene and, therefore, expresses no endogenous GALT. Transformants were tested for restoration of GALT activity both indirectly, by cell growth on galactose, and directly, by analysis of enzyme activity in cell extracts. The results of both tests were striking; wild-type human GALT functioned in yeast almost as well as the endogenous enzyme. In contrast, cells transformed with either human or yeast GALT sequences engineered to carry a common human GALT mutation, Q188R (changing Gln188 to Arg), exhibited essentially no detectable GALT activity and failed to grow on galactose. Lymphoblasts from patients homozygous for the Q188R mutation similarly exhibited essentially no detectable GALT activity in parallel assays. The results reported here establish the utility of the yeast-based expression system for human GALT and set the stage for more detailed studies of this important enzyme and its role in galactosemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banroques J., Schapira F., Grégori C., Dreyfus J. C. Molecular studies on galactose 1 phosphate uridylyl transferase from normal and mutant subjects. An immunological approach. Ann Hum Genet. 1983 Jul;47(Pt 3):177–185. doi: 10.1111/j.1469-1809.1983.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Fikes J. D., Guarente L. A cDNA encoding a human CCAAT-binding protein cloned by functional complementation in yeast. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1968–1972. doi: 10.1073/pnas.88.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bertoli D., Segal S. Developmental aspects and some characteristics of mammalian galactose 1-phosphate uridyltransferase. J Biol Chem. 1966 Sep 10;241(17):4023–4029. [PubMed] [Google Scholar]
- Flach J. E., Reichardt J. K., Elsas L. J., 2nd Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1990 Aug;7(4):365–369. [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Holton J. B. Galactose disorders: an overview. J Inherit Metab Dis. 1990;13(4):476–486. doi: 10.1007/BF01799505. [DOI] [PubMed] [Google Scholar]
- Johnston S. A., Hopper J. E. Isolation of the yeast regulatory gene GAL4 and analysis of its dosage effects on the galactose/melibiose regulon. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6971–6975. doi: 10.1073/pnas.79.22.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., Howard B. H. A rapid method for recombination and site-specific mutagenesis by placing homologous ends on DNA using polymerase chain reaction. Biotechniques. 1991 Jan;10(1):62–66. [PubMed] [Google Scholar]
- Kelley R. I., Harris H., Mellman W. J. Characterization of normal and abnormal variants of galactose-1-phosphate uridylyltransferase (EC 2.7.7.12) by isoelectric focusing. Hum Genet. 1983;63(3):274–279. doi: 10.1007/BF00284663. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Leslie N. D., Immerman E. B., Flach J. E., Florez M., Fridovich-Keil J. L., Elsas L. J. The human galactose-1-phosphate uridyltransferase gene. Genomics. 1992 Oct;14(2):474–480. doi: 10.1016/s0888-7543(05)80244-7. [DOI] [PubMed] [Google Scholar]
- Nadler H. L., Chacko C. M., Rachmeler M. Interallelic complementation in hybrid cells derived from human diploid strains deficient in galactose-1-phosphate uridyl transferase activity. Proc Natl Acad Sci U S A. 1970 Oct;67(2):976–982. doi: 10.1073/pnas.67.2.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant T. R. Expression of human lysosomal beta-hexosaminidase in yeast vacuoles. Biochem Biophys Res Commun. 1990 Jul 16;170(1):383–390. doi: 10.1016/0006-291x(90)91285-z. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Belmont J. W., Levy H. L., Woo S. L. Characterization of two missense mutations in human galactose-1-phosphate uridyltransferase: different molecular mechanisms for galactosemia. Genomics. 1992 Mar;12(3):596–600. doi: 10.1016/0888-7543(92)90453-y. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1988 Apr;5(2):107–122. [PubMed] [Google Scholar]
- Reichardt J. K., Levy H. L., Woo S. L. Molecular characterization of two galactosemia mutations and one polymorphism: implications for structure-function analysis of human galactose-1-phosphate uridyltransferase. Biochemistry. 1992 Jun 23;31(24):5430–5433. doi: 10.1021/bi00139a002. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Packman S., Woo S. L. Molecular characterization of two galactosemia mutations: correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase. Am J Hum Genet. 1991 Oct;49(4):860–867. [PMC free article] [PubMed] [Google Scholar]
- Reichardt J. K., Woo S. L. Molecular basis of galactosemia: mutations and polymorphisms in the gene encoding human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2633–2637. doi: 10.1073/pnas.88.7.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Broach J. R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989 Dec;16(5-6):339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schneider J. C., Guarente L. Vectors for expression of cloned genes in yeast: regulation, overproduction, and underproduction. Methods Enzymol. 1991;194:373–388. doi: 10.1016/0076-6879(91)94028-b. [DOI] [PubMed] [Google Scholar]
- Segawa T., Fukasawa T. The enzymes of the galactose cluster in Saccharomyces cerevisiae. Purification and characterization of galactose-1-phosphate uridylyltransferase. J Biol Chem. 1979 Nov 10;254(21):10707–10709. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Steffan J. S., Minard K. I., McAlister-Henn L. Expression and function of heterologous forms of malate dehydrogenase in yeast. Arch Biochem Biophys. 1992 Feb 14;293(1):93–102. doi: 10.1016/0003-9861(92)90370-c. [DOI] [PubMed] [Google Scholar]
- Torchia T. E., Hamilton R. W., Cano C. L., Hopper J. E. Disruption of regulatory gene GAL80 in Saccharomyces cerevisiae: effects on carbon-controlled regulation of the galactose/melibiose pathway genes. Mol Cell Biol. 1984 Aug;4(8):1521–1527. doi: 10.1128/mcb.4.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner D. D., Buist N. R., Donnell G. N. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis. 1990;13(6):802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- Williams V. P. Purification and some properties of galactose 1-phosphate uridylyltransferase from human red cells. Arch Biochem Biophys. 1978 Nov;191(1):182–191. doi: 10.1016/0003-9861(78)90080-2. [DOI] [PubMed] [Google Scholar]