Abstract
Sphingosine-1 phosphate (S1P) is involved in a variety of cellular processes via activation of S1P receptors (S1PRs; S1PR1 to S1PR5) that are highly expressed in the brain. It has been shown that the level of S1P is reduced in the brain of Alzheimer's disease (AD) patients. However, there is no study designed to evaluate the expression of S1PRs in AD brains. The objectives of the present work are (1) to examine the expression of S1PR1-3 in the hippocampus of beta amyloid (Aβ) 1-42 injected rats and (2) to clarify the effects of chronic S1PR1 activation on S1PR1-3 levels, spatial memory deficit and hippocampal damage in AD rats. SEW2871, the S1PR1 selective agonist, repeatedly was injected intraperitoneally during a period of two weeks. Upon Western Blot data bilateral intrahippocampal injection of Aβ1-42 decreased the expression of S1PR1 while increased S1PR2 level and did not affect that of S1PR3. We found that chronic administration of SEW2871 inhibited the reduction of S1PR1 expression and ameliorated spatial memory impairment in the Morris water maze task in rats. In addition, SEW2871 attenuated the Aβ1-42-induced hippocampal neuronal loss according to Nissl staining findings. Data in the current study highlights the importance of S1PR1 signaling pathway deregulation in AD development and suggests that activation of S1PR1 may represent a potential approach for developing new therapeutics to manage memory deficit and apoptosis associated with neurodegenerative disorders such as AD.
Keywords: SEW2871, cognitive function, sphingosine-1 phosphate receptors, Alzheimer's disease
Introduction
Sphingosine 1-phosphate (S1P) is a sphingolipid with important roles in and out of the cells. S1P regulates cell growth (Olivera and Spiegel, 1993[41]) and suppresses the apoptosis (Cuvillier et al., 1996[9]). The biological activities of S1P are mostly mediated by G-protein coupled receptors family, termed as S1P receptors (S1PRs). Among S1PRs (S1PR1-5), S1PR1, S1PR2 and S1PR3 are widely expressed in several tissues at various levels (Young and Van Brocklyn, 2006[53]). Except S1PR4, all S1PR subtypes are expressed in the central nervous system (CNS) and S1PR1-3 levels are substantially high in the brain (MacLennan et al., 1994 [33]; Zhang et al., 1999[54]). Among these, S1PR1 is highly expressed in white matter, hippocampus and the cerebellum (Chae et al., 2004[7]) suggesting a critical role for S1PR1 in neural functions in these regions.
There is a confirmed connection between deregulation of S1P activity and some neurodegenerative diseases, nevertheless little is known about the relevance with Alzheimer's disease (AD). The S1PR1 has been shown to regulate lymphocytes egress from secondary lymphatic organs (Matloubian et al., 2004[35]) and to confer some beneficial effects on ischemia conditions (Wei et al., 2010[50], Liesz et al., 2011[30]). Thus some S1PR modulators like FTY720, have been approved as a first-line treatment for relapsing remitting multiple sclerosis (Chiba et al., 2011[8]). SEW2871, a selective agonist of S1PR1, also attenuates the expression of proinflammatory molecules and tissue injury under inflammatory conditions such as renal ischemia/reperfusion injury (Jo et al., 2005[19], Awad et al., 2006[2], Lien et al., 2006[29]), diabetic nephropathy and emphysema (Awad et al., 2006[2], Hofmann et al., 2009[16], Diab et al., 2010[11]).
AD is one of the neurodegenerative disorders that is linked to neuro-inflammation and is characterized by progressive cognitive decline and abnormalities in behavior. Accumulation of beta amyloid (Aβ) peptides and neurofibrillary tangles are pathological hallmarks of AD (Steinerman et al., 2008[47]; McNaull et al., 2009[36]; Bhaskar and Lamb, 2012[4]). Soluble Aβ oligomers activate sphingomyelinases, increase the level of ceramide (Obeid et al., 1993[39]; Malaplate-Armand et al., 2006[34]) while AD patients brains shows reduced levels of the S1P (Brinkmann et al., 2010[6]). Enhanced ratio of ceramids to S1P seems to be in favor of neuronal death in AD brains.
According to the in vitro experiments, shifting the balance between S1P synthesizing (sphingosine kinases-1) and degrading (S1P-lyase or -phosphatase) enzymes toward high levels of S1P (Van Veldhoven, 2000[49]; Le Stunff et al., 2002[27]; Hla, 2003[15]; Chae et al., 2004[7]) is associated with decreased levels of ceramide and sphingosine and inhibits ceramide-induced apoptosis (Edsall et al., 2001[12]; Reiss et al., 2004[46]). S1PR1 activations seem to account for S1P anti-apoptotic effects (Cuvillier et al., 1996[9]).
The present study aims to clarify the impact of S1PR1 signaling on AD progress and outcomes. According to some reports indicating that S1PRs expressions alter in some inflammatory conditions like renal ischemia-reperfusion injury (Awad et al., 2006[2]), we explored whether S1PRs levels changes in AD brain. Then the influence of chronic administration of SEW2871, the S1PR1 selective agonist, was evaluated on the corresponding S1PRs expressions, hippocampal neuronal survival and on spatial learning and memory in Aβ1-42 injected rats.
Materials and Methods
Drugs
Aβ1-42 (Sigma-Aldrich, USA) stock solution was diluted by PBS to a concentration of 1 µg/µl and was incubated at 37 °C for 7 days to allow the formation of fibril aggregation. SEW2871 was purchased from Cayman Chemical and dissolved in Dimethyl sulfoxide (DMSO) to the final concentration of 1 mg/ml.
Animals and experimental groups
The rats used in this study were males of the Wistar albino strain weighing 250-300 g that were housed in cages (four per cage) with ad libitum food and water. The animals were maintained under a controlled environment (12 h light-dark cycle with lights on from 07:00 to 19:00 h and 25 ± 2 °C room temperature). All animal manipulations were carried out according to the Ethical Committee for the use and care of laboratory animals of Shahid Beheshti University of medical sciences in compliance with the standards of the European Communities Council directive (86/609/EEC).
Animals were randomly divided into 4 groups as following (n=10 per each):
Control: the rats receiving intrahippocampal physiological buffer solution (PBS) (Aβ vehicle) injection and daily i.p. injection of DMSO (SEW2871 vehicle)
SEW: the rats receiving intrahippocampual PBS injection and daily i.p. injection of SEW2871
Aβ: the rats receiving intrahippocampal Aβ1-42 injection and daily i.p. injection of DMSO
Aβ+SEW: the rats receiving intrahippocampal Aβ1-42 injection and daily i.p. injection of SEW2871
Drug administration
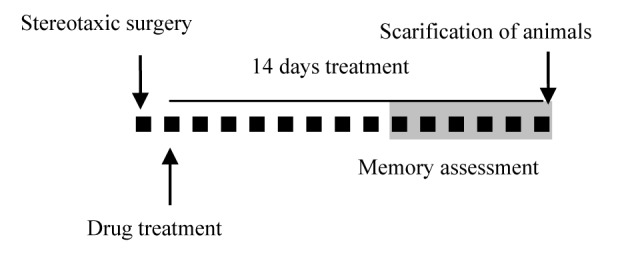
The reagents used for AD induction or to stimulate S1PR1 in animals, were administered according to experimental schedule summarized in Figure 1(Fig. 1).
Figure 1. Diagrammatic representation of the study protocol. One day after the stereotaxic surgery, daily treatment with SEW2871 or vehicle started, which continued for 14 days. Each small black square represents one day and the “gray mark” shows the time of the Morris water maze test performance. Scarification of animals was done 14 days after the surgery.
Aß1-42 preparation and intrahippocampal injection
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic instrument (Stoelting, USA).
The following coordinates were used for bilateral intrahippocampal injection: AP: -3.84 mm, L: ± 2.2 mm and V: 2.5 mm according to the atlas of rat brain (Paxinos and Watson, 2007[44]). A solution containing 2 µg aggregated Aβ1-42 or PBS (for control and SEW groups) of the same volume was injected. Solutions were administrated via Hamilton microsyringe. All injections were made at the rate of 2 μl/4min. To minimize leakage of the solutions, the needle was kept in the position for 2 min after injection and the scalp was then closed with a suture.
SEW2871 chronic treatment
Started on the first day after the stereotaxic surgery, rats were injected with i.p. DMSO (the SEW2871 solvent) or SEW2871 (0.5 mg/kg) for 14 consecutive days (Park et al., 2010[43]). At the end of drug treatment period (14th day after surgery) animals were sacrificed.
Morris water maze test
The behavioral studies were carried out on days 9-14 after the surgery. A circular water tank (140 cm diameter, 55 cm high) was divided into four equally spaced quadrants. A transparent platform was set at the southeast quadrant of the tank, 1 cm below the surface of the water. Extra-maze cues such as some racks, bookshelves, pictures on the walls, surrounded the room where the water maze was performed. On day 9th, rats were habituated to the pool by allowing them to perform a 120 s swimming without the platform.
The task was conducted for four consecutive days. In each trial (that constitutes four trains), a rat was placed in the water at one of the four starting positions, with the sequence of the positions being selected randomly. The rat was allowed to swim until it found the platform or till the 90 s had elapsed. In this latter case, the trial was terminated and the animal was put on the platform for 20 s. The distance moved (cm) and escape latency (s) to reach the platform was recorded by a computer.
On day 14th, the platform was removed from the pool and animals underwent a 60 s spatial probe trial. The time spent (s) in the target quadrant that the platform was formerly located was recorded and compared between groups (Moradpour et al., 2006[38]).
Western blotting
After behavioral tests, rats were sacrificed and hippocampus (n=6) was used to determine the S1PR1-3 and cleaved caspase-3 expression by Western blotting method. Hippocampal tissues were homogenized in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5 % Nonidet P-40, 50 mM NaF, 20 mM EDTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 0.1 % SDS, and 2 mM phenylmethylsulfonyl fluoride). The homogeneate tissues were then centrifuged at 5,000 rpm for 10 min at 4 °C. The supernatants were collected as total protein extracts. Protein concentration was determined by using Bradford's method (Bradford, 1976[5]). Individual samples were subjected to SDS polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membranes. Blotting membranes were incubated with nonfat milk and then incubated overnight at 4 °C with the primary antibodies against cleaved caspase-3 (Cell Signalling, Beverly, MA, USA; 1:1000), S1PR1 (Abcam, USA; 1:1500), S1PR2 (Cayman Chemical, USA; 1:1500), S1PR3 (Abcam, USA; 1:1500). Then the membranes were incubated with secondary antibodies and processed with enhanced chemiluminescence reagent kit (Amersham Biosciences Europe GmbH, Germany). The images were scanned and analyzed semiquantitatively in a blind fashion using the Image J software. The expression of β-actin was used as an internal control for every experiment.
Tissue processing and Nissl's staining of hippocampus neurons
After the behavioral assessment, 6 rats of experimental groups were anesthetized and sacrificed by decapitation. The brains of animals were removed and one hemisphere was fixed in 4 % paraformaldehyde and embedded in paraffin. Serial coronal sections of 5 µm thickness were cut (beginning from 2.7 mm posterior to Bregma through the hippocampus), a total of 9 sections were collected from each brain and Nissl's staining was performed. Briefly, the sections were dipped into aqueous solution of cresyl violet (1 %) for 7 min and then washed with distilled water followed by placing in 95 % ethanol. The sections were then dehydrated in absolute alcohol, cleared and mounted with permount mounting medium and analyzed under the light microscope. The number of survived pyramidal cells was counted in a 1 mm length of CA1 region of hippocampus under ×40 magnification of light microscopy as performed previously (Xu et al., 2009[51]) and the number of neurons in this region was counted in different groups and percentage of neuronal loss was compared.
Statistical analysis
All data were expressed as mean ± SEM. Data were analyzed by using one-way ANOVA, followed by Tukey HSD post hoc analysis and P<0.05 was assumed to be statistically significant. All statistical studies were carried out with SPSS program (version 16.0, SPSS Inc., USA).
Results
Intrahippocampal injection of Aβ1-42 modifies S1PR1,2 expressions
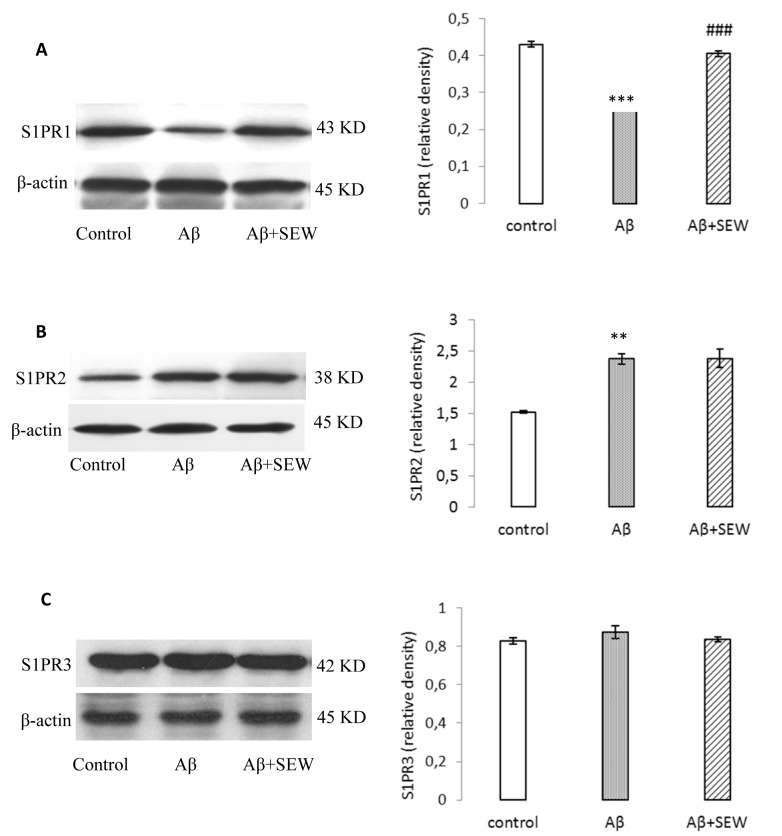
In order to determine the effect of intrahippocampal injection of Aβ1-42 on S1PRs protein levels, the expressions of S1PR1-3 proteins (that are highly expressed in the brain) were evaluated by Western blotting method. Compared with control, the expression of S1PR1 was reduced prominently in rats hippocampus two weeks after Aβ1-42 (P<0.001) (Figure 2A(Fig. 2)), while the expression of S1PR2 was significantly elevated then (P<0.05) (Figure 2B(Fig. 2)) and no change was observed in S1PR3 expression (Figure 2C(Fig. 2)).
Figure 2. Effect of SEW2871 on S1PR1-3 expression in Aβ1-42 injected rats evaluated by Western blotting method. 15 days after the stereotaxic procedure, the expression of S1PR1-3 in hippocampus was detected. Results showed decreasing of S1PR1 (A) and increasing of S1PR2 (B) in AD model rats. Treatment of AD model rats with SEW2871 resulted in elevated levels of S1PR1 (A) in hippocampus and had no effect on S1P2 (B). The expression of S1P3 did not show any change between groups (C). β-actin protein was used here as an internal control. One representative Western blot is shown; n = 6. The band density values were calculated and the values from the control were used as 1. Values are the mean ± S.E.M., **P<0.01 and ***P<0.001 vs. control group; ### P<0.001 vs. Aβ group.
SEW2871 chronic administration prevents the Aß1-42-induced decrease in S1PR1 level
The expression of S1PR1-3 was also determined in AD model rats chronically treated with SEW2871. According to the S1PR1-3 immunoblotting results, the expression of S1PR1 increased significantly in Aβ+SEW group compared with Aβ group (P<0.01) (Figure 2A(Fig. 2)). However, treatment with SEW2871 could not affect the S1PR2 and S1PR3 levels (Figure 2B, C(Fig. 2)).
Regarding the positive influence of the SEW2871 on S1PR1 expression in Aβ1-42 injected rats, we decided to examine the effects of chronic administration of SEW2871 on cognitive deficit in AD model rats.
SEW2871 improves memory performance of AD model rats in Morris water maze test
In order to survey the spatial learning and memory performance in rats, the Morris water maze task was accomplished. Results showed that, comparing with control group, learning and memory ability of Aβ1-42 injected rats were significantly impaired. The average escape latency in searching for the hidden underwater platform, decreased with the increase of training days. Injection of Aβ1-42 caused a significant decline in spatial learning as indicated by significant increases in distance moved and escape latency (P<0.001) in searching for the underwater platform (Figure 3A, B(Fig. 3)). In addition, the time spent in the target quadrant (in the probe test) decreased significantly (P<0.001) (Figure 3C(Fig. 3)). These results showed that the application of Aβ1-42 impaired rat's spatial learning and memory. The effects of SEW2871 administration on spatial learning and memory are shown in Figure 3(Fig. 3). In the hidden platform test, administration of SEW2871 slightly improved the learning task in Aβ1-42 injected rats. The distance moved and escape latency to reach the hidden platform were slightly shorter in SEW2871-administrated AD model animals on 4th day of training, compared with rats were injected with Aβ1-42 only (P<0.05) (Figure 3A, B(Fig. 3)). In addition, after removing the platform, the time spent in the target quadrant increased significantly in Aβ injected animals which received SEW2871 compared with AD rats that did not receive the drug (P<0.05) (Figure 3C)(Fig. 3).
Figure 3. Effect of SEW2871 on spatial learning and memory performance in Aβ1-42 injected rats in Morris water maze test. Intrahippocampal injection of PBS or Aβ1-42 (2 μg/2 μL/ rat hippocampus) was performed and from the next day, rats were treated with a daily intraperitoneal injection of SEW2871 (0.5 mg/kg) or DMSO (vehicle). Hidden platform test was carried out on days 11-14 after the injection of Aβ1-42 and a probe trial was conducted in the day after the final training trial. Significant deficit in learning and memory performance was observed in Aβ injected group and chronic treatment with SEW2871 improved learning and memory task. Distance moved to reach platform (cm) (A) Escape latency in target quadrant (sec) (B) and Time spent in target quadrant (sec) (C) are shown and compared between the experimental groups. Data are expressed as mean ± S.E.M., n=10. *P<0.05, ***P<0.001 vs. control; #P<0.05, ###P<0.001 vs. Aβ group.
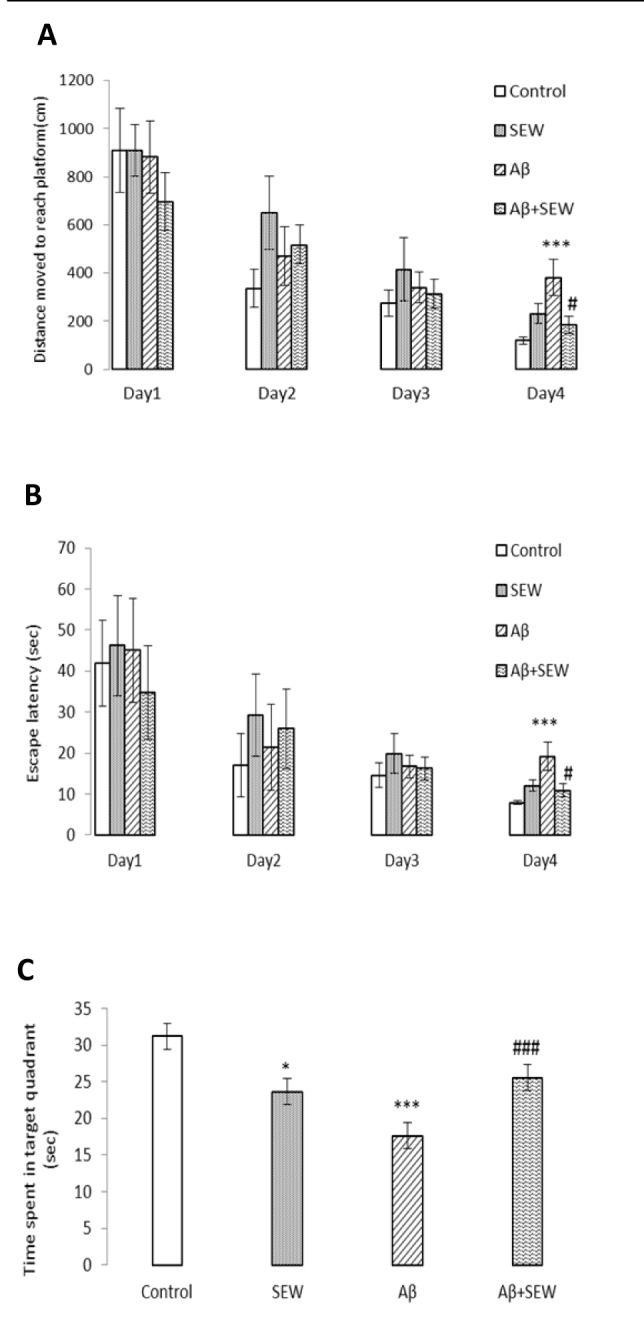
Chronic administration of SEW2871 to PBS injected group resulted in no significant change in learning task, but caused deficit in the memory performance. In probe test, the time spent in the target quadrant in PBS injected rats which chronically received SEW2871 was reduced significantly, compared with control group (P<0.05) (Figure 3C(Fig. 3)).
SEW2871 protects from apoptosis induced by Aβ1-42 exposure in CA1 region of hippocampus
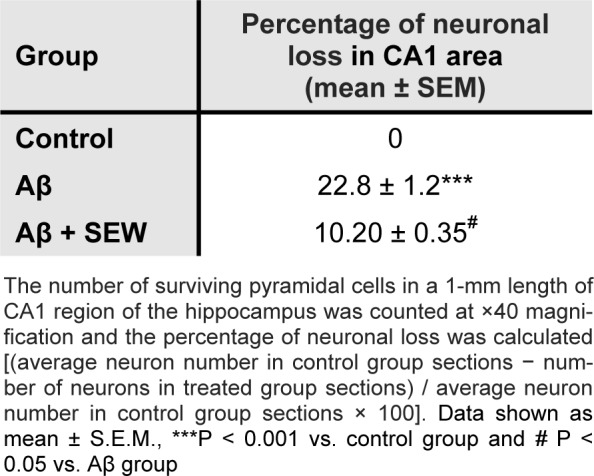
The results of microscopic examinations of the hippocampus sections showed that Aβ injection reduces neuronal layer thickness in CA1 area of hippocampus and administration of SEW2871 prevents the Aβ-induced damage (Figure 4(Fig. 4)). Furthermore, intrahippocampal Aβ injection resulted in significant neural loss in CA1 area compared with control animals (P<0.001) which was markedly attenuated by SEW2871 treatment (P<0.05) (Table 1(Tab. 1)).
Figure 4. Effect of SEW2871 on Aβ-induced damage in CA1 region of rat hippocampus. Investigation of Nissl stained photomicrographs from CA1 region of hippocampus showed that the pyramidal cells layer in CA1 area of Aβ1-42-injected rats are markedly thinner than that in the control group. While in AD rats which chronically received SEW2871 it was as thick as that in control group. From left to right: control, Aβ, Aβ + SEW groups. Photos represent ×400 magnification.
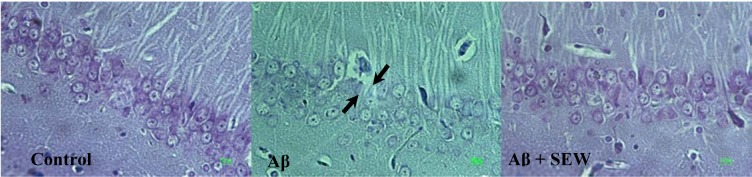
Table 1. Effect of chronically treatment of SEW2871 on Aβ1-42-induced neuronal loss in CA1 region of hippocampus.
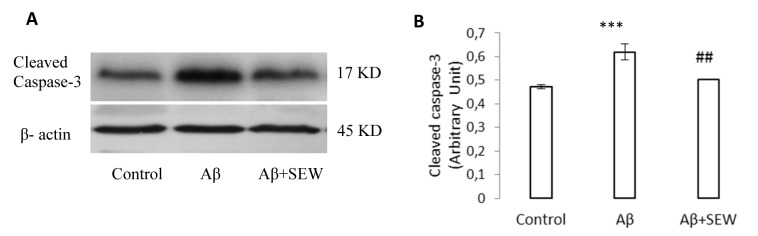
To suggest about the apoptotic pathways, the intracellular levels of cleaved caspase-3 was measured to estimate caspase-3 activation. The hippocampi Western blot analysis showed a significant increase in Aβ injected rats (P<0.001), while administration of SEW2871 in AD rats reduced the Aβ1-42 induced caspase-3 cleavage (p<0.05) (Figure 5(Fig. 5)). All together, these results suggest that both Aβ apoptotic effects and SEW2871 anti-apoptotic benefits may be mediated at least in part through caspase-3 modifications.
Figure 5. Effect of SEW2871 on level of cleaved caspase-3 in the hippocampus of Aβ1-42 injected rats. The expression of cleaved caspase-3 was determined by Western blotting method. The level of cleaved caspase-3 in control, Aβ and Aβ + SEW groups (One representative Western blot is shown; n=6) (A). The relative densities of corresponding bands were measured and compared (B). Each point shows the mean ± S.E.M.,***P < 0.001 vs. control group and ## P< 0.01 vs. Aβ group.
Discussion
Revealing the S1PRs signaling effects and interactions in the context of pathological conditions may help to suggest new therapeutic approaches. Here we determined the changes occur in S1PRs levels in the hippocampus following exposure to Aβ1-42 fibrils. Then we pointed out that chronic administration of SEW2871 results in enhanced S1PR1 expression and reduced S1PR2 levels in AD model rats. SEW2871 also attenuated Aβ-induced caspase-3 activation, hippocampal damage and cognitive impairment in AD animals.
S1P decrease and ceramide increase in AD patient brains have been reported in some studies (He et al., 2010[14]; de la Monte, 2012[10]). The apoptotic effects of Aβ are mimicked by ceramides (Liu et al., 1998[31]; Yang et al., 2004[52]). Although S1P may act as a non-competitive ceramide synthase inhibitor (Laviad et al., 2008[26]) and inhibits the sphingomyelinase activity (Malaplate-Armand et al., 2006[34]; Brinkmann et al., 2010[6]), even low concentrations of ceramide cause cell death in mature hippocampal neurons (Mitoma et al., 1998[37]). The S1P-ceramide imbalance could result in neuronal death and S1PRs agonists application could be introduced as a useful therapeutic approach to reduce Aβ-induced brain damages.
As compared with other S1PRs, S1PR1 is particularly abundant in hippocampus of rodents (Lee et al., 2010[28]). It has been suggested that the S1P exerts its anti-apoptotic effects mostly via S1PR1 activation (Young and Van Brocklyn, 2006[53]). The present study indicates a significant decline in the S1PR1 level in AD model rats. Taken together, S1PR1 could be assumed as a potential inhibitor of Aβ-induced cell death in hippocampus. To investigate this hypothesis, the AD model rats were treated chronically with SEW2871.
Upon our data, SEW2871 inhibited the Aβ-induced reduction in S1PR1 expression. This confirms some previous evidences indicating that S1PR1 activation induces production of growth factors and increases S1PRs mRNA expression (Pitson et al., 2005[45]; Landeen et al., 2007[25]).
According to our histological studies along with cleaved caspase-3 immuno-blotting results, SEW2871 inhibites the Aβ-induced neural loss in CA1 region of hippo-campus probably via attenuating caspase-3 pathway. However the current results could not suggest about the underlying mechanisms, these anti-apoptotic effects are inconsistent with some studies examined S1PR1's protective role under inflammatory conditions like diabetic nephropathy and emphysema (Awad et al., 2006[2], Hofmann et al., 2009[16], Diab et al., 2010[11]). S1PR1 signaling has also been shown to be involved in stem/progenitor cells migration to damaged area of the CNS (Kimura et al., 2007[24]) but it seems less likely to account for the improved neural survival in just two weeks.
The spatial memory deficit is an important hallmark of AD (Forsyth and Ritzline, 1998[13]). This may be the probable consequence of events following Aβ accumulation in pyramidal neurons, such as: glutamatergic synaptic transmission depression (Kamenetz et al., 2003[21]), reduced glutamate receptors (Almeida et al., 2005[1]; Liu et al., 2010[32]) and facilitation of the long-term depression (LTD) of synaptic transmission (Berdyshev et al., 2009[3]). In the present work, we used Morris water maze to investigate cognitive abilities in animals and demonstrated that intrahippocampal injection of Aβ1-42 induced learning and memory deficit in animals as an important component of valid animal models of AD.
S1P is involved in memory formation and glutamate release that seems to be partly mediated by S1PR1 and S1PR3 (Kajimoto et al., 2007[20]). Long term potentiation is impaired in sphingosine kinase-1 (S1P synthesizing enzyme) knockout mice leading to spatial memory deficits (Kanno et al., 2010[23]). In addition, S1PR1 induces Gi activation and cytoskeletal rearrangements through small G-protein Rac (Okamoto et al., 2000[40]) which facilitates the transmitter secretion (Humeau et al., 2002[17]) and may play a role in memory formation. Consistently, data from the present work indicates that S1PR1 activation may prevent cognitive deficit progress in AD. Upon our results, chronic administration of SEW2871, the selective agonist of S1PR1, improved the spatial learning and memory in animals after Aβ1-42.
Some beneficial effects have also been demonstrated for S1PR3 activation in glutamate transmission and cell survival (Brinkmann et al., 2010[6]), nevertheless we did not observe any change in S1PR3 expression not in Aβ neither in Aβ+ SEW2871 animals as compared to control rats.
However we did not use any specific S1PR2 agonist here, some previous reports have demonstrated that S1PR2 activation may induce apoptosis and necrosis in human proximal tubule cells (Park et al., 2012[42]) and neurite retraction (Toman and Spiegel, 2002[48]). Cell rounding and neurite retraction has been observed in cultered hippocampal neurons exposed to Aβ aggregates (Ivins et al., 1998[18]). Upon our results S1PR2 expression increases in Aβ1-42 injected hippocampi and is not affected with SEW2871 treatment in AD rats. In agreement with this, enhanced levels of S1PR2 mRNA expression previously have been identified in Aβ treated monocytes (Kaneider et al., 2004[22]). Considering reduced S1P amounts in AD brains (He et al., 2010[14]) it seems probable that S1PR2 expression may be the result of compensatory mechanisms in the brain which may intensify neurodegeneration in the brain.
Briefly the present study may suggest some significant interplay between S1PRs which may play a key role in AD progress. Our data confirms the importance of S1PR1 signaling and emphasizes on the appropriate agonists as potential new therapeutics for AD. However these experiments do not rule out the S1PR2 involvement. According to the enhanced S1PR2 levels in AD animals in this study, it is highly suggested to evaluate the advantages of S1PR2 pharmacological blockage in animal models of AD.
Acknowledgements
The authors thank the Shaheed Beheshti University of Medical Science research fund for providing us with financial support on this work.
References
- 1.Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, et al. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:1516–1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 3.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, et al. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar K, Lamb BT. The role of Aβ and Tau oligomers in the pathogenesis of Alzheimer’s Disease. In: Rahimi F, Bitan G, editors. Non-fibrillar amyloidogenic protein assemblies-common cytotoxins underlying degenerative diseases. New York: Springer; 2012. pp. 135–188. [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 7.Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–150. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Chiba K, Kataoka H, Seki N, Maeda Y, Sugahara K. Fingolimod (FTY720), the sphingosine 1-phosphate receptor modulator, as a new therapeutic drug in multiple sclerosis. Inflamm Regen. 2011;31:167–174. [Google Scholar]
- 9.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM. Triangulated mal-signaling in Alzheimer's disease: roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J Alzheimers Dis. 2012;30:231–249. doi: 10.3233/JAD-2012-111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. 2010;181:344–352. doi: 10.1164/rccm.200906-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001;76:1573–1584. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- 13.Forsyth E, Ritzline PD. An overview of the etiology, diagnosis, and treatment of Alzheimer disease. Phys Ther. 1998;78:1325–1331. doi: 10.1093/ptj/78.12.1325. [DOI] [PubMed] [Google Scholar]
- 14.He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiol Aging. 2010;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res. 2003;47:401–407. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia–reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 17.Humeau Y, Popoff MR, Kojima H, Doussau F, Poulain B. Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J Neurosci. 2002;22:7968–7981. doi: 10.1523/JNEUROSCI.22-18-07968.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivins KJ, Bui ETN, Cotman CW. [beta]-Amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5:365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- 19.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol. 2007;27:3429–3440. doi: 10.1128/MCB.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 22.Kaneider NC, Lindner J, Feistritzer C, Sturn DH, Mosheimer BA, Djanani AM, et al. The immune modulator FTY720 targets sphingosine–kinase-dependent migration of human monocytes in response to amyloid beta-protein and its precursor. FASEB J. 2004;18:1309–1311. doi: 10.1096/fj.03-1050fje. [DOI] [PubMed] [Google Scholar]
- 23.Kanno T, Nishizaki T, Proia R, Kajimoto T, Jahangeer S, Okada T, et al. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience. 2010;171:973–980. doi: 10.1016/j.neuroscience.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, et al. Essential roles of sphingosine 1‐phosphate/s1p1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25:115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- 25.Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol Heart Circ Physiol. 2007;292:2698–2711. doi: 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- 26.Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH., Jr Characterization of ceramide synthase 2. J Biol Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 27.Le Stunff H, Peterson C, Thornton R, Milstien S, Mandala SM, Spiegel S. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J Biol Chem. 2002;277:8920–8927. doi: 10.1074/jbc.M109968200. [DOI] [PubMed] [Google Scholar]
- 28.Lee DH, Jeon BT, Jeong E, Kim JS, Cho YW, Kim HJ, et al. Altered expression of sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 in mouse hippocampus after kainic acid treatment. Biochem Biophys Res Commun. 2010;393:476–480. doi: 10.1016/j.bbrc.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Lien YHH, Yong K, Cho C, Igarashi S, Lai L. S1P1-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 30.Liesz A, Sun L, Zhou W, Schwarting S, Mracsko E, Zorn M, et al. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PloS one. 2011;6:e21312. doi: 10.1371/journal.pone.0021312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-α-induced cell death. J Biol Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Chang L, Roselli F, Almeida OFX, Gao X, Wang X, et al. Amyloid-β induces caspase-dependent loss of PSD-95 and synaptophysin through NMDA receptors. J Alzheimers Dis. 2010;22:541–556. doi: 10.3233/JAD-2010-100948. [DOI] [PubMed] [Google Scholar]
- 33.MacLennan AJ, Browe CS, Gaskin AA, Lado DC, Shaw G. Cloning and characterization of a putative G-protein coupled receptor potentially involved in development. Mol Cell Neurosci. 1994;5:201–209. doi: 10.1006/mcne.1994.1024. [DOI] [PubMed] [Google Scholar]
- 34.Malaplate-Armand C, Florent-Béchard S, Youssef I, Koziel V, Sponne I, Kriem B, et al. Soluble oligomers of amyloid-β peptide induce neuronal apoptosis by activating a cPLA2-dependent sphingomyelinase-ceramide pathway. Neurobiol Dis. 2006;23:178–189. doi: 10.1016/j.nbd.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 36.McNaull BB, Todd S, McGuinness B, Passmore AP. Inflammation and anti-inflammatory strategies for Alzheimer’s disease – a mini-review. Gerontology. 2009;56:3–14. doi: 10.1159/000237873. [DOI] [PubMed] [Google Scholar]
- 37.Mitoma J, Ito M, Furuya S, Hirabayashi Y. Bipotential roles of ceramide in the growth of hippocampal neurons: Promotion of cell survival and dendritic outgrowth in dose‐and developmental stage–dependent manners. J Neurosci Res. 1998;51:712–722. doi: 10.1002/(SICI)1097-4547(19980315)51:6<712::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Moradpour, F, Naghdi, N, Fathollahi, Y Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav Brain Res. 2006;175:223–232. doi: 10.1016/j.bbr.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto H, Takuwa N, Yokomizo T, Sugimoto N, Sakurada S, Shigematsu H, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20:9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 42.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:266–280. doi: 10.1681/ASN.2011050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SW, Kim M, Chen SWC, Brown KM, D'Agati VD, Lee HT. Sphinganine-1-phosphate protects kidney and liver after hepatic ischemia and reperfusion in mice through S1P1 receptor activation. Lab Invest. 2010;90:1209–1224. doi: 10.1038/labinvest.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th. New York: Academic Press; 2007. [Google Scholar]
- 45.Pitson SM, Xia P, Leclercq TM, Moretti PAB, Zebol JR, Lynn HE, et al. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiss U, Oskouian B, Zhou J, Gupta V, Sooriyakumaran P, Kelly S, et al. Sphingosine-phosphate lyase enhances stress-induced ceramide generation and apoptosis. J Biol Chem. 2004;279:1281–1290. doi: 10.1074/jbc.M309646200. [DOI] [PubMed] [Google Scholar]
- 47.Steinerman JR, Irizarry M, Scarmeas N, Raju S, Brandt J, Albert M, et al. Distinct pools of {beta}-Amyloid in Alzheimer disease-affected brain: a clinicopathologic study. Arch Neurol. 2008;65:906–912. doi: 10.1001/archneur.65.7.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toman RE, Spiegel S. Lysophospholipid receptors in the nervous system. Neurochem Res. 2002;27:619–627. doi: 10.1023/a:1020219915922. [DOI] [PubMed] [Google Scholar]
- 49.Van Veldhoven PP. Sphingosine-1-phosphate lyase. Methods Enzymol. 2000;311:244–254. doi: 10.1016/s0076-6879(00)11087-0. [DOI] [PubMed] [Google Scholar]
- 50.Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2010;69:119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Wang S, Lin Y, Cao L, Wang R, Chi Z. Ghrelin protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. Neurosci Lett. 2009;453:58–61. doi: 10.1016/j.neulet.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 52.Yang DI, Yeh CH, Chen S, Xu J, Hsu CY. Neutral sphingomyelinase activation in endothelial and glial cell death induced by amyloid beta-peptide. Neurobiol Dis. 2004;17:99–107. doi: 10.1016/j.nbd.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Young N, Van Brocklyn JR. Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. Scient World J. 2006;6:946–966. doi: 10.1100/tsw.2006.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang G, Contos JJA, Weiner JA, Fukushima N, Chun J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]