Abstract
Elevated level of plasma homocysteine (Hcy) has been identified as an independent risk factor for coronary artery disease (CAD). Furthermore, numerous studies have documented the influences of a common polymorphism (C677T) of methylenetetrahydrofolate reductase (MTHFR) on homocysteine levels. However the relationship between this mutation and cardiovascular diseases (CVD) has remained as a controversial issue. The present study was undertaken to investigate the relationship between C677T polymorphism of MTHFR gene, plasma total Hcy levels and the number of affected vessels as a criterion for the extent of CAD. MTHFR genotypes and plasma homocysteine (HCY) concentrations were examined in 231 patients and 300 healthy subjects who underwent diagnostic coronary angiography. A multiple linear regression analysis was performed to identify the predictors of Hcy levels whereas logistic regression model was built to determine the association of Hcy quartiles with the risk of CAD adjusted for risk factors. The prevalence of MTHFR genotypes was similar between CAD patients and non-CAD individuals while the geometric mean of Hcy values was significantly higher in patient group (14.13 ± 4.11 μmol/l) than in control group (10.19 ± 3.52 μmol/l) (P < 0.001). Moreover, unlike the MTHFR polymorphism, Hcy concentration increased with increasing number of stenosed vessels and the CAD risk increased about 2 folds in the top two Hcy quartiles (≥ 17.03 and 13.20-17.02 μmol/l) compared with the lowest quartile (≤ 9.92 μmol/l) after controlling for conventional risk factors (P<0.001 for both). Our data suggest that hyperhomocysteinaemia (HHcy) is significantly associated to CAD risk increase as well as to the extent of coronary atherosclerosis.
Keywords: methylene tetrahydrofolate reductase, homocystein, coronary artery disease, vessel score
Introduction
Higher plasma homocysteine (Hcy) concentrations are considered as an independent risk factor for coronary artery disease (CAD) (reviewed in De Bree et al., 2002[12]; Ford et al., 2002[14]; Parnetti et al., 2002[37]). Hyperhomocysteinaemia (HHcy) induced atherosclerosis is characterized by endothelial oxidative damage and dysfunction followed by platelet activation, promotion of platelet aggregation, alteration of the normal procoagulant-anticoagulant balance and thrombosis (Durand et al., 1997[13]; Hajjar et al., 1998[20]; Visioli et al., 2002[51]). Elevations of plasma Hcy has been associated with genetic defects in enzymes involved in its metabolism and/or with nutritional deficiencies of vitamin B6, B12, and folic acid (Brouwer et al., 1999[9]; Kullo et al., 2006[25]).
The 5,10-methylene tetrahydrofolate reductase (MTHFR) catalyzes the reduction of 5,10-methylene tetrahydrofolate to 5-methyltetrahydrofolate. The latter is the methyl donor in the conversion reaction of Hcy to methionine which is catalyzed by the vitamin B12-dependent methionine synthase. MTHFR-encoding gene has been reported to be a genetic determinant of HHcy (Franken et al., 1996[15]). A common substitution in this gene produces a thermolabile variant of MTHFR with specific decreased enzymatic activity which in turn leads to higher total Hcy (tHcy) concentrations in plasma (Frosst et al., 1995[16]). The molecular basis of this thermolability is a substitution of a cytosine to thymine at nucleotide 677 (rs1801133) in exon 4 of the MTHFR gene that converts an alanine to a valine codon (Frosst et al., 1995[16]).
Since the coronary atherosclerotic disease is the leading cause of death and disability among Iranian population (Hatmi et al., 2007[22]), the present study sought to investigate whether there is an association between C667T polymorphism of MTHFR gene and plasma Hcy levels, as well as to evaluate the relationship of this polymorphism and the tHcy concentrations with the extension of the coronary artery disease.
Materials and Methods
Study subjects
The study population comprised 300 healthy controls and 231 patients with premature CAD. All subjects in this study were recruited at three major university hospitals (Saadi, Kowsar and Nemazee) in Shiraz between July 2010 and March 2012. Women and men enrolled in the current investigation were all under 55 and 50 years of age respectively. All study participants were informed about the study's objectives, and an informed consent was obtained from those who agreed to participate. The Human Ethics Committee at the Medical University of Shiraz, Iran, approved the study protocol. At the time of subject enrollment, information about the standard risk factors of CAD and relevant data on past medical history such as diabetes mellitus, hypertension, smoking habits, family history and drug therapy was collected from all individuals. Diabetics were recognized as those with FBS levels higher than 126 mg/dl or subjects who were using oral hypoglycemic agents or insulin. Liver dysfunction, renal failure, chronic or acute infectious disease, pernicious anemia, hypothyroidism, use of anti-inflammatory drugs, steroids and vitamins supplementation (which might influence homocysteine levels) were accepted as exclusion criteria. The data of hypertension, smoking habits, diabetes mellitus, and family history of heart disease (defined as the incidence of myocardial infarction (MI), coronary artery disease and coronary artery bypass graft (CABG) in at least one of their first or second degree relatives) were registered as a two level variable. Smoking status was coded as ever and never smokers.
All of the subjects were of Iranian ancestry and none were first- or second-degree relatives.
Premature coronary artery disease
All participants underwent coronary angiography on account of premature CAD as a verified illness or presumptive diagnosis. Coronary angiography was carried out by the Judkins technique, with images recorded on compact disks. Subjects with more than 50 % stenosis in one or more of their three major coronary arteries (left anterior descending (LAD), left circumflex (LCx), and right coronary artery (RCA)) or their primary branches were considered as patients. All vessels were assessed from multiple angles by two experienced interventional cardiologists blinded to the patients' MTHFR genotype and tHcy concentration. Based on the number of involved coronary arteries (0-3), participants were sub-classified as follows: No vessel disease (NVD) who had no significantly stenosed vessels (< 50 % luminal stenosis); single vessel disease (SVD), who had one significantly stenosed vessel; double vessel disease (DVD), who had two significantly stenosed vessels; and triple vessel disease (TVD), who had severe CAD involving all the three major arteries.
Biochemical analysis
10 mL of venous blood samples (5 mL in EDTA and 5 mL without anticoagulant) were collected from each subject after overnight fasting. Blood samples without anticoagulant were freshly used for measuring the participants' lipid and sugar profiles by standard enzymatic assays (Pars Azmoon, diagnostic kits, Iran). EDTA containing portions which had been kept on ice in the dark were centrifuged within 90 min at 2500 rpm for 10 min at 4 oC. Resulted plasma fractions were aliquoted and stored at -70 oC until Hcy and folate estimation. High performance liquid chromatography (HPLC) with a fluorescent detector was used for determining the tHcy levels according to Araki and Sako (1987[2]) whereas the plasma concentrations of folate were obtained by an automated chemiluminescence method (Rozen, 1997[39]). HHcy was defined as a plasma tHcy level ≥15 μmol/L.
Genotype determination
Genomic DNA was extracted from peripheral blood leukocytes using salting out method (Miller et al., 1988[30]). Detection of the C677T polymorphism in the MTHFR gene was performed by PCR-RFLP analysis according to protocol conditions and primer sequences reported previously (Frosst et al., 1995[16]). Briefly, a 198 bp fragment surrounding the supposed mutation site was obtained by the following primers:
forward:
5'-TGAAGGAGAAGGTGTCTGCGGGA-3';
reverse:
5'-AGGCGGTGCGGTGAGAGTG-3'.
The PCR mixture (20 ml) contained 50 ng of genomic DNA, 10 pmol of each primer, 1.5 mM MgCl2, 2.5 mM of each dNTP, 1X PCR buffer and 1 U of Taq polymerase (Fermentas). The cycle parameters were 3 min initial denaturation at 94 °C, followed by 35 subsequent cycles of amplification at 94 °C for 45 sec, at 65 °C for 45 sec, and 72 °C for 45 sec. Final extension step was performed at 72 °C for 7 min. The PCR products were then subjected to HinfI (Fermentas) digestion by overnight incubation at 37 °C. Fragments were size-separated by gel electrophoresis using 4 % (w/v) agarose. Allele C was not digested and remained 198 bp after digestion whereas allele T was cut into two fragments of 175 bp and 23 bp, thus heterozygous subjects showed three fragments of 198, 175 and 23 bp.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) and were compared by Student's t-test or ANOVA for more than two groups. Scheffe's post-hoc test was utilized to discriminate the significant differences among a group of means because it is fairly conservative and therefore very robust to the F-test outcome (Snedecor and Cochran, 1980[43]). Kolmogorov-Smirnov test was used to assess the normality of distribution of continuous variables. Because of the right-skewed distribution of tHcy and folate, analyses were performed using log-transformed data for these variables to reduce kurtosis. Thus geometric means of the mentioned variable are presented. Chi-square test was employed to compare categorical variables as well as to assess the Hardy-Weinberg equilibrium. We used univariate analyses to estimate the association of Hcy values with other variables. For this purpose, Pearson correlation coefficients were computed to evaluate the relationships between continuous variables and Hcy concentrations whereas Student's t-test was used to compare the mean values of Hcy in dichotomous variables (Hypertension, familial history of heart disease, smoking habit, diabetes and sex). Subsequently, significantly associated variables were further analyzed by a multiple linear regression analysis to determine independent predictors of plasma tHcy levels. A logistic regression model was fitted to examine the independent impact of different clinical and biochemical factors on premature CAD. Furthermore, to evaluate the graded effect of Hcy concentrations on the risk of CAD, another logistic regression analysis - with Hcy quartiles as independent variables and the lowest quartile as reference - was carried out. Respective odds ratios (OR) were calculated for an unadjusted analysis as well as for a adjusted model which had been controlled for parameters that may contribute to the risk for CAD such as MTHFR genotype, diabetes, sex, hypertension, age, lipid profile, familial history and smoking status. Statistical analyses were all performed by the software package SPSS 17.0 (Statistical Package for the Social Science, SPSS, Inc., Chicago, Illinois) and a probability value ≤0.05 was used to establish statistical significance.
Results
Clinical and biochemical parameters
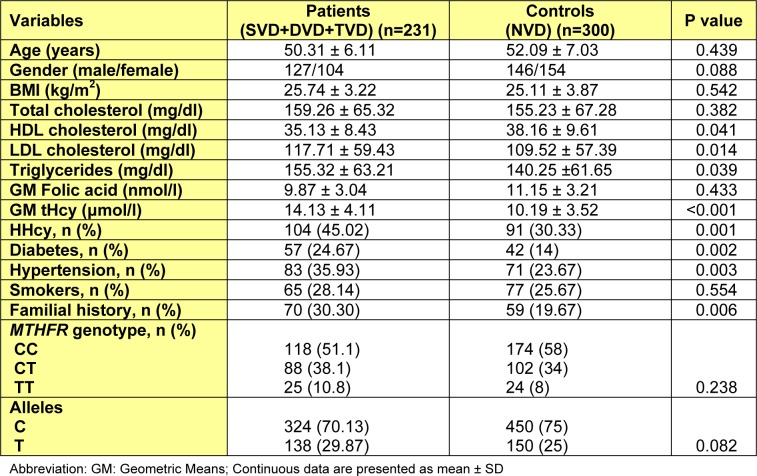
Baseline characteristics and clinical biochemistry parameters of the patients and controls are summarized in Table 1(Tab. 1). Altogether 273 men (aged 21-50 years; mean 47.44) and 258 women (aged 27-55 years; mean 51.13) were successfully genotyped in this study. As expected, the premature CAD group showed higher concentrations of LDL, triglyceride and Hcy, and lower concentrations of HDL compared with the control group. A significantly higher prevalence of diabetes, HHcy, familial history of heart disease and hypertension was also observed in patients. Plasma folate concentration did not differ significantly between premature CADs and healthy subjects. The two groups were matched for age (P=0.439) and sex (p=0.088). The relative frequencies of males in the patient and control groups were 54.98 % and 48.67 % respectively.
Table 1. Biological characteristics of participants in patient and control groups.
C677T polymorphism, premature CAD and homocysteine levels
The prevalence of the MTHFR genotypes was in the range of Hardy-Weinberg equilibrium both in the control (χ2=2.61, df=2, P=0.106) and patient (χ2=1.9, df=2, P=0.168) groups. C677T mutation frequency has been also presented in Table 1(Tab. 1) for the patient and control groups. There was no significant difference between the two groups regarding genotype (χ2 = 2.874, df = 2, P=238) and allele (χ2=3.132, df=1, P=082) frequencies. The TT genotype was observed in 8 % of the controls and 10.8 % of the patient group. No significant difference was observed in the prevalence of either any genotype or allele frequencies between male and female subjects (P0.05).
The premature CAD group consisted of 231 patients (aged 29-55 years; mean 50.31 years), including 78 (33.77 %) with one-, 62 (26.84 %) with two-, and 91 (39.40 %) with three-vessel disease while no vessel disease group (aged 21-55 years; mean 52.09 years) comprised of 300 subjects. Although a trend for higher prevalence of TT genotype was observed across the four subgroups of involved vessels, C677T polymorphism did not show a significant correlation with the extent of premature CAD (P=0.641) (Table 2(Tab. 2)). On the other hand, as Table 2(Tab. 2) displays, tHcy concentrations were significantly higher in patients with TVD (vs. SVD and NVD) and DVD (vs. NVD) (P<0.05 for both). In addition, the prevalence of HHcy was significantly lower in NVD subjects compared to DVD (P=0.027) and TVD (P=0.001) groups. Other investigated parameters had statistically similar distributions among different patient subgroups (i.e. SVD, DVD and TVD) (data not shown). Chi-square analysis also failed to find any significant difference in the number of diseased vessels between males and females (P>0.05).
Table 2. Distribution of MTHFR genotypes, HHcy and Hcy concentrations in studied population with different numbers of stenotic coronary artery.
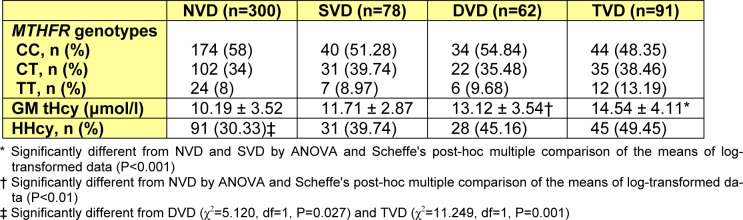
Table 3(Tab. 3) presents the distribution of biochemical and clinical factors according to the MTHFR genotypes in the patient group. No significant association was observed between investigated factors and MTHFR genotypes except for the prevalence of HHcy and mean tHcy concentration. Patients with TT genotype had significantly higher concentrations of tHcy compared to the wild type homozygotes (P<0.001) and heterozygotes (P<0.05). A same trend was observed for HHcy (Table 3(Tab. 3)) as patients with thermolabile homozygous genotype (TT) were 1.894 times more likely to have HHcy than patients with C allele carriers (recessive genetic model: TT vs. CT+CC; OR=1.894, 95 % CI: 1.198-2.994, P=0.006).
Table 3. Biochemical and clinical parameters in premature CAD subjects (SVD+DVD+TVD) with different MTHFR genotypes.
Determinants of tHcy concentrations
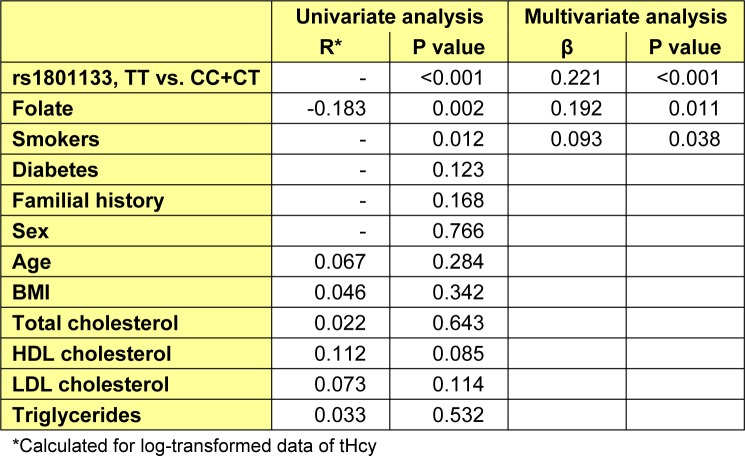
In univariate analysis plasma tHcy levels were correlated with folate level (r=-0.183, P=0.002). Likewise, the mean tHcy level was found to be higher in smokers and those with thermolabile homozygous genotype (recessive model; TT vs. CC+CT). Finally, the stepwise multiple linear regression analysis which was adjusted for folate and smoking status (adjusted R2=0.38, P<0.001), showed that rs1801133 in the recessive model was a significantly independent predictor of tHcy levels (P < 0.001; Table 4(Tab. 4)).
Table 4. Multivariate regression analysis for tHcy levels.
Predictors of premature coronary artery disease
In order to identify effective factors predisposing to premature CAD, a logistic regression analysis with backward selection strategy was conducted. Categorical variables (hypertension, gender, diabetes, MTHFR genotypes, familial history of heart disease and tobacco use) and continuous factors (tHcy, folate, TC, TG, HDL, LDL, BMI and age) were included in the model as independent variables. Results showed an independent association between tHcy and the risk of premature CAD (OR=9.04, 95% CI: 2.64-26.11; P =0.001). Hypertension, diabetes, familial history of heart disease and LDL cholesterol were also independently associated with premature CAD (Table 5(Tab. 5)).
Table 5. Risk factors for premature CAD with logistic regression.

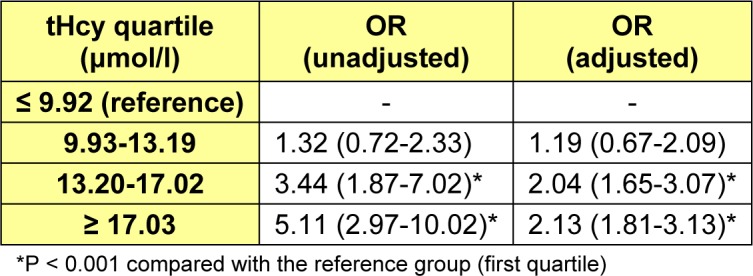
Additionally, to evaluate the graded impact of tHcy levels on the premature CAD, a logistic regression analysis was performed with the independent variable being Hcy quartiles. In our population 25th, 50th and 75th percentiles of Hcy (ranged from min=3.50 to max=69 μmol/l) were 9.92, 13.19 and 17.02 μmol/l respectively. Table 6(Tab. 6) presents association results between homocysteine quartiles and risk of premature CAD in two models: unadjusted model and a model adjusted for conventional risk factors of coronary atherosclerotic disease such as smoking, hypertension, lipids, age, diabetes, positive familial history of heart disease, MTHFR and sex. Apparently the odds ratio (OR) for premature CAD increased with increasing quartiles of Hcy (Ref=lowest quartile) both before and after adjustment. The adjusted model showed that subjects in the top two homocysteine quartiles (≥17.03 and 13.20-17.02 μmol/l) are 2.04 (95 % CI, 1.65-3.07) and 2.13 (95 % CI, 1.81-3.13) times more likely to have premature CAD than those in the first quartile (≤9.92 μmol/L) (P<0.001 for both) (Table 6(Tab. 6)).
Table 6. Association between tHcy quartiles and risk of premature CAD.
Discussion
Homocysteine hypothesis indicates that mild to moderate hyperhomocysteinemia is an independent predictor for the development of cardiovascular disease (CVD). However, there are also studies which have shed doubt on the role of this thiol-containing amino acid in the pathogenesis of atherosclerotic vascular disease (reviewed in Ueland et al., 2000[49]; Lentz and Haynes, 2004[28]). These controversial results brought up a homocysteine controversy instead of the homocysteine hypothesis (reviewed in Smulders and Blom, 2011[42]). In addition, several numbers of studies, from the early 1990s, have introduced various evidences for the genetic basis of elevated levels of Hcy (reviewed in Trabetti, 2008[47]). Among the candidate genes, the MTHFR is of particular medical interest as being a risk factor for hyperhomocysteinemia as well as for CVD. Although some meta-analyses have found an association between the MTHFR 677T allele and atherosclerosis, vascular disease and myocardial infarction (Wald et al., 2002[53]; Cronin et al., 2005[11]; Laraqui et al., 2007[26]), others failed to detect such a relationship (Brattstrom et al., 1998[8]; Klerk et al., 2002[24]). Ethnicity differences could be a responsible parameter for at least some of these controversial results.
The result of this investigation did not support an independent association of the MTHFR C677T polymorphism either with the presence of premature CAD or with its extent. However, a positive relationship was observed between the TT MTHFR genotype and elevated plasma tHcy levels. Folic acid and smoking habits were also among the significant predictors of tHcy levels in the current study. Folate status is the most important determinant of plasma homocysteine levels in the general population (Nygard et al., 1998[34]; Selhub et al., 1999[41]), and its low intake or blood concentrations increase the risk of CVD (Morrison et al., 1996[33]; Rimm et al., 1998[38]). Several mechanisms such as lipid peroxidation, platelet activation and aggregation, reduced VonWillebrand factor, inflammation, hypertension, endothelial damage and vasoconstriction (Harker et al., 1976[21]; Fryer et al., 1993[17]; Bartecchi et al., 1994[4]; MacKenzie et al., 1994[29]) have been suggested to explain the causal role of smoking on coronary atherosclerosis so far. Due to these results, the presence of a direct and consistent association between the gene variant and plasma tHcy levels is admitted in the studied population.
On the other hand, in the current study tHcy level was positively associated not only with the presence of premature CAD but also with the number of involved vessels which is in agreement with some previous reports regarding the correlation between tHcy levels and number of atherosclerotic vessels (von Eckardstein et al., 1994[52]; Montalescot et al., 1997[31]; Chao et al., 1999[10]; Tokgozoglu et al., 1999[46]; Yoo et al., 1999[54]; Kerkeni et al., 2006[23]; Alam et al., 2008[1]). There are also a few case-control studies in the literature which were unable to verify such a relationship (Bozkurt et al., 2003[7]; Guerzoni et al., 2009[19]). There is still a study in which tHcy levels showed a significant correlation with the extent of coronary atherosclerosis in patients with low cardiovascular risk profiles unlike in patients with high risk profiles (Tsai et al., 2000[48]). These controversial results involving the relation between plasma tHcy levels and the extent of CAD could be partly attributed to the variable stenosis criteria used in defining CAD by different studies. Some investigators have considered ≥ 50 % stenosis as the cut off point for CAD diagnosis (von Eckardstein et al., 1994[52]; Tokgozoglu et al., 1999[46]; Kerkeni et al., 2006[23]; Guerzoni et al., 2009[19]), whereas in other studies CAD criterion has been taken to be ≥ 70-75 % stenosis (Montalescot et al., 1997[31]; Chao et al., 1999[10]; Yoo et al. 1999[54]; Alam et al., 2008[1]). Moreover homocysteine circulating levels are affected by some various factors that differ in each population such as genetic factors, dietary habits, geographic and demographic differences and life style (Bayer et al., 2002[5]; Bozkurt et al., 2003[7]). Additionally independent risk factors predisposing individuals to atherosclerosis may also vary among various populations.
Furthermore, like some previous reports (Verhoef et al., 1997[50]; Chao et al., 1999[10]; Selhub et al., 2000[40]; Pajunen et al., 2002[35]; Panayiotou et al., 2009[36]) we found evidence of a graded effect of Hcy quartiles on the risk of premature CAD. Significant odds ratios of top two Hcy quartiles in comparison to the lowest quartile even after controlling for other conventional risk factors of CVD together with the robust association of tHcy levels with TT genotypes of MTHFR gene makes it reasonable to suggest that MTHFR mutation mediates its influence on atherosclerosis by means of HHcy.
In conclusion, these data suggest that an elevated plasma Hcy level, but not MTHFR mutation, is associated with the extent of premature coronary artery disease. In addition, our findings admit the role of the MTHFR T allele as an independent determinant of plasma tHcy concentration. However, the present study has some limitations. Pathologic studies have shown that arterial thrombosis and plaque rupture are episodic events which could significantly affect the severity of stenosis at the time of angiography (Sullivan et al., 1990[45]). On the other hand, it has been revealed that more than 50 % of the coronary surface is usually covered by raised plaque (Solberg and Strong, 1983[44]) and considerable portion of coronary events could be resulted from their progression (Azen et al., 1996[3]). Thus, estimating the extent of CAD by the number of involved vessels would contribute to underestimate the risk of atherosclerosis and may not provide the best reflection of this process. There are multiple scoring systems (Leaman et al., 1981[27]; Gensini, 1983[18]; Sullivan et al., 1990[45]; Montalescot et al., 1994[32]; Birnie et al., 1998[6]) which can evaluate the severity or extent of CAD much better than traditional vessel score approach used here. Besides, some other key determinants of plasma tHcy levels such as vitamins B6 and B12, caffeine consumption, creatinine concentration and exercise were not taken into account in the present investigation. Since TT genotype, smoking status and folic acid levels can only predict 38 % of tHcy changes in our population (Table 4(Tab. 4)), assessment of other Hcy predictors seem indispensible.
Acknowledgements
The authors wish to thank Prof. J. Dehbozorgian for referring patients into the study and Miss Z. Azmudeh for her help with data collection. We also thank the nursing staff of the Saadi, Nemazee and Kowsar hospitals. This work was funded by Fars Province Branch of Iranian Academic Center for Education, Culture & Research (ACECR).
References
- 1.Alam MA, Husain SA, Narang R, Chauhan SS, Kabra M, Vasisht S. Association of polymorphism in the thermolabile 5,10-methylene tetrahydrofolate reductase gene and hyperhomocysteinemia with coronary artery disease. Mol Cell Biochem. 2008;310:111–117. doi: 10.1007/s11010-007-9671-7. [DOI] [PubMed] [Google Scholar]
- 2.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 3.Azen SP, Mack WJ, Cashin-Hemphill L, LaBree L, Shircore AM, Selzer RH, et al. Progression of coronary artery disease predicts clinical coronary events. Long-term follow-up from the Cholesterol Lowering Atherosclerosis Study. Circulation. 1996;93:34–41. doi: 10.1161/01.cir.93.1.34. [DOI] [PubMed] [Google Scholar]
- 4.Bartecchi CE, MacKenzie TD, Schrier RW. The human costs of tobacco use (1) N Engl J Med. 1994;330:907–912. doi: 10.1056/NEJM199403313301307. [DOI] [PubMed] [Google Scholar]
- 5.Bayer IM, Caniggia I, Adamson SL, Langille BL. Experimental angiogenesis of arterial vasa vasorum. Cell Tissue Res. 2002;307:303–313. doi: 10.1007/s00441-002-0512-4. [DOI] [PubMed] [Google Scholar]
- 6.Birnie DH, Holme ER, McKay IC, Hood S, McColl KE, Hillis WS. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J. 1998;19:387–394. doi: 10.1053/euhj.1997.0618. [DOI] [PubMed] [Google Scholar]
- 7.Bozkurt A, Toyaksi H, Acarturk E, Tuli A, Cayli M. The effects of hyperhomocysteinemia on the presence, extent, and severity of coronary artery disease. Jpn Heart J. 2003;44:357–368. doi: 10.1536/jhj.44.357. [DOI] [PubMed] [Google Scholar]
- 8.Brattstrom L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation. 1998;98:2520–2526. doi: 10.1161/01.cir.98.23.2520. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer IA, van Dusseldorp M, Thomas CM, Duran M, Hautvast JG, Eskes TK, et al. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J Clin Nutr. 1999;69:99–104. doi: 10.1093/ajcn/69.1.99. [DOI] [PubMed] [Google Scholar]
- 10.Chao CL, Tsai HH, Lee CM, Hsu SM, Kao JT, Chien KL, et al. The graded effect of hyperhomocysteinemia on the severity and extent of coronary atherosclerosis. Atherosclerosis. 1999;147:379–386. doi: 10.1016/s0021-9150(99)00208-7. [DOI] [PubMed] [Google Scholar]
- 11.Cronin S, Furie KL, Kelly PJ. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke. 2005;36:1581–1587. doi: 10.1161/01.STR.0000169946.31639.af. [DOI] [PubMed] [Google Scholar]
- 12.De Bree A, Verschuren WM, Kromhout D, Kluijtmans LA, Blom HJ. Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev. 2002;54:599–618. doi: 10.1124/pr.54.4.599. [DOI] [PubMed] [Google Scholar]
- 13.Durand P, Lussier-Cacan S, Blache D. Acute methionine load-induced hyperhomocysteinemia enhances platelet aggregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J. 1997;11:1157–1168. [PubMed] [Google Scholar]
- 14.Ford ES, Smith SJ, Stroup DF, Steinberg KK, Mueller PW, Thacker SB. Homocyst(e)ine and cardiovascular disease: a systematic review of the evidence with special emphasis on case-control studies and nested case-control studies. Int J Epidemiol. 2002;31:59–70. doi: 10.1093/ije/31.1.59. [DOI] [PubMed] [Google Scholar]
- 15.Franken DG, Boers GH, Blom HJ, Cruysberg JR, Trijbels FJ, Hamel BC. Prevalence of familial mild hyperhomocysteinemia. Atherosclerosis. 1996;125:71–80. doi: 10.1016/0021-9150(96)05849-2. [DOI] [PubMed] [Google Scholar]
- 16.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 17.Fryer RH, Wilson BD, Gubler DB, Fitzgerald LA, Rodgers GM. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler Thromb. 1993;13:1327–1333. doi: 10.1161/01.atv.13.9.1327. [DOI] [PubMed] [Google Scholar]
- 18.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 19.Guerzoni AR, Biselli PM, Godoy MF, Souza DR, Haddad R, Eberlin MN, et al. Homocysteine and MTHFR and VEGF gene polymorphisms: impact on coronary artery disease. Arq Bras Cardiol. 2009;92:263–268. doi: 10.1590/s0066-782x2009000400003. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar KA, Mauri L, Jacovina AT, Zhong F, Mirza UA, Padovan JC, et al. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J Biol Chem. 1998;273:9987–9993. doi: 10.1074/jbc.273.16.9987. [DOI] [PubMed] [Google Scholar]
- 21.Harker LA, Ross R, Slichter SJ, Scott CR. Homocystine-induced arteriosclerosis. The role of endothelial cell injury and platelet response in its genesis. J Clin Invest. 1976;58:731–741. doi: 10.1172/JCI108520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatmi ZN, Tahvildari S, Gafarzadeh Motlag A, Sabouri Kashani A. Prevalence of coronary artery disease risk factors in Iran: a population based survey. BMC Cardiovasc Disord. 2007;7:32. doi: 10.1186/1471-2261-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerkeni M, Addad F, Chauffert M, Myara A, Gerhardt M, Chevenne D, et al. Hyperhomocysteinaemia, methylenetetrahydrofolate reductase polymorphism and risk of coronary artery disease. Ann Clin Biochem. 2006;43:200–206. doi: 10.1258/000456306776865232. [DOI] [PubMed] [Google Scholar]
- 24.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 25.Kullo IJ, Ding K, Boerwinkle E, Turner ST, Mosley TH, Jr., Kardia SL, et al. Novel genomic loci influencing plasma homocysteine levels. Stroke. 2006;37:1703–1709. doi: 10.1161/01.STR.0000225929.96190.b3. [DOI] [PubMed] [Google Scholar]
- 26.Laraqui A, Allami A, Carrie A, Raisonnier A, Coiffard AS, Benkouka F, et al. Relation between plasma homocysteine, gene polymorphisms of homocysteine metabolism-related enzymes, and angiographically proven coronary artery disease. Eur J Intern Med. 2007;18:474–483. doi: 10.1016/j.ejim.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Leaman DM, Brower RW, Meester GT, Serruys P, van den Brand M. Coronary artery atherosclerosis: severity of the disease, severity of angina pectoris and compromised left ventricular function. Circulation. 1981;63:285–299. doi: 10.1161/01.cir.63.2.285. [DOI] [PubMed] [Google Scholar]
- 28.Lentz SR, Haynes WG. Homocysteine: is it a clinically important cardiovascular risk factor? Cleve Clin J Med. 2004;71:729–734. doi: 10.3949/ccjm.71.9.729. [DOI] [PubMed] [Google Scholar]
- 29.MacKenzie TD, Bartecchi CE, Schrier RW. The human costs of tobacco use (2) N Engl J Med. 1994;330:975–980. doi: 10.1056/NEJM199404073301406. [DOI] [PubMed] [Google Scholar]
- 30.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montalescot G, Ankri A, Chadefaux-Vekemans B, Blacher J, Philippe F, Drobinski G, et al. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int J Cardiol. 1997;60:295–300. doi: 10.1016/s0167-5273(97)00099-5. [DOI] [PubMed] [Google Scholar]
- 32.Montalescot G, Viossat I, Chabrier PE, Sotirov I, Detienne JP, Drobinski G, et al. Endothelin-1 in patients with coronary heart disease undergoing cardiac catheterization. J Am Coll Cardiol. 1994;24:1236–1241. doi: 10.1016/0735-1097(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 33.Morrison HI, Schaubel D, Desmeules M, Wigle DT. Serum folate and risk of fatal coronary heart disease. JAMA. 1996;275:1893–1896. doi: 10.1001/jama.1996.03530480035037. [DOI] [PubMed] [Google Scholar]
- 34.Nygard O, Refsum H, Ueland PM, Vollset SE. Major lifestyle determinants of plasma total homocysteine distribution: the Hordaland Homocysteine Study. Am J Clin Nutr. 1998;67:263–270. doi: 10.1093/ajcn/67.2.263. [DOI] [PubMed] [Google Scholar]
- 35.Pajunen P, Syvanne M, Nieminen MS, Kareinen A, Viitanen L, Lehto S, et al. Serum homocysteine, creatinine, and glucose as predictors of the severity and extent of coronary artery disease in asymptomatic members of high-risk families. Eur J Clin Invest. 2002;32:472–478. doi: 10.1046/j.1365-2362.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 36.Panayiotou A, Nicolaides A, Griffin M, Tyllis T, Georgiou N, Martin R, et al. Serum total homocysteine, folate, 5,10-methylenetetrahydrofolate reductase (MTHFR) 677C-->T genotype and subclinical atherosclerosis. Expert Opin Ther Targets. 2009;13:1–11. doi: 10.1517/14728220802560281. [DOI] [PubMed] [Google Scholar]
- 37.Parnetti L, Caso V, Amici S, Lanari A, Gallai V, Bottiglieri T. Hyperhomocyst(e)inemia: a risk factor for cerebrovascular disease. Clin Exp Hypertens. 2002;24:501–509. doi: 10.1081/ceh-120015326. [DOI] [PubMed] [Google Scholar]
- 38.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, et al. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–364. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 39.Rozen R. Genetic predisposition to hyperhomocysteinemia: deficiency of methylenetetrahydrofolate reductase (MTHFR) Thromb Haemost. 1997;78:523–526. [PubMed] [Google Scholar]
- 40.Selhub J, Jacques PF, Bostom AG, Wilson PW, Rosenberg IH. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Public Health Rev. 2000;28:117–145. [PubMed] [Google Scholar]
- 41.Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, et al. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991-1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131:331–339. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 42.Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2011;34:93–99. doi: 10.1007/s10545-010-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snedecor GW, Cochran WG. Statistical methods. Des Moines, Iowa: Iowa State University Press; 1980. Statistical methods; pp. 215–237. [Google Scholar]
- 44.Solberg LA, Strong JP. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis. 1983;3:187–198. doi: 10.1161/01.atv.3.3.187. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan DR, Marwick TH, Freedman SB. A new method of scoring coronary angiograms to reflect extent of coronary atherosclerosis and improve correlation with major risk factors. Am Heart J. 1990;119:1262–1267. doi: 10.1016/s0002-8703(05)80173-5. [DOI] [PubMed] [Google Scholar]
- 46.Tokgozoglu SL, Alikasifoglu M, Unsal, Atalar E, Aytemir K, Ozer N, et al. Methylene tetrahydrofolate reductase genotype and the risk and extent of coronary artery disease in a population with low plasma folate. Heart. 1999;81:518–522. doi: 10.1136/hrt.81.5.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–282. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 48.Tsai WC, Li YH, Tsai LM, Chao TH, Lin LJ, Chen TY, et al. Correlation of homocytsteine levels with the extent of coronary atherosclerosis in patients with low cardiovascular risk profiles. Am J Cardiol. 2000;85:49–52. doi: 10.1016/s0002-9149(99)00605-0. [DOI] [PubMed] [Google Scholar]
- 49.Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72:324–332. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- 50.Verhoef P, Kok FJ, Kruyssen DA, Schouten EG, Witteman JC, Grobbee DE, et al. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:989–995. doi: 10.1161/01.atv.17.5.989. [DOI] [PubMed] [Google Scholar]
- 51.Visioli F, Smith A, Zhang W, Keaney JF, Jr , Hagen T, Frei B. Lipoic acid and vitamin C potentiate nitric oxide synthesis in human aortic endothelial cells independently of cellular glutathione status. Redox Rep. 2002;7:223–227. doi: 10.1179/135100002125000604. [DOI] [PubMed] [Google Scholar]
- 52.von Eckardstein A, Malinow MR, Upson B, Heinrich J, Schulte H, Schonfeld R, et al. Effects of age, lipoproteins, and hemostatic parameters on the role of homocyst(e)inemia as a cardiovascular risk factor in men. Arterioscler Thromb. 1994;14:460–464. doi: 10.1161/01.atv.14.3.460. [DOI] [PubMed] [Google Scholar]
- 53.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo JH, Park JE, Hong KP, Lee SH, Kim DK, Lee WR, et al. Moderate hyperhomocyst(e)inemia is associated with the presence of coronary artery disease and the severity of coronary atherosclerosis in Koreans. Thromb Res. 1999;94:45–52. doi: 10.1016/s0049-3848(98)00197-2. [DOI] [PubMed] [Google Scholar]