Abstract
Purpose
We tested whether 18 polymorphisms in 16 genes (GSTP1, COX2, IL-10, EGFR, EGF, FGFR4, CCDN1, VEGFR2, VEGF, CXCR2, IL-8, MMP3, ICAM1, ERCC1, RAD51 and XRCC3) would predict disease-free-survival (DFS), Overall survival (OS) and toxicity in the INT0144 trial, which was designed to investigate different postoperative regimen of 5-FU-based chemoradiation in locally advanced rectal cancers: Arm1 consisted of bolus 5-FU followed by 5-FU protracted venous infusion (PVI) with radiotherapy; Arm2 was induction and concomitant PVI 5-FU with radiotherapy Arm3 was induction and concomitant bolus 5-FU with radiotherapy.
Patients and Methods
DNA from 746 stage II/III rectal patients enrolled in the SWOG S9304 phase III trial was analyzed. Genomic DNA was extracted from FFPE tumor tissue. The polymorphisms were analyzed using direct DNA-sequencing or PCR-RFLP.
Results
GSTP1-Ile105Val (rs1695) was significantly associated with DFS and OS and its effect did not vary by treatment arm. The 5-year DFS and OS were 53% and 58%, respectively, for G/G, 66% and 72% for G/A and 57% and 66% for A/A patients. In Arm2, IL8-251A/A genotype (rs4073) was associated with a lower risk of toxicities (p=0.04). The VEGFR2 H472Q Q/Q genotype (rs1870377) was associated with a higher risk of grade 3–5 proximal upper gastrointestinal tract (PUGIT) mucositis (p=0.04) in Arm 2. However, in Arm 1 this genotype was associated with a lower risk of PUGIT mucositis (p=0.004).
Conclusion
rs1695 may be prognostic in patients with rectal cancer treated with adjuvant chemoradiation. rs4073 and rs1870377 may exhibit different associations with toxicity, according to the 5-FU schedule.
Keywords: rectal cancer, polymorphism, prognostic, GSTP1
INTRODUCTION
The efficacy of 5-fluorouracil (5-FU)-based chemoradiation (CRT) in rectal cancer has been well demonstrated by several large prospective studies, improving both local control and survival when compared to surgery alone or post-operative radiotherapy.(1,2) Consequently, post-operative 5-FU-based CRT was widely adopted for stage II and III rectal cancers in North America. More recently, based on the publication of two large phase 3 trials, 5-FU-based CRT was shifted to the neoadjuvant setting.(3,4) However, while the CRT sequence with regard to surgery has been shown to be important for local recurrence, its impact on overall survival (OS) is currently unclear. With regards to the chemotherapeutic agents, 5-FU remains the cornerstone agent that is combined with radiotherapy.
The Southwest Oncology Group (SWOG) initiated a clinical trial (S9304 /INT 0144) on behalf of the Gastrointestinal Intergroup, comparing adjuvant CRT therapy using different 5-FU based regimens to clarify their impact on clinical outcome and toxicity in patients with stage II and III rectal cancer. The SWOG S9304 study is the largest study reported so far in patients with locally advanced rectal cancer, with 1917 registered patients. Patients were randomized to 3 treatment arms: bolus 5-FU before and after radiotherapy with 5-FU protracted venous infusion (PVI) during radiotherapy (RT), 5-FU PVI only and bolus 5-FU only with leucovorin and levamisole. All arms provided similar disease-free survival (DFS), 3-year DFS of 67–69%, and OS, 3-year OS of 81–83%. In contrast, toxicity profiles were different with more grade 3 to 4 hematologic toxicities in the two bolus 5-FU arms (arm 1, 49% and arm 3, 55% vs 4% in arm 2).(5)
In contrast to the growing number of predictive biomarkers for anti-cancer agents, there are no established biomarkers to select patients who will benefit most from chemo-radiation, ultimately leading to higher cure rates.(6) Patients having a worse outcome despite 5-FU-based CRT may need treatment intensification and more efficient monitoring strategies that could detect early recurrence and allow curative resection. It is recognized that germline variations in genes involved in the host microenvironment, DNA repair and drug metabolisms, as opposed to somatic mutations in the tumor, have a great influence for treatment efficacy or toxicity.(7) We hypothesized that genetic variation within critical genes involved in major biologic capabilities of the rectal tumors might have prognostic value in patients treated with 5-FU based CRT. We further assessed if these genetic variations would be associated with treatment toxicity. We evaluated 18 polymorphisms in 16 genes involved in detoxification (GSTP1, rs1695), angiogenesis (VEGFR2, rs1870377; VEGF, rs2010963 and rs3025039; CXCR2, rs2230054; IL-8, rs4073), DNA repair (ERCC1, rs11615; RAD51, rs1801320; XRCC3, rs861539), adhesion (ICAM1, rs5498; MMP3, promoter 5A/6A), inflammation (COX2, rs20417 and rs5275; Il-10, rs1800896), and tumor growth (EGFR, rs2227983; EGF, rs4444903; FGFR4, rs351855; CCDN1, rs17852153). The polymorphisms were selected based on functionality and/or based on documented association with radiation, 5-FU-based chemoradiation or colorectal cancer patients’ risk or prognosis (Supplemental Table 1).
METHODS
The SWOG S9304 trial
The SWOG S9304 has been previously reported. (5) A total of 1917 patients with non-metastatic rectal adenocarcinoma (T3-4, N0 or T1-4, N1-3) were enrolled in the SWOG S9304 study from March 1994 to August 2000. Patients were randomly allocated to one of three chemotherapy regimens and stratified according to nodal involvement, type of surgery, primary tumor stage, and time from surgery to registration.
Arm 1 consisted of induction bolus 5-FU followed by combined 5-FU PVI and radiation. Bolus 5-FU was then administered for two cycles.
Arm 2 consisted of induction 5-FU PVI followed by combined 5-FU PVI and radiation. Additional 5-FU PVI was given after completion of radiation.
Arm 3 consisted of induction bolus 5-FU, leucovorin (LV) and levamisole. Radiation was combined with bolus 5-FU and LV. Patients then received additional bolus 5-FU, LV and levamisole.
Radiation consisted in 45 Gy in single daily 1.8 Gy fractions followed by a boost volume of 5.4 Gy in 1.8 Gy fractions. Patients’ characteristics, treatment schedules and outcomes have been previously described.(5)
Assessment of chemotherapy toxicity
The SWOG S9304 study used the Common Toxicity Criteria for Adverse Events (CTCAE) version 2.0. For this correlative study, we defined the clinical outcome of toxicity as any CTCAE grade ≥3 toxicity reported over the entire trial duration. Because of the distinct reported toxicity profile of each treatment regimen, toxicity outcomes were analyzed separately within each treatment arm. In addition to the overall toxicity, we explored the association of the polymorphisms with specific toxicities defined as: any grade ≥3 hematological toxicity; any grade ≥3 gastrointestinal toxicity; and any grade ≥3 mucositis of the proximal upper gastrointestinal tract (PUGIT) defined by occurrence of any grade ≥3 stomatitis, pharyngitis or esophagitis.
Molecular assessment
All patients signed an informed consent prior to entering this study that included information regarding the use of their tumor tissue to explore relevant molecular parameters. Formalin-fixed, paraffin-embedded (FFPE) surgically resected tumor tissue from the primary tumor was available for 746 patients. Blocks or 10-um thick unstained slides were anonymized, and then forwarded to the laboratory, which was blind to treatment and outcomes. Samples were sent to the laboratory through multiple shipments over an extended period of time (from 2007 to 2010). Therefore, a screen testing with 241 available patients was performed and reported in 2008.(8) Polymorphisms with at least a trend for an association with outcome were expanded for an additional 505 patients. Based on newly available data, promising polymorphisms were tested in the latest cohort and correlated with survival and toxicity. Additional testing in the screen cohort was not conducted because of the lack of sufficient tumor tissue. Toxicity analysis for the polymorphisms evaluated solely in the screen cohort was not performed because of the resulting insufficient number of patients within each arm. This study was conducted adhering to the REporting recommendations for tumor MARKer prognostic studies (REMARK). The reference single nucleotide polymorphisms (SNP) identification numbers, forward and reverse primer and assay type are summarized in supplemental Table 1.
Genotyping
Two 10-um thick unstained slides were macro dissected by sterilized surgical scalpel. FFPE tumor tissue were collected in a sterilized eppendoff tube. DNA isolation was done by conventional proteinase K-phenol/chloroform -- ethanol method using the QIAamp extraction kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Extracted DNA was stored at −80°C. Polymorphisms were determined using the Polymerase chain reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) technique. Briefly, 100ng genomic DNA was amplified by PCR, followed by restriction enzyme digestion. Resulting DNA fragments were visualized on 3–4% ethidium bromide-stained agarose gels. If no matching restriction enzyme could be found, samples were analyzed by direct sequencing.. For quality control purposes, 5% of the samples were analyzed by direct DNA-sequencing for each polymorphism to double-check the quality of the PCR-RFLP essays. Genotype concordance was >98%.
mRNA quantification
GSTP1 mRNA expression levels were measured at Response Genetics, Inc, a CLIA approved laboratory. Manual micro-dissection using a light microscope was performed on all tumor samples to ensure >80% tumor cells were dissected. After RNA isolation, cDNA was prepared from each sample as described previously.(9,10) Quantitation of GSTP1 and an internal reference (β-actin) cDNA were performed using a fluorescence-based real-time detection method (ABI PRISM 7900HT Sequence detection System [TaqMan®] Applied Biosystems, Foster City, CA) as previously described.(11) Primers and probe for detectection of GSTP1 mRNA gene expression levels as following: probe; TCACCTGGGCCGCACCCTTG; forward primer, CCTGTACCAGTCCAATACCATCCT; reverse primer, TCCTGCTGGTCCTTCC-CATA. All samples were amplified in triplicate. mRNA quantification was done by using the average of the triplicates.
Statistical Analysis
The primary end-points of this study were OS, DFS, relapse patterns and toxicity. OS was defined as the date from registration until date of death from any cause. Patients still known to be alive at the time of analysis were censored at the last known date of contact. DFS was defined as date from registration to the first development of recurrent disease, or of death from any cause, whichever came first. Patients still known to be alive without recurrence at the time of analysis were considered to be censored at their last follow-up time. OS and DFS estimates and curves were generated using the Kaplan-Meier method. Polymorphisms were modeled using indicator variables for the heterozygous and homozygous variant genotypes. The associations of polymorphisms with OS and DFS were analyzed univariately using the Cox proportional hazards model and adjusting for the variables used in stratification at time of randomization: the type of surgery (abdominoperineal resection vs. anterior resection), nodal involvement (N0 vs. N1 vs. N2-3), primary tumor stage (T1-2 vs. T3 vs. T4b) , and time from surgery to registration (20 – 45 days vs. 46 – 70 days), both in comparing pairwise genotype levels as well as the overall effect of the polymorphism on OS and DFS
Toxicities, using the National Cancer Institute Common Toxicity Criteria (version 2.0), were categorized by maximum grade, evaluating at Grades 0–2 vs Grades 3–5. The maximum grade of all reported toxicities was identified, as well as the maximum grade of specific toxicities including hematologic, gastrointestinal and stomatitis or esophagitis. The association between the polymorphisms and toxicity was tested using chi-square test. Given the number of analyses and comparisons, all unadjusted p-values at or below the 0.05 level were adjusted for SNP-wise error rates using the Bonferroni-Holm method.(12)
RESULTS
Patients’ characteristics and outcomes included in this correlative study were similar to the overall SWOG S9304 population, and are summarized in Table 1. The large majority of studied patients were Caucasian (92%). At the time of data analysis, 383 out of 746 patients were still alive with a median follow-up of 123 months.
Table 1.
Patients’ characteristics
| Samples Available (N=746) | No samples Available (N=1108) | p-value | |
|---|---|---|---|
|
| |||
| Median Age (range) | 61 years (19–86) | 61 years (21–85) | 0.30 |
|
| |||
| Gender | |||
| Male | 479 (64%) | 718 (65%) | 0.80 |
| Female | 267 (36%) | 390 (35%) | |
|
| |||
| Race | |||
| White | 692 (93%) | 1002 (90%) | 0.09 |
| Non-white | 54 (7%) | 106 (10%) | |
|
| |||
| Spanish/Hispanic Origin | |||
| No | 701(94%) | 1034 (93%) | 0.63 |
| Yes/Unknown | 45 (6%) | 74 (7%) | |
|
| |||
| Performance Status | |||
| 0/1 | 727 (97%) | 1070 (97%) | 0.34 |
| 2 | 19 (3%) | 38 (3%) | |
|
| |||
| Type of surgery | |||
| APR | 283 (38%) | 399 (36%) | 0.40 |
| Anterieur resection | 463 (62%) | 709 (64%) | |
|
| |||
| Treatment Arm | |||
| Arm1 | 257 (34%) | 368 (33%) | 0.55 |
| Arm 2 | 233 (31%) | 373 (34%) | |
| Arm 3 | 256 (35%) | 367 (33%) | |
|
| |||
| N stage | |||
| N0 | 225 (30%) | 341 (31%) | 0.59 |
| N1 | 316 (42%) | 444 (40%) | |
| N2-3 | 205 (28%) | 323(29%) | |
|
| |||
| T stage | |||
| T1-2 | 111 (15%) | 191 (17%) | 0.25 |
| T3-T4a | 589 (79%) | 838 (76%) | |
| T4b | 46 (6%) | 79 (7%) | |
|
| |||
| Stage | |||
| 2 | 225 (30%) | 341 (31%) | 0.78 |
| 3 | 521 (70%) | 767 (69%) | |
|
| |||
| TFSR | |||
| 20–45 days | 477 (64%) | 683 (62%) | 0.33 |
| 46–70 days | 269 (36%) | 425 (38%) | |
|
| |||
| 3-Year OS (95% CI) | 80% (77% – 83%) | 83% (81% – 86%) | 0.38 |
|
| |||
| 3-Year DFS (95% CI) | 67% (64% – 70%) | 70% (67% – 72%) | 0.42 |
Abbreviations: DFS, disease-free survival; N, number; OS, overall survival; TFSR, Time From Surgery to Registration
Association of polymorphisms with DFS and OS
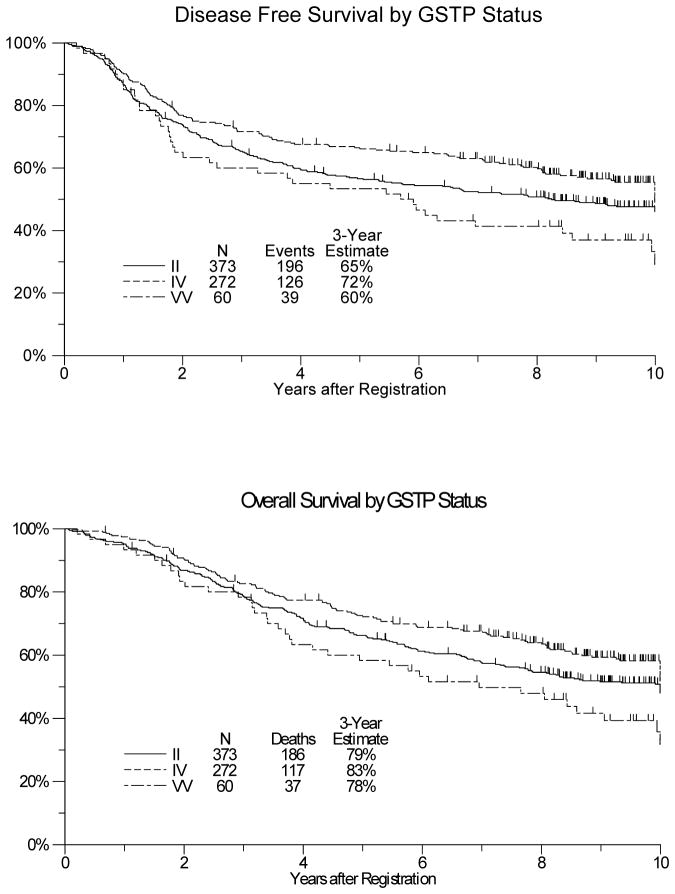
In univariate analyses, GSTP1-Ile105Val (rs1695) was significantly associated with DFS and OS. The 3-year and 5-year DFS was 60% and 53% for the low activity genotype (G/G), 72% and 66% for G/A and 65% and 57% for A/A patients (p=0.02; Figure 1A). The 3-year and 5-year OS was 78% and 58% for the low activity genotype (G/G), 83% and 72% for G/A and 79% and 66% for A/A patients (p=0.02; Figure 1B). In the multivariate model adjusting for type of surgery, nodal status, primary tumor stage and time from surgery until registration, rs1695 remained associated with both DFS and OS. (Table 2;p=0.01 and p=0.01). The effect of rs1695 did not vary by treatment arm (pinteraction=0.13) nor by gender (p=0.10). Furthermore, its effect did not vary by either age or stage (stage 2 vs 3). None of the other tested polymorphisms were significantly associated with DFS or OS (Table 2).
Figure 1.
Disease-free survival (figure 1A) and overall survival (figure 1B) by gluthathione S-transferase pi 1 (GSTP1) I105V polymorphism (rs1695) in 705 patients treated with adjuvant 5-fluorouracil-based chemoradiation within the SWOG S9304 trial.
Table 2.
Polymorphisms in Relation to Overall-Survival and Disease-Free Survival
| Overall Survivala
|
Disease-Free Survivala
|
|||||
|---|---|---|---|---|---|---|
| Polymorphism | Genotype | N | HR (95% CI) | p valueb | HR (95% CI) | p valueb |
|
|
|
|||||
| rs11615 | CC | 226 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 301 | 0.98 (0.76 – 1.27) | 0.90 | 0.98 (0.76 – 1.26) | 0.86 | |
| TT | 182 | 1.00 (0.75 – 1.33) | 0.98 | 0.99 (0.75 – 1.31) | 0.97 | |
| Peffect = 0. 99 | Peffect = 0. 99 | |||||
| rs1695 | AA | 373 | 1.0 (reference) | – | 1.0 (reference) | – |
| AG | 272 | 0.83 (0.65 – 1.05) | 0.13 | 0.80 (0.63 – 1.01) | 0.06 | |
| GG | 60 | 1.46 (1.01 – 2.11) | 0.04 | 1.38 (0.97 – 1.98) | 0.08 | |
| Peffect = 0. 01¥ | Peffect = 0.01¥ | |||||
| rs1801320 | GG | 170 | 1.0 (reference) | – | 1.0 (reference) | – |
| CG | 31 | 0.87 (0.50 – 1.53) | 0.63 | 0.95 (0.55 – 1.64) | 0.85 | |
| CC | 4 | 5.23 (1.06 – 25.69) | 0.04 | 4.19 (0.94 – 18.71) | 0.06 | |
| Peffect = 0.12 | Peffect = 0.19 | |||||
| rs178522153 | GG | 259 | 1.0 (reference) | – | 1.0 (reference) | – |
| GA | 296 | 0.98 (0.76 – 1.26) | 0.88 | 0.98 (0.77 – 1.25) | 0.86 | |
| AA | 166 | 1.19 (0.89 – 1.57) | 0.24 | 1.23 (0.94 – 1.62) | 0.14 | |
| Peffect = 0. 37 | Peffect = 0.21 | |||||
| rs5498 | CC | 146 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 274 | 0.86 – (0.65 – 1.15) | 0.31 | 0.86 (0.65 – 1.14) | 0.29 | |
| TT | 216 | 0.73 (0.53 – 1.00) | 0.05 | 0.75 (0.55 – 1.02) | 0.07 | |
| Peffect = 0.14 | Peffect = 0.19 | |||||
| rs861539 | CC | 103 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 76 | 0.79 (0.51 – 1.20) | 0.27 | 0.79 (0.52 – 1.21) | 0.29 | |
| TT | 30 | 0.78 (0.43 – 1.40) | 0.40 | 0.74 (0.41 – 1.32) | 0.31 | |
| Peffect = 0.46 | Peffect = 0.43 | |||||
| rs20417 | GG | 476 | 1.0 (reference) | – | 1.0 (reference) | – |
| GC | 188 | 1.11 (0.87 – 1.42) | 0.41 | 1.12 (0.89 – 1.43) | 0.33 | |
| CC | 38 | 1.00 (0.59 – 1.71) | 0.99 | 1.07 (0.64 – 1.80 ) | 0.79 | |
| Peffect = 0.70 | Peffect = 0.63 | |||||
| rs5275 | CC | 61 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 186 | 0.81 (0.51 – 1.28) | 0.36 | 0.87 (0.55 – 1.37) | 0.54 | |
| TT | 249 | 0.89 (0.58 – 1.39) | 0.62 | 0.92 (0.60 – 1.43) | 0.72 | |
| Peffect = 0.63 | Peffect = 0.82 | |||||
| rs1800896 | GG | 60 | 1.0 (reference) | – | 1.0 (reference) | – |
| GA | 96 | 1.01 (0.63 – 1.62) | 0.97 | 0.97 (0.61 – 1.54) | 0.89 | |
| AA | 63 | 1.40 (0.84 – 2.36) | 0.20 | 1.19 (0.71 – 1.99) | 0.51 | |
| Peffect = 0.30 | Peffect = 0.65 | |||||
| rs4444903 | GG | 114 | 1.0 (reference) | – | 1.0 (reference) | – |
| AG | 207 | 0.80 (0.56 – 1.14) | 0.22 | 0.75 (0.54 – 1.05) | 0.09 | |
| AA | 183 | 0.86 (0.60 – 1.24) | 0.42 | 0.82 (0.58 – 1.15) | 0.24 | |
| Peffect = 0.47 | Peffect = 0.25 | |||||
| rs2227983 | GG | 266 | 1.0 (reference) | – | 1.0 (reference) | – |
| AG | 186 | 1.05 (0.79 – 1.41) | 0.73 | 1.04 (0.79 – 1.37) | 0.79 | |
| AA | 42 | 0.99 (0.59 – 1.67) | 0.97 | 0.94 (0.57 – 1.55) | 0.80 | |
| Peffect = 0.94 | Peffect = 0.92 | |||||
| rs351855 | GG | 108 | 1.0 (reference) | – | 1.0 (reference) | – |
| GA | 65 | 0.84 (0.53 – 1.32) | 0.44 | 0.86 (0.55 – 1.33) | 0.49 | |
| AA | 41 | 1.06 (0.62 – 1.83) | 0.83 | 1.06 (0.61 – 1.82) | 0.84 | |
| Peffect = 0.65 | Peffect = 0. 69 | |||||
| rs2010963 | GG | 226 | 1.0 (reference) | – | 1.0 (reference) | – |
| CG | 183 | 0.80 (0.59 – 1.09) | 0.15 | 0.82 (0.61 – 1.09) | 0.18 | |
| CC | 79 | 0.78 (0.52 – 1.18) | 0.25 | 0.82 (0.55 – 1.21) | 0.31 | |
| Peffect = 0.26 | Peffect = 0.32 | |||||
| rs1870377 | AA | 36 | 1.0 (reference) | – | 1.0 (reference) | – |
| AT | 200 | 0.97 (0.57 – 1.65) | 0.92 | 0.94 (0.56 – 1.56) | 0.80 | |
| TT | 262 | 0.87 (0.51 – 1.47) | 0.60 | 0.86 (0.52 – 1.42) | 0.56 | |
| Peffect = 0.72 | Peffect = 0. 75 | |||||
| rs3025039 | CC | 489 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 195 | 0.99 (0.77 – 1.26) | 0.91 | 0.94 (0.74 – 1.20) | 0.63 | |
| TT | 15 | 0.63 (0.26 – 1.55) | 0.32 | 0.56 (0.23 – 1.37) | 0.20 | |
| Peffect = 0.56 | Peffect = 0.35 | |||||
| rs4073 | AA | 163 | 1.0 (reference) | – | 1.0 (reference) | – |
| AT | 334 | 0.97 (0.73 – 1.29) | 0.84 | 0.99 (0.75 – 1.29) | 0.92 | |
| TT | 221 | 1.15 (0.85 – 1.56) | 0.35 | 1.18 (0.88 – 1.58) | 0.27 | |
| Peffect = 0. 40 | Peffect = 0.33 | |||||
| rs2230054 | TT | 4 | 1.0 (reference) | – | 1.0 (reference) | – |
| CT | 144 | 2.18 (0.90 – 16.07) | 0.44 | 0.97 (0.23 – 4.06) | 0.97 | |
| CC | 357 | 1.97 (0.27 – 14.39) | 0.50 | 0.87 (0.21 – 3.62) | 0.85 | |
| Peffect = 0.59 | Peffect = 0.77 | |||||
| MMP3 | 5A/5A | 59 | 1.0 (reference) | – | 1.0 (reference) | – |
| 5A/6A | 103 | 1.17 (0.74 – 1.87) | 0.50 | 1.45 (0.91 – 2.31) | 0.12 | |
| 6A/6A | 62 | 1.15 (0.67 – 1.96) | 0.61 | 1.32 (0.77 – 2.26) | 0.31 | |
| Peffect = 0.79 | Peffect = 0.28 | |||||
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio;
Adjusted for type of surgery, lymph node involvement, primary tumor size, and time from surgery to registration.
multivariate P–value from Cox regression analysis comparing pairwise genotypes within each polymorphism.
Peffect: multivariate P–value from Cox regression comparing overall effect of polymorphism on OS and DFS.
P–value adjusted for multiple comparisons p=0.18.
Association of polymorphisms with site of relapse
The site of relapse of the studied population was similar to the overall SWOG S9304 population (Supplemental Table 2). rs1695 was associated with a significantly different rate of distant relapse (p=0.03), consistent with the overall effect on DFS and OS (Supplemental Table 3). rs1695 was not associated with pelvic failure risk. Also, MMP3 polymorphism showed a similar association that should be interpreted with caution, given the small number of patients and the lack of effect on DFS and OS.
Association of GSTP1 mRNA levels with GSTP1 variants
Among patients with both rs1695 genotypes and gene expression levels assessed, there were 376 eligible patients with both genotype and expression data available. The distribution of rs1695 genotypes in these 376 patients was 171 (45%), 163 (43%) and 42 (11%) for A/A, G/A and G/G respectively. There was no significant association between rs1695 genotypes and gene expression levels (Supplemental Table 4).
Association of polymorphisms with toxicity
In Arm 2, the homozygous IL8 -251A/A genotype (rs4073) was associated with a lower risk of any grade 3-5 toxicities compared to A/T or T/T genotypes (p=0.04; Supplemental Table 5). None of the evaluated polymorphisms predicted a significant relation with the incidence of overall toxicity in the two arms with bolus 5-FU-based CRT. Despite not being significantly associated with overall toxicity, several polymorphisms were related to specific grade 3–5 toxicities (Supplemental Table 5). Given the relatively small number of patients and events, these findings should be interpreted with caution. Overall, stomatitis and pharyngitis represented most of the PUGIT mucositis (stomatitis and pharyngitis = 425; esophagitis =17). The homozygous VEGFR2 H472Q A/A genotype (rs1870377) was associated with a higher risk of grade 3–5 PUGIT mucositis compared to A/T or T/T genotypes in Arm 2 (p=0.04). However, the rs1870377 A/A genotype was significantly associated with a lower risk of PUGIT mucositis in Arm 1 (p=0.004). The EGFR +497G>A A/A genotype (rs2227983) was associated with higher risk of PUGIT mucositis only in Arm 1 (p=0.05). While patients heterozygous for the EGF +61A>G polymorphism (rs4444903) experienced more haematological toxicities in the Arm 1 (p=0.04), they experienced less PUGIT mucositis in Arm 3 (p=0.05). And finally, patients with the COX2 +8743 C/C genotype (rs5275) experienced less gastrointestinal toxicities in Arm 1 (p=0.03).
Multiple testing for the association of polymorphisms with DFS, OS, site of relapse and toxicity
After adjusting rs1695 for multiple testing, the association did not remain univariately significant for OS, DFS (p=0.36 for both) and site of relapse (p=0.54). In the multivariate model adjusted for type of surgery, lymph node involvement, primary tumor stage or time from surgery to registration, the p-value was 0.18 for both OS and DFS. MMP3 polymorphism did not remain significant for site of relapse (p=0.68). Also none of the polymorphisms remained significantly associated with toxicity .
DISCUSSION
This study identified rs1695 as a prognostic biomarker, significantly associated with both DFS and OS. At five years, depending on the patient’s genotype, this SNP could determine 3 distinct subgroups with a 14% and 6% absolute difference for the 5-year OS between the high (G/G) and low-risk (G/A) groups and the intermediate (A/A) and low (Val/Ile) risk-group, respectively. This association was independent of stratification factors that included prognostic factors such as tumor and nodal stage. Its effect did not vary by treatment arm nor by gender. We further showed that rs1695 was not associated with GSTP1 gene expression in the tumor, consistent with the known SNP’s function affecting enzymatic activity.
The glutathione S-transferase (GST) superfamily is involved in cellular detoxification processes by catalysing the conjugation of glutathione to a wide variety of xenobiotics and to reactive oxygen species (enzymatic role). The GST detoxification ability plays an important role in cellular protection, and is being implicated in drug resistance and, to a lesser degree, radiation resistance.(13) The GSTPi class, encoded by a the single gene (GSTP1), is the most highly expressed GST in human tumors, including colon cancers.(14) Importantly, GSTP1 acts also as a regulator of the mitogen-activator protein (MAP) pathway (non-enzymatic role) by protein interaction (e.g. JNK1).(15) This mechanism has been postulated for GSTP1-overexpressed drug-resistant cells when the drug is not a GSTP1 substrate, such as antimetabolites (e.g. 5-FU). In colon cancer, both GSTP1 enzymatic and non-enzymatic roles are critical, promoting proliferation and survival.(16)
rs1695 leads to an amino acid change within the active site of the GSTPi protein, changing the GSTP1 catalytic activity in a substrate-specific manner. The catalytic activity and detoxification capacity in individuals with the G allele as compared to individuals with A allele was reduced for 1-chloro-2,4-dinitrobenzene.(17) In contrast, the G allele conferred a higher catalytic efficiency for polycyclic aromatic hydrocarbon diol epoxides.(18) Moreover, A-containing and G-containing haplotypes were shown to have differential effects on proliferation and apoptosis.(19) These pre-clinical observations provide an explanation as to why the heterozygote G/A genotype has a differential outcome when compared to the homozygous genotypes. Most previous data are difficult to compare with this study because they included patients given platinum-based treatments, and included colon cancer or advanced diseases.(20–25) However, our group previously reported clinical data supporting rs1695 allele-specific activity in stage II and III rectal cancer.(26)
Since the loco-regional failure (LRF) occurred in only approximately 10% in each arm and convincing studies testing the role of GSTP1 and radiation resistance were lacking, we speculated that rs1695 effect was either linked to the systemic therapy (antimetabolites) or to the tumor-inherent biologic behaviour (prognostic effect). Consistently, with our hypothesis, we were able to demonstrate that the frequency of failure by rs1695 genotype was different only for distant relapses.
The higher rs4073 expression genotype (A/A) predicted a lower risk of overall high-grade toxicity only in patients given PVI 5-FU. It is critical to point out that fluoropyrimidines have different antitumor effects according to the mode of administration,(27) which translates to specific side effects such as mucositis, stomatitis and hand-foot syndrome. Il-8 is a pro-inflammatory chemokine that is involved is several signalling pathways promoting cell survival, angiogenesis and proliferation.(28) We have previously published that overexpression of Il-8 leads to cell proliferation and survival through the activation of the transcription factor NF-kappaB(29) and its inhibition overcame resistance to prolonged 5-FU exposure.(30) We therefore postulate that high germline Il-8 expression protects against cell death from PVI 5-FU through the activation of NF-kappaB. Alternatively, it may be related to the Il-8 pro-angiogenic effect, as Il-8 overexpression leads to higher vascular density.(29) Increased VEGF-independent angiogenesis may translate to better healing processes and would therefore be particularly relevant for continuous 5-FU, given that its toxicity profile ispredominant in the mucosae.
As bolus 5-FU is being replaced by infusional 5-FU in the treatment of colorectal cancer, we focused on the toxicity profiles on Arm 2. Only rs1870377 was associated with PUGIT mucositis in patients given infusion 5-FU with RT. The homozygous A genotype associated with a lower binding affinity for VEGF (31) predicted a higher risk of mucositis. VEGFR2 is mostly expressed on vascular endothelial cells and is a crucial regulator of angiogenesis (32) and in wound healing processes.(33). Lower VEGF pathway activation may therefore interfere with this process and be associated with more important toxicities. Interestingly, our data suggest that this association depends on the mode of 5-FU administration, and is relevant only for PVI 5-FU, which is known for its mucosal toxicity. In contrast, for bolus 5-FU, with low mucosal toxicity, lower VEGF pathway activation was associated with lower mucosal toxicity. We speculated that lower VEGF pathway activation, affecting vascularity density or vascular permeability,(34) led to poor drug distribution, reducing the risk of upper GI toxicities. However, the small number of events and the fact that no significant association was found in Arm 3 raise the possibility of false positive results.
This study has several limitations. Patients included in the SWOG S9304 trial were treated with post-operative 5-FU-based chemoradiation. While current standard of care in locally advanced rectal adenocarcinoma is pre-operative 5-FU-based or capecitabine-based chemoradiation because of better local control, it has never been shown to be associated with better distant control and longer overall survival. As rs1695 was linked only with distant relapses, it is therefore likely that its prognostic role can be translated to current practice. However, clinicians should be cautious when interpreting the toxicity data, as they may differ according to the fluoropyrimidine (e.g. capecitabine). Despite the biomarker evaluation being performed in a large prospective and well-designed phase III study, we acknowledge that we could collect only a subset of the 1917 patients included in the trial and that we cannot exclude a sampling bias. However, we showed that patients’ characteristics and outcomes included in this correlative study were similar to the overall SWOG S9304 population. Because of the number of studied variables, we adjusted our results for multiple testing. Whenever adjustments are made for multiple comparisons, the p-values increase due to spreading 5% chance of error over more than one (multiple) comparison. None of the evaluated biomarkers remained significant raising the possibility of false positive results (finding by chance). Nevertheless, in the context of a hypothesis-driven (non-agnostic) approach and because multiple testing adjusting techniques also increase false negative results, we believe that rs1695 should be further studied. Therefore, given this limitation and despite the need of biomarkers in rectal cancer, we propose further validation of our results in another large adequately powered clinical trial before going into prospective validation. Prognostic biomarkers such as rs1695 are important, as they may help clinicians to evaluate and discuss the need of post-operative oxaliplatin-based chemotherapy based on the risk of distant relapse. While the relative gain does not differ, the absolute gain is much higher when the prognosis worsens. Furthermore, patients with the unfavourable rs1695 G/G genotype may be encouraged to take part in clinical trials. Finally, prognostic biomarkers are often the key to find drugable targets in the biomarker itself or its related pathway.
In summary, our data show that the rs1695 is significantly associated with both DFS and OS in a representative subgroup of the large prospective phase III S9304 study. Additional clinical studies and further pre-clinical research should be performed to validate our data and better characterize this biomarker. Better understanding of rs1695 variants may help to optimize anti-cancer treatments for rectal cancers. Both rs4073 and rs1870377 may be associated with toxicity in patients given infusional 5-FU with RT.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
In the era of personalized treatments, the identification of prognostic biomarkers in early stages of rectal cancer is of critical importance for risk-adapted treatments/follow-up and for drug development. To the best of our knowledge, this is by far the largest correlative study evaluating the role of the microenvironment and hereditary variables in rectal cancer treated with 5-FU-based CRT. This study was conducted in a well-designed prospective clinical trial with the researchers blinded to the statistical analyses. We showed that GSTP1-Ile105Val SNP (rs1695) was able to determine clinically meaningful difference in both OS and DFS. Our data support that this association is mainly driven by distant recurrences. Assessment of rs1695 may therefore guide the type and intensity of systemic chemotherapy in stage II and III rectal cancer.
Acknowledgments
Supported by: This work was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA058882, CA142558, CA21115, CA17145 CA25224, CA077202, CA32291, CCSRI021039 and CCSRI015469; and by NIH grants 5R01CA105145 and NIH 5P30CA014089-27S1. Pierre Bohanes was supported by a research grant from “Ligue Suisse contre le Cancer” and by the Daniel Butler Memorial Research Fund.
Footnotes
Disclosures: the authors do not have potential conflicts of interest.
Clinical Trials Registry: ClinicalTrials.gov Identifier: NCT00972751 and NCT00002551
References
- 1.Douglass HO, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315:1294–5. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 2.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high–risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease–free survival in patients with carcinoma of the rectum: NSABP R–03. J Clin Oncol. 2009;27:5124–30. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smalley SR, Benedetti JK, Williamson SK, Robertson JM, Estes NC, Maher T, et al. Phase III trial of fluorouracil–based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol. 2006;24:3542–7. doi: 10.1200/JCO.2005.04.9544. [DOI] [PubMed] [Google Scholar]
- 6.Cobo M, Isla D, Massuti B, Montes A, Sanchez JM, Provencio M, et al. Customizing cisplatin based on quantitative excision repair cross–complementing 1 mRNA expression: a phase III trial in non–small–cell lung cancer. J Clin Oncol. 2007;25:2747–54. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich CM, Robien K, McLeod HL. Cancer pharmacogenetics: polymorphisms, pathways and beyond. Nat Rev Cancer. 2003;3:912–20. doi: 10.1038/nrc1233. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Rankin C, Nagashima F, Ulrich CM, Holmes RS, Makar KW, et al. J Clin Oncol; 2008 Gastrointestinal Cancers Symposium; 2008. p. 300. [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single–step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Lord RV, Salonga D, Danenberg KD, Peters JH, De Meester TR, Park JM, et al. Telomerase reverse transcriptase expression is increased early in the Barrett's metaplasia, dysplasia, adenocarcinoma sequence. J Gastrointest Surg. 2000;4:135–42. doi: 10.1016/s1091-255x(00)80049-9. [DOI] [PubMed] [Google Scholar]
- 11.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT–PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 12.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics. 1979:65–70. [Google Scholar]
- 13.Tew KD. Glutathione–associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–20. [PubMed] [Google Scholar]
- 14.Ranganathan S, Tew KD. Immunohistochemical localization of glutathione S–transferases alpha, mu, and pi in normal tissue and carcinomas from human colon. Carcinogenesis. 1991;12:2383–7. doi: 10.1093/carcin/12.12.2383. [DOI] [PubMed] [Google Scholar]
- 15.Townsend DM, Tew KD. The role of glutathione–S–transferase in anti–cancer drug resistance. Oncogene. 2003;22:7369–75. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang DT, Chen F, Kohli M, Rago C, Cummins JM, Dang LH. Glutathione S–transferase pi1 promotes tumorigenicity in HCT116 human colon cancer cells. Cancer Res. 2005;65:9485–94. doi: 10.1158/0008-5472.CAN-05-1930. [DOI] [PubMed] [Google Scholar]
- 17.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S–transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–80. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 18.Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, et al. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1–1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–6. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 19.Holley SL, Fryer AA, Haycock JW, Grubb SE, Strange RC, Hoban PR. Differential effects of glutathione S–transferase pi (GSTP1) haplotypes on cell proliferation and apoptosis. Carcinogenesis. 2007;28:2268–73. doi: 10.1093/carcin/bgm135. [DOI] [PubMed] [Google Scholar]
- 20.Stoehlmacher J, Park DJ, Zhang W, Yang D, Groshen S, Zahedy S, et al. Association between glutathione S–transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94:936–42. doi: 10.1093/jnci/94.12.936. [DOI] [PubMed] [Google Scholar]
- 21.Kweekel DM, Koopman M, Antonini NF, Van der Straaten T, Nortier JWR, Gelderblom H, et al. GSTP1 Ile105Val polymorphism correlates with progression–free survival in MCRC patients treated with or without irinotecan: a study of the Dutch Colorectal Cancer Group. Br J Cancer. 2008;99:1316–21. doi: 10.1038/sj.bjc.6604654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun MS, Richman SD, Quirke P, Daly CL, Meade AM, Adlard JW, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol. 2008;26:2690–8. doi: 10.1200/JCO.2007.15.5580. [DOI] [PubMed] [Google Scholar]
- 23.Funke S, Risch A, Nieters A, Hoffmeister M, Stegmaier C, Seiler CM, et al. Genetic Polymorphisms in Genes Related to Oxidative Stress (GSTP1, GSTM1, GSTT1, CAT, MnSOD, MPO, eNOS) and Survival of Rectal Cancer Patients after Radiotherapy. J Cancer Epidemiol. 2009;2009:302047. doi: 10.1155/2009/302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarate R, Rodriguez J, Bandres E, Patino–Garcia A, Ponz–Sarvise M, Viudez A, et al. Oxaliplatin, irinotecan and capecitabine as first–line therapy in metastatic colorectal cancer (mCRC): a dose–finding study and pharmacogenomic analysis. Br J Cancer. 2010;102:987–94. doi: 10.1038/sj.bjc.6605595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paez D, Salazar J, Pare L, Pertriz L, Targarona E, Del Rio E, et al. Pharmacogenetic Study in Rectal Cancer Patients Treated With Preoperative Chemoradiotherapy: Polymorphisms in Thymidylate Synthase, Epidermal Growth Factor Receptor, GSTP1, and DNA Repair Genes. Int J Radiat Oncol Biol Phys. 2011;81:1319–27. doi: 10.1016/j.ijrobp.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MA, Gil J, Lu B, Zhang W, Zang D, Yun J, et al. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- 27.Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer––a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997;15:368–81. doi: 10.1200/JCO.1997.15.1.368. [DOI] [PubMed] [Google Scholar]
- 28.Waugh DJ, Wilson C. The interleukin–8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 29.Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. Interleukin–8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–49. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodach LL, Bos CL, Duran N, Peppelenbosch MP, Ferreira CV, Hardwick JC. Violacein synergistically increases 5–fluorouracil cytotoxicity, induces apoptosis and inhibits Akt–mediated signal transduction in human colorectal cancer cells. Carcinogenesis. 2006;27:508–16. doi: 10.1093/carcin/bgi307. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, et al. Polymorphisms of KDR gene are associated with coronary heart disease. J Am Coll Cardiol. 2007;50:760–7. doi: 10.1016/j.jacc.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 32.Olsson AK, Dimberg A, Kreuger J, Claesson–Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 33.Bao P, Kodra A, Tomic–Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–58. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor–2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–12. doi: 10.1016/j.cellsig.2007.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.