Abstract
Cerebral amyloid angiopathy (CAA) occurs in nearly every individual with Alzheimer’s disease (AD) and Down’s syndrome, and is the second largest cause of intracerebral hemorrhage. Mouse models of CAA have demonstrated evidence for increased gliosis contributing to CAA pathology. Nearly two thirds of Americans are overweight or obese, with little known about the effects of obesity on the brain, although increasingly the vasculature appears to be a principle target of obesity effects on the brain. In the current study we describe for the first time whether diet induced obesity (DIO) modulates glial reactivity, amyloid levels, and inflammatory signaling in a mouse model of CAA. In these studies we determine that DIO does not significantly alter gross Aβ levels, astrocyte (GFAP) or microglial (IBA-1) gliosis in the CAA mice. However, within the hippocampal gyri a localized increase in reactive microglia were increased in the CA1 and stratum oriens relative to CAA mice on a control diet. DIO was observed to selectively increase IL-6 in CAA mice, with IL-1β and TNF-α not increased in CAA mice in response to DIO. Taken together, these data show that prolonged DIO does not significantly alter gross levels of Aβ or gliosis in CAA mice, but appears to elevate some localized microglial reactivity within the hippocampal gyri and selective markers of inflammatory signaling. These data are consistent with many of the studies in the existing literature using other models of Aβ pathology, which surprisingly show a mixed profile of DIO effects towards pathological processes in mouse models of neurodegenerative disease. The importance for considering the potential impact of ceiling effects in Aβ pathogenesis within the various mouse models, the lack of consistency for DIO based experimentation, and the current experimental limitations for DIO in mice to fully replicate metabolic dysfunction present in human obesity, are discussed.
Keywords: Adiposity, Alzheimer’s disease, Astrocyte, Diabetes, Hippocampus, Inflammation, Microglia
1. Introduction
Despite the fact nearly two thirds of Americans are overweight or obese, and the fact that obesity is known to contribute to multiple morbidities and dysfunction of multiple tissues, relatively little is known in terms of the effect of obesity on the brain [1,2]. Epidemiological and cross sectional studies, while not uniform, suggest that presence of obesity increases the incidence of numerous neurodegenerative conditions including Alzheimer’s disease (AD), vascular dementia, and cerebral stroke [2–7]. The presence of prolonged obesity, starting in midlife, is particularly linked to the development of neurodegenerative disorders such as AD [8–13]. Despite such advances, very little is known in terms of the potential mechanisms by which obesity potentially promotes the development of neurodegenerative diseases, or which specific neuropathological features may potentially be exacerbated by the presence of obesity.
A large and robust number of publications involving findings from rodent studies have been insightful in understanding the molecular and pathophysiological basis for obesity, and the metabolic complications of obesity [14–23]. While the impact of obesity on multiple tissues of the body are well established in human and rodent studies (muscular, hepatic, cardiac, nephritic systems), relatively little is known in terms of how obesity impacts the central nervous system. In particular, there is a paucity of data on the effects of diet induced obesity (DIO) as modulators of specific neuropathological processes in established mouse models of neurodegenerative disease. Such data is essential in order to develop a mechanistic understanding as to if, and how, obesity modulates neurodegenerative processes involved in diseases such as AD.
Cerebral amyloid angiopathy (CAA) occurs in nearly all cases of Alzheimer’s disease (AD), Down’s syndrome, and is the second leading cause of intracerebral hemorrhage [24–28]. CAA is caused by the accumulation of amyloid within the cerebrovasculature, and is associated with numerous alterations relevant to AD and other neurodegenerative conditions including neuroinflammation, oxidative stress, and gliosis. No studies to date have examined the impact of DIO towards CAA. In the current study, we examined the effects of prolonged DIO towards multiple neuropathological processes in an established CAA mouse model [29–31].
Taken together, our data demonstrate that prolonged DIO does not exacerbate any of the gross measures of Aβ levels or glial reactivity. Localized changes in microglial reactivity, and a selective elevation in IL-6 (but not IL-1 or TNF-α) was observed in CAA mice in response to DIO. The lack of gross DIO effects towards Aβ pathogenesis is surprisingly consistent with a number of studies in the literature [32–36], and points to a need to carefully consider the current experimental limitations in studying the effects of DIO on the brain. The potential for ceiling effects in Aβ pathogenesis within the various mouse models, the lack of consistency for DIO based experimentation, and the current experimental limitations for DIO in mice to fully replicate metabolic dysfunction present in human obesity, are discussed.
2. Materials and Methods
2.1. Animals and Dietary Treatments
All animal experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Male and female C57BL/6 mice (“Control”; Charles River Laboratories) and male and female Tg-SwDI (“CAA”) mice were studied. The TgSwDI mouse was generated as described previously [29]. The CAA and corresponding control mice were maintained and genotyped as described previously [29–31,37]. Briefly, the transgenic mouse model expressed the human AβPP770 isoform including the Swedish, Dutch and Iowa mutations under the Thy-1 promoter. Both genotypes were randomly divided and assigned to either a high fat diet (HFD; D12492, Research Diets) or control diet (CD; D12450B) at 2–3 months of age. The HFD provided 60% kcals from fat while the CD provided 10% kcals from fat. The CD was developed as an appropriate, balanced control to the HFD: both contained the necessary mineral and vitamin mixes, 20% kcals from protein and the fat source was soybean oil and lard. These diets were administered for 9–11 months. Mice were housed in standard caging with a 12:12 light/dark cycle and ad libitum access to water and their respective diet unless otherwise noted. Total body fat content was measured via nuclear magnetic resonance (NMR) spectroscopy (Minispec, Brucker Optics, Billerica, MA).
2.2. Immunohistochemistry
The formalin-perfused mouse brain tissues were kept in formalin for 24–48 hours and then processed for paraffin embedding. Samples were sectioned at 5 µm. For immunohistochemistry staining, paraffin was removed by washing slides three times in xylenes, tissue was re-hydrated in a series of alcohol washes (100%, 95%, 70%, and 50%), and then tissue was treated for heat-mediated antigen retrieval. Slides were washed, blocked in 10% serum and incubated overnight with primary antibody anti-collagen IV (Cat. #: ab6586, Abcam, Cambridge, MA, USA) in 3% serum. The next day slides were washed, incubated with biotinylated secondary antibody (Vector, Burlingame, CA, USA), and developed using DAB Peroxidase Substrate Kit (Vector). For double labeling, the slides were re-blocked again in 10% serum and incubated overnight with primary antibody anti-IBA-1(Cat. #: 019-19741, Wako, Osaka, Japan) or anti-GFAP (Cat. #: ab48050, Abcam) in 3% serum. The next day slides were washed, incubated with biotinylated secondary antibody, and developed using VIP Peroxidase Substrate Kit (Vector). Slides were scanned using a Hamamatsu NanoZoomer Digital Slide Scanning System (Hamamatsu City, Japan) at 20× magnification.
2.3. RT-PCR
RT-PCR experiments were performed as done previously by our laboratory [38]. Total RNA from 30 mg mouse striatum was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions with minor modifications. The corresponding cDNA was made from 2 µg of extracted total RNA by M-MuLV transcriptase (New England Biolabs, Ipswich, MA) using 20 µL of the reverse transcription system according to the manufacturer’s instructions. For quantitative real-time PCR (qPCR) analysis, aliquots of cDNA were subjected to qPCR in 20 µl of 1×Brilliant II QPCR & QRT-PCR Reagents (Agilent Technologies, Santa Clara, CA), 1× primers and TaqMan probe (6-FAM/ZEN/IBFQ mode) and 10 ng of cDNA. Primers and probes for TNF-α, IL-1β, IL-6 and Gapdh were ordered through the PrimeTime Pre-designed qPCR Assays system of Integrated DNA Technologies (IDT, Coralville, IA). Each sample was loaded in triplicate, negative and positive controls were included. Amplification of Gadph was used as an internal reference gene. PCR amplifications were performed as follows: 50°C for 2 minutes, 95°C for 15 minutes, and 40 cycles each with 95°C for 15 seconds and 60°C for 45 seconds using an ABI PRISM 7000 sequence detector according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). For data analysis, the ΔΔCt method was used. Relative mRNA expression of each gene was expressed as the mean and STD of 6 independent total RNA extractions and real-time PCR analyses.
2.4. Western Blotting
Protein lysates from mouse striatum were made in RIPA buffer using a tissue homogenizer and the amount of protein in lysates was estimated using BCA reagent (Thermo Fisher Scientific, Rockford, IL, USA). Protein samples were analyzed by SDS-PAGE and then transferred onto a nitrocellulose membrane. The membrane was then probed with antibodies anti- IBA1, GFAP as described previously and β-actin (Cat. #: sc-47778, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). All electrophoresis and immunoblotting reagents were purchased from Bio-Rad Laboratories (Hercules, CA, USA). The HRP-conjugated secondary antibodies were purchased from the Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
2.5. Aβ analysis
The amount of PBS and SDS soluble Aβ was quantified as outlined previously in numerous studies from our laboratories [39–42].
2.6. Statistical Analysis
Statistical significance between two groups was determined using a paired t-test. All data are shown as mean ± standard error of measurement. For all other analyses, statistical significance was determined by ANOVA followed by Fisher's least significant difference post hoc test. P < 0.05 was considered statistically significant (labeled as *).
3. Results
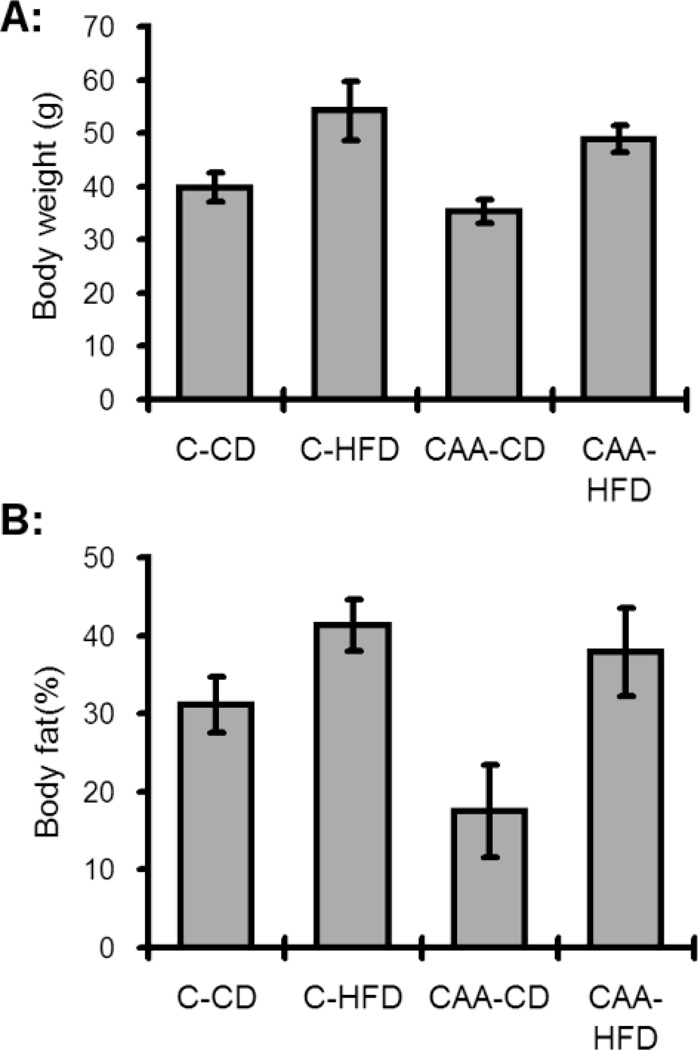
Placement of CAA and control mice on a DIO regiment resulted in the predicted increase in both body weight and adiposity (Fig. 1), as compared to maintaining animals on a control diet (CD). It is important to point out that the current study differed from our recent publication examining the effects of DIO on adiposity in CAA mice [43], in the fact the diet duration in the current study was nearly 2 times longer duration, and the fact the previous study used only male mice (current study an equal mix of male/female mice). We next analyzed the ability of DIO to alter the levels of Aβ40 and Aβ42. As anticipated CAA mice exhibited significant increases in both Aβ40 and Aβ42 (Table 1), as compared to control mice. Surprisingly, prolonged DIO did not significantly alter the levels of in the Aβ40/Aβ42 in either the soluble (PBS) or insoluble (SDS) fractions (Table 1).
Fig. 1. Body weights and body fat following diet induced obesity.
(A) Control HFD mice (C-HFD) were significantly heavier than all other groups (p<0.05) while CAA control diet (CAA-CD) mice weighed significantly less than all other groups (p<0.05). (B) Body fat as measured by NMR revealed increased total body fat (p<0.05) for both groups fed the HFD. The CAA-CD mice had less fat compared to all other groups (p<0.05).
Table 1.
Dietary induced obesity effects on Aβ levels in the context of a mouse model of cerebral amyloid angiopathy
| PBS | SDS | |||
|---|---|---|---|---|
| Aβ40 | Aβ42 | Aβ40 | Aβ42 | |
| CAA Control Diet | 202 (37) | 1,006 (168) | 92,040 (8,216) | 12,399 (832) |
| CAA High Fat Diet | 98 (41) | 746 (205) | 84,564 (7,358) | 10,251 (1,050) |
| WT Control Diet | 70 (13) | 497 (125) | 1,091 (62) | 10,892 (518) |
| WT High Fat Diet | 12 (12) | 542 (70) | 862 (46) | 11,847 (539) |
Data presented as the mean (nM) and SEM of soluble (PBS) and insoluble (SDS) levels of Aβ.
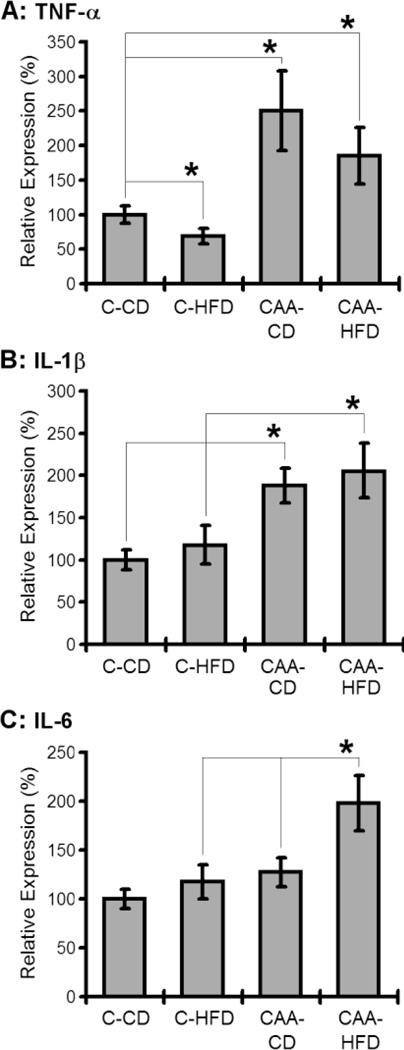
Next we analyzed the effects of DIO towards gross inflammatory signaling in CAA mice. Interestingly, HFD exposure selectively increased IL-6 in CAA mice (Fig. 2), and did not significantly alter either TNF-α or IL-1β in CAA mice following administration of DIO (Fig. 2).
Fig. 2. Effects of the diet induced obesity on the gross inflammatory signaling pathway in mouse brains.
The mRNA level of TNF-α (A), IL-1 (B) and IL-6 (C) in different mouse brain was examined. HFD exposure selectively increased IL-6 in CAA mice (p<0.05).
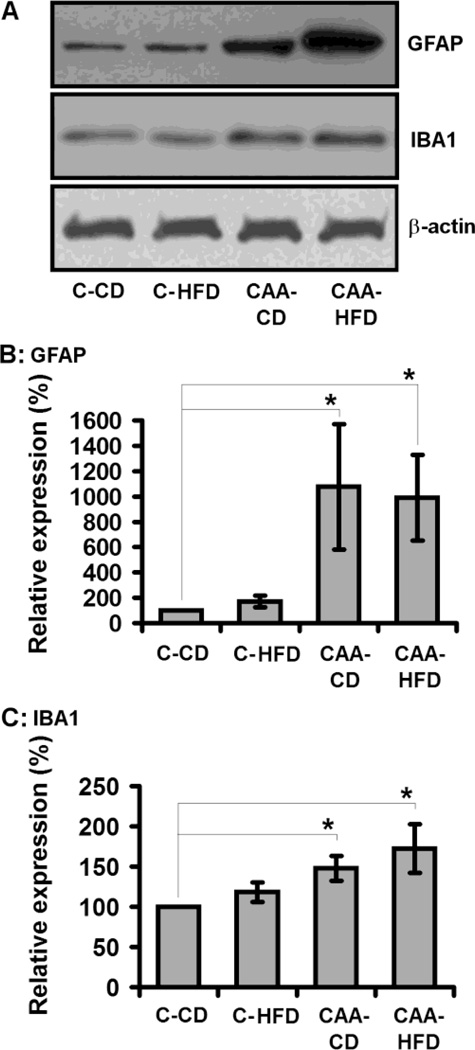
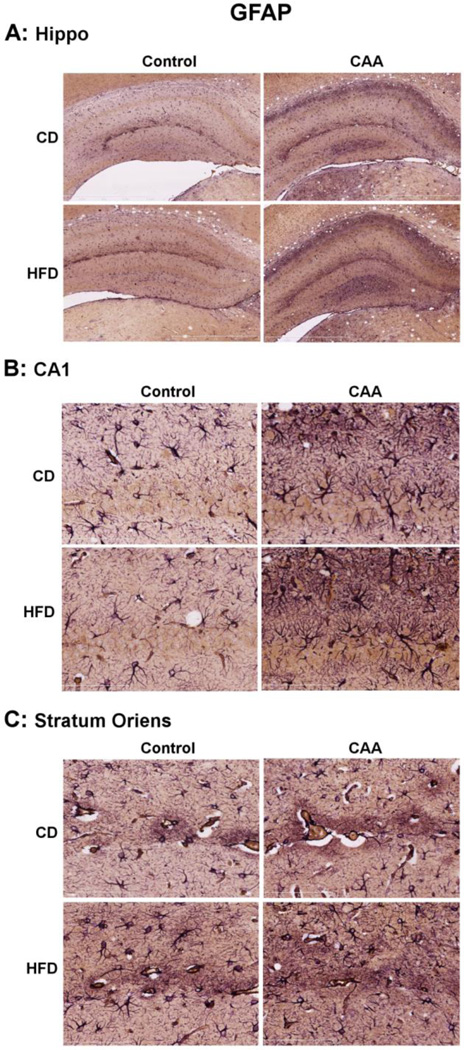
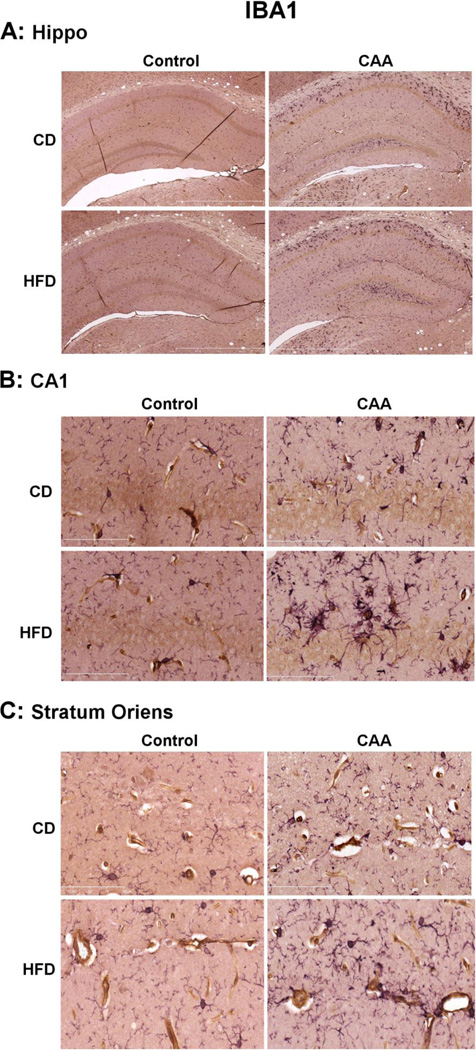
We next analyzed the impact of DIO towards the levels of microglial and astrocyte gliosis in CAA mice. As expected, CAA mice exhibited a significant increase in both IBA-1 and GFAP levels as compared to control mice (Fig. 3); consistent with elevated levels of microglial and astrocyte reactivity, respectively. DIO did not significantly alter the gross levels of either IBA-1 or GFAP in CAA mice (Fig. 3). While gross levels of GFAP and IBA-1 were not altered by DIO, we next sought to elucidate if any change in the level of these markers could be detected using immunohistochemistry. In these studies we observed that, consistent with our Western blot analysis no significant change in the levels or distribution of GFAP immuoreactivity was observed in CAA mice response to DIO (Fig. 4). This observation was consistent whether analyzing the entire hippocampal gyri, as well as a subset of analysis in CA1 and stratum oriens within the hippocampal gyri. We next analyzed IBA-1 immunoreactivity and observed an apparent increase in the number of IBA-1 labeled, were observed in stratum oriens of the hippocampal gyri (Fig. 5). The increase in large phagocytic microglia near cerebral arterioles was selective for stratum oriens and not seen in the CA1 or other areas of the hippocampal gyri (Fig. 5).
Fig. 3. Effects of the diet induced obesity on astrocyte and microglial reactivity.
Diet induced obesity did not significantly alter the gross protein levels of either astrocyte (GFAP) or microglial (IBA-1) reactivity.
Fig. 4. Diet induced obesity did not significantly alter immunoreactivity and distribution of GFAP in the hippocampus of CAA mice.
The levels of astrocyte reactivity were measured in the brain using GFAP immunoreactivity (purple) and co-localized with cerebral blood vessels using collagen IV immunoreactivity (brown).
Fig. 5. Effects of diet induced obesity on the immuoreactivity and distribution of IBA1 in the hippocampus of CAA mice.
The levels of microglial reactivity were measured in the brain using IBA-1 immunoreactivity (purple) and co-localized with cerebral blood vessels using collagen IV immunoreactivity (brown).
4. Discussion
Our results indicate that DIO does not significantly alter gross Aβ levels in either soluble or insoluble fractions of CAA mice. These data are consistent with previous studies which identified that DIO in mouse models of Aβ neurodegeneration do not alter the gross levels of Aβ [33–35]. Interestingly, studies which combine obesity with hypercholesterolemia are known to more consistently increase Aβ pathology in mice [44–46]. Taken together, these data suggest that more than just obesity may be required to consistently alter gross levels of Aβ levels in mice. These observations are particularly important when considering the preclinical use of mice for the study of obesity-Aβ interactions. For example, it is well known that mouse models of DIO do not recapitulate the pleotropic array of metabolic syndrome observed in obese humans. Obesity in humans is accompanied by the development of metabolic syndrome [47–49] including the presence of hypercholesterolemia, diabetes, hyperlipidemia and often accompanied by morbidities such as atherosclerosis and hypertension [48]. In mice, most of these modifications do not occur in response to DIO, and those that do are only modestly elevated (insulin resistance, hyperlipidemia). For these reasons, the preclinical studies of DIO effects on brain neuropathology will likely require an approach in which obesity is accompanied by hypercholesterolemia or other cardiovascular disease risk factor. Despite the differences between mice and human DIO studies, it appears that the vasculature is a key target of obesity induced effects on the various tissues of the body [50–52]. Most studies to date have focused on the effects of obesity on the vasculature of the heart and peripheral organs, although it is highly likely cerebrovasculature will be a principle target of DIO effects on the brain.
Studies are needed in mice to identify which aspects of Aβ regulation (synthesis, processing, degradation, oligomerization, deposition) are consistently altered by obesity in humans and clinically relevant mouse models. Currently, little to nothing is known in terms of the effects of obesity on key regulatory Aβ enzymes such as BACE and γ-secretase, nor have studies identified if obesity promotes meaningful changes the rates/levels of Aβ assembly and/or deposition. In the current study while we did not see a significant change in Aβ levels in either the soluble or insoluble pools, it is important to point out that these studies were conducted following prolonged DIO, and the CAA mice at this age are known to have near maximal levels of Aβ. These studies raise the likelihood of ceiling effects (in terms of potentially maximal Aβ levels/changes) reducing the chances for identifying DIO modulation of Aβ. Careful consideration of ceiling effects for pathology are clearly an important consideration in the designing of future studies looking at the ability of DIO to modulate Aβ pathogenesis. Studies in the future need to carefully design studies (time points, diet duration, Aβ model) in a manner in which there is significant room in the modulation of Aβ levels to identify the ability of DIO to increase, as well as decrease Aβ levels in the brain.
Additional concerns for studies of DIO effects on the brain include the fact that there is little consistency in the types of diets used, the duration of diets, the control diets used for the majority of studies in the literature (Table 2). The importance of control diets utilized is critical as the use of chow diet as controls in DIO studies does not allow for the control of the types of macronutrients utilized (source, quality, etc). This can make a huge difference in terms of experimental outcomes and the interpretation of whether DIO is capable of modulating specific aspects of AD related pathogenesis. Pair feeding studies, in which the amount of calories is held constant between a control diet and high fat diet, can be useful in identifying whether it is an increase in obesity or dietary fat which is responsible for mediating a given aspect of pathology. To date, pair feeding studies have not been used in studies of brain pathogenesis, although it is important to point out that pair feeding studies do have a potential confound in the fact that access to food is restricted. This restriction of food access is necessary to control for calories, but can induce stress in rodents, with stress potentially modulating brain homeostasis.
Table 2.
Diet induced obesity and alterations in mouse brain homeostasis
| Diet | Duration | Mouse Strain | End Point & Results | Ref. |
|---|---|---|---|---|
| 60%-HFD, 10%-CD | 16 weeks | C57BL/6 | increased reactive oxygen species (ROS); decreased cognition | [43] |
| 60%-HFD, 5%-CD | 16 weeks | C57BL/6 | increased IR, ROS; no AD histopathology, no increase in Aβ or phospho-tau, no impairments in IGF signaling or acetylcholine homeostasis | [33] |
| 10% sucrose in water | 25 weeks | B6C3-Tg(APPswe, PSEN1dE9)85Dbo/J | Metabolic dysfunction, memory impairment, increased apoE and aggregation of Aβ in the brain | [34] |
| 40%-WD, 10%-CD | 4 weeks | APPswe/PS1M146V; PS1M146V; C57BL/6 | increased cerebral ROS, alterations in brain Aβ | [36] |
| 60%-HFD | 16 weeks | C57BL/6 | impairment of fear-conditioning test | [53] |
| 60%-HFD, 10%-CD | 20 weeks | APPSwe/Ind | metabolic dysfunction; decreased cognition | [54] |
| 21.2%-HFD, 5.5%-CD | 22 weeks | C57BL/6 | increased pro-inflammatory factors and APP expression in brains and fat tissues | [55] |
| 55% -HFD | 8 weeks | WT, PSEN1, APP/PSEN1 | AD increases body weight gain, glucose intolerance and insulin resistance induced by HFD in mice | [56] |
| 60%-HFD | 20 weeks | C57BL/6J, MC4R−/−, Tie2-GFP | enhanced GFAP-immunoreactivity in the medial preoptic, paraventricular and dorsomedial nuclei of the hypothalamus. | [57] |
| 60%-HFD | 22 weeks | C57BL/6 | impaired hippocampus-dependent spatial memory, alteration in the expression of corresponding genes | [58] |
| 58%-HFD, 10.5%-CD | 12 weeks | C57BL/6 | promotion of negative emotional states and depressive-like symptomology | [59] |
| 45%-HFD, 10%-CD | 14 weeks | C57BL/6 | HFD selectively protects against the effects of chronic social stress in the mouse | [60] |
It is well established that the different mouse strains exhibit considerable variability in regards to their susceptibility to DIO [33–36]. Additionally, even within the same strain there is known to be considerable heterogeneity in body weight gain following DIO that appears to be due to genetic influences [61–63]. While traditionally, these variances have been the focus of understanding the causes of obesity, they have considerable influence on how mouse studies are used to study the consequences of DIO on multiple tissues, including the brain. For example, recent studies have demonstrated that increases in APP occur in response to obesity in both brain and adipose tissue [55–64], with decreases in obesity contributing to lower APP levels [65]. These studies did not share significant overlap in their design, nor did they account for the influences of genetic susceptibility to DIO, making it difficult to understand what is truly occurring in terms of DIO effects on APP levels.
Similar to Aβ, we believe that the lack of an effect of DIO on gross inflammatory signaling and glial reactivity in CAA mice is also due in large part to a ceiling effect in the level of Aβ pathology in CAA mice. Previous studies from our laboratory and others has firmly established the ability of DIO to modulate both glial reactivity and inflammatory signaling in the rodent brain [55,66–70]. It is highly likely that the modulation of these two aspects of brain homeostasis contributes to the mechanism by which DIO modulates brain pathology and brain function, with studies currently underway in our laboratory to establish this experimentally in mouse models of Aβ pathogenesis. Both microglia and astrocytes are key to the neurovascular unit, raising the potential for DIO modulating neurovascular unit homeostasis [71–73] and potentially serve as the basis for DIO increasing susceptibility to Alzheimer’s disease [74,75].
It is important to point out that in humans and rodent studies, increases in visceral adiposity are preferentially linked to the morbidities and mortality associated with obesity [76–78]. The ability of visceral adiposity to preferentially mediate the negative effects of DIO appears to be due to both the fact it is biochemically distinct from visceral adiposity, as well as the fact it is near vital internal organs of the body. Establishing the specific role of different adipose depots in mediating the negative effects of DIO on the brain is a critical area of future research. Similarly, it is well established that gastric surgery in humans and rodents (gastric sleeve, gastric bypass, roux en y) has significant beneficial effects on mobidity and mortality [79–82]. Interestingly, these benefits often occur before significant weight loss has occurred, and in individuals who remain morbidly obese. It is presumed these rapid beneficial effects are mediated by changes in gut hormones such as GLP-1, although it remains to be determined what beneficial effects of gastric surgery are on Aβ pathogenesis and other aspects of neurodegenerative disease.
Adipose tissue is the largest endocrine tissue in most Americans, and the adipokines secreted by adipose tissue almost certainly contribute to brain homeostasis. Understanding the influence of adipokines like leptin on brain homeostasis fairly limited to date. Interestingly, studies are showing complex roles for these adipokines in regulating Aβ processing and Aβ levels [2,83–85]. Understanding the effects of physiological levels of adipokines and their targets in the brain, as related to neurodegenerative disease, is another crucial area of research. Db/Db and Ob/Ob mice have been useful in understanding adipose biology and adipose modulation of other tissues of the body, in particular the role of leptin signaling in different aspects of biology and pathological processes [14,20]. Studies involving crossing Ob/Ob diabetic mice with mouse models of Aβ pathology have demonstrated evidence for accelerated neurodegeneration and cognitive impairment [86]. It is important to point out that these studies involve acute experimentation (animals less than 6 months old) because of the severity of obesity and extreme insulin resistance. The magnitude of metabolic disturbances and obesity appear to limit the use of this model to study obesity mediated effects on the brain, and make it difficult to discern whether the effects on the brain are a byproduct of a mouse with a myriad of morbidities and short life span.
Acknowledgments
This work utilized the facilities of the Cell Biology and Bioimaging Core that are supported in part by COBRE (NIH 2P20-RR021945) and NORC (NIH 2P30-DK072476) center grants from the National Institutes of Health, the Pennington Animal Metabolism and Behavior Core. The work was supported by funds from the NIH and Hibernia National Bank/Edward G Schleider Chair to JNK. Further support (TLB, MPM) was provided by NIA (5P01-AG005119). The authors also thank Dr William Van Nostrand for supplying original founder CAA mice used for the generation of mice in the current study.
References
- 1.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 2.Lee EB. Obesity, leptin, and Alzheimer's disease. Ann. N. Y. Acad. Sci. 2012;1243:15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 4.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch. Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitmer RA. The epidemiology of adiposity and dementia. Curr. Alzheimer Res. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 6.Barton M, Baretella O, Meyer MR. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br. J. Pharmacol. 2012;165:591–602. doi: 10.1111/j.1476-5381.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Béjot Y, Giroud M. Stroke in diabetic patients. Diabetes Metab. 2010;36(Suppl 3):S84–S87. doi: 10.1016/S1262-3636(10)70472-9. [DOI] [PubMed] [Google Scholar]
- 8.Gustafson DR, Backman K, Waern M, Ostling S, Guo X, Zandi P, Mielke MM, Bengtsson C, Skoog I. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch. Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 11.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr. Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 12.Chiang CJ, Yip PK, Wu SC, Lu CS, Liou CW, Liu HC, Liu CK, Chu CH, Hwang CS, Sung SF, Hsu YD, Chen CC, Liu SI, Yan SH, Fong CS, Chang SF, You SL, Chen CJ. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am. J. Geriatr. Psychiatr. 2007;15:762–771. doi: 10.1097/JGP.0b013e318050c98f. [DOI] [PubMed] [Google Scholar]
- 13.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 14.Panchal SK, Brown L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25:1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 16.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, Wuytack F, Holvoet P, Flameng W, Balligand JL, Herijgers P. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc. Res. 2008;77:371–379. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 17.Dobrzyn P, Dobrzyn A, Miyazaki M, Ntambi JM. Loss of stearoyl-CoA desaturase 1 rescues cardiac function in obese leptin-deficient mice. Journal of Lipid Research. 2010;51:2202–2210. doi: 10.1194/jlr.M003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigorgne AE, Bouchet-Delbos L, Naveau S, Dagher I, Prévot S, Durand-Gasselin I, Couderc J, Valet P, Emilie D, Perlemuter G. Obesity induced lymphocyte hyperresponsiveness to chemokines: a new mechanism of fatty liver inflammation in obese mice. Gastroenterology. 2008;134:1459–1469. doi: 10.1053/j.gastro.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Ae Park S, Choi MS, Cho SY, Seo JS, Jung UJ, Kim MJ, Sung MK, Park YB, Lee MK. Genistein and daidzein modulate hepatic glucose and lipid regulating enzyme activities in C57BL/KsJ-db/db mice. Life Sciences. 2006;79:1207–1213. doi: 10.1016/j.lfs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Ge F, Zhou S, Hu C, Lobdell H, IV, Berk PD. Insulin and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. American Journal of Physiology. 2010;299:G855–G866. doi: 10.1152/ajpgi.00434.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarda A, Franzin C, Milan G, Sanna M, Dal Prà C, Pagano C, Boldrin L, Piccoli M, Trevellin E, Granzotto M, Gamba P, Federspil G, De Coppi P, Vettor R. Increased adipogenic conversion of muscle satellite cells in obese Zucker rats. International Journal of Obesity. 2010;34:1319–1327. doi: 10.1038/ijo.2010.47. [DOI] [PubMed] [Google Scholar]
- 22.Van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc. Diabetol. 2009;8 doi: 10.1186/1475-2840-8-39. article 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, Recchia F, Stanley W, Wolin MS, Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am. J. of Physiol. Heart Circ. Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann. Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 25.Kumar-Singh S. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes Brain Behav. 2008;7(Suppl 1):67–82. doi: 10.1111/j.1601-183X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Zhang JH. Cerebral amyloid angiopathy-related microhemorrhages in Alzheimer's disease: a review of investigative animal models. Acta. Neurochir. Suppl. 2011;111:15–17. doi: 10.1007/978-3-7091-0693-8_3. [DOI] [PubMed] [Google Scholar]
- 28.Pezzini A, Del Zotto E, Volonghi I, Giossi A, Costa P, Padovani A. Cerebral amyloid angiopathy: a common cause of cerebral hemorrhage. Curr. Med. Chem. 2009;16:2498–24513. doi: 10.2174/092986709788682047. [DOI] [PubMed] [Google Scholar]
- 29.Davis J, Xu F, Deane R, Romanov G, Previti ML, Vasek M, Davis J, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 30.Van Nostrand WE, Xu F, Rozemuller AJ, Colton CA. Enhanced capillary amyloid angiopathy-associated pathology in Tg-SwDI mice with deleted nitric oxide synthase 2. Stroke. 2010;41:S135–S138. doi: 10.1161/STROKEAHA.110.595272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Vickle GD, Esh CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, Walker DG, Lue LF, Beach TG, Davis J, Van Nostrand WE, Castaño EM, Roher AE. Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy. Am. J. Pathol. 2008;173:483–493. doi: 10.2353/ajpath.2008.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 33.Moroz N, Tong M, Longato L, Xu H, de la Monte SM. Limited Alzheimer-type neurodegeneration in experimental obesity and type 2 diabetes mellitus. J. Alzheimer. Dis. 2008;15:29–44. doi: 10.3233/jad-2008-15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Dis. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 35.Hiltunen M, Khandelwal VK, Yaluri N, Tiilikainen T, Tusa M, Koivisto H, Krzisch M, Vepsäläinen S, Mäkinen P, Kemppainen S, Miettinen P, Haapasalo A, Soininen H, Laakso M, Tanila H. Contribution of genetic and dietary insulin resistance to Alzheimer phenotype in APP/PS1 transgenic mice. J. Cell. Mol. Med. 2012;16:1206–1222. doi: 10.1111/j.1582-4934.2011.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hommet C, Mondon K, Constans T, Beaufils E, Desmidt T, Camus V, Cottier JP. Review of cerebral microangiopathy and Alzheimer's disease: relation between white matter hyperintensities and microbleeds. Dement. Geriatr. Cogn. Disord. 2011;32:367–378. doi: 10.1159/000335568. [DOI] [PubMed] [Google Scholar]
- 36.Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, Markesbery WR, Murphy MP, Keller JN. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP × PS1 knock-in mice. J. Neurochem. 2009;108:860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Nostrand WE. Cellular and transgenic animal models of cerebrovascular amyloidosis. Mol. Med. Today. 2000;6:373–374. doi: 10.1016/s1357-4310(00)01762-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Ebenezer PJ, Dasuri K, Fernandez-Kim SO, Francis J, Mariappan N, Gao Z, Ye J, Bruce-Keller AJ, Keller JN. Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am. J. Physiol. Endocrinol. Metab. 2011;301:E599–E607. doi: 10.1152/ajpendo.00059.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckett TL, Webb RL, Niedowicz DM, Holler CJ, Matveev S, Baig I, Levine H, Iii, Keller JN, Murphy MP. Postmortem Pittsburgh Compound B (PiB) Binding Increases with Alzheimer's Disease Progression. J. Alzheimers Dis. 2012;32:127–138. doi: 10.3233/JAD-2012-120655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niedowicz DM, Beckett TL, Matveev S, Weidner AM, Baig I, Kryscio RJ, Mendiondo MS, LeVine HL, Keller JN, Murphy MP. Pittsburgh compound B and the postmortem diagnosis of Alzheimer’s disease. Ann. Neurol. 2012 doi: 10.1002/ana.23633. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St Clair D, Keller JN. Cognitive impairment in humanized APP×PS1 mice is linked to Aβ(1–42) and NOX activation. Neurobiol. Dis. 2011;44:317–326. doi: 10.1016/j.nbd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. A beta solubility and deposition during AD progression and in APP×PS-1 knock-in mice. Neurobiol. Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Freeman LR, Zhang L, Dasuri K, Fernandez-Kim SO, Bruce-Keller AJ, Keller JN. Mutant Amyloid Precursor Protein Differentially Alters Adipose Biology under Obesogenic and Non-Obesogenic Conditions. PLoS One. 2012;7:e43193. doi: 10.1371/journal.pone.0043193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umeda T, Tomiyama T, Kitajima E, Idomoto T, Nomura S, Lambert MP, Klein WL, Mori H. Hypercholesterolemia accelerates intraneuronal accumulation of Aβ oligomers resulting in memory impairment in Alzheimer's disease model mice. Life Sci. 2012 doi: 10.1016/j.lfs.2011.12.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, Bhat NR. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J. Neurochem. 2008;106:475–485. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 47.Garber AJ. Obesity and type 2 diabetes: which patients are at risk? Diabetes Obes. Metab. 2012;14:399–408. doi: 10.1111/j.1463-1326.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- 48.Flegal KM, Carroll MD, Ogden CL, Curtin R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 49.Bays HE, Chapman RH, Grandy S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int. J. Clin. Pract. 2007;61:737–747. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J. Vasc. Surg. 2011;54:819–831. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vykoukal D, Davies MG. Biology of metabolic syndrome in a vascular patient. Vascular. 2012;20:156–165. doi: 10.1258/vasc.2011.201201. [DOI] [PubMed] [Google Scholar]
- 52.Gregory SM, Headley SA, Wood RJ. Effects of dietary macronutrient distribution on vascular integrity in obesity and metabolic syndrome. Nutr. Rev. 2011;69:509–519. doi: 10.1111/j.1753-4887.2011.00390.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamada-Goto N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, Nakao K. Impairment of fear-conditioning responses and changes of brain neurotrophic factors in diet-induced obese mice. J. Neuroendocrinol. 2012;24:1120–1125. doi: 10.1111/j.1365-2826.2012.02327.x. [DOI] [PubMed] [Google Scholar]
- 54.Maesako M, Uemura K, Kubota M, Kuzuya A, Sasaki K, Hayashida N, Asada-Utsugi M, Watanabe K, Uemura M, Kihara T, Takahashi R, Shimohama S, Kinoshita A. Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 2012;287:23024–23033. doi: 10.1074/jbc.M112.367011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One. 2012;7:e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mody N, Agouni A, McIlroy GD, Platt B, Delibegovic M. Susceptibility to diet-induced obesity and glucose intolerance in the APP (SWE)/PSEN1 (A246E) mouse model of Alzheimer's disease is associated with increased brain levels of protein tyrosine phosphatase 1B (PTP1B) and retinol-binding protein 4 (RBP4), and basal phosphorylation of S6 ribosomal protein. Diabetologia. 2011;54:2143–2151. doi: 10.1007/s00125-011-2160-2. [DOI] [PubMed] [Google Scholar]
- 57.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J. Comp. Neurol. 2012 doi: 10.1002/cne.23233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, Sweatt JD. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol. Learn Mem. 2012;98:25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. (Lond) 2012 doi: 10.1038/ijo.2012.48. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 60.Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 61.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int. J. Obes. (Lond) 2010;34(Suppl 1):S23–S27. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]
- 63.Korach-André M, Archer A, Barros RP, Parini P, Gustafsson JÅ. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc. Natl. Acad. Sci. USA. 2011;108:403–408. doi: 10.1073/pnas.1017884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity. 2008;16:1493–1500. doi: 10.1038/oby.2008.267. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. Faseb. J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- 66.Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buckman LB, Thompson MM, Moreno HN, Ellacott KL. Regional astrogliosis in the mouse hypothalamus in response to obesity. J. Comp. Neurol. 2012 doi: 10.1002/cne.23233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta S, Knight AG, Gupta S, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic. Biol. Med. 2010;49:22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurol. Res. 2012;34:325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willis CL. Glia-induced reversible disruption of blood-brain barrier integrity and neuropathological response of the neurovascular unit. Toxicol. Pathol. 2011;39:172–185. doi: 10.1177/0192623310385830. [DOI] [PubMed] [Google Scholar]
- 73.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta. Physiol. (Oxf.) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 74.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huffman DM, Barzilai N. Contribution of adipose tissue to health span and longevity. Interdiscip. Top Gerontol. 2010;37:1–19. doi: 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- 77.Muzumdar R, Allison DB, Huffman DM, Ma X, Atzmon G, Einstein FH, Fishman S, Poduval AD, McVei T, Keith SW, Barzilai N. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp. Gerontol. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson TD, Hutter MM. Morbidity and effectiveness of laparoscopic sleeve gastrectomy, adjustable gastric band, and gastric bypass for morbid obesity. Adv. Surg. 2012;46:255–268. doi: 10.1016/j.yasu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 2012 doi: 10.1111/nmo.12034. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int. J. Obes. (Lond) 2011;35:642–651. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tezapsidis N, Johnston JM, Smith MA, Ashford JW, Casadesus G, Robakis NK, Wolozin B, Perry G, Zhu X, Greco SJ, Sarkar S. Leptin: a novel therapeutic strategy for Alzheimer's disease. J. Alzheimers Dis. 2009;16:731–740. doi: 10.3233/JAD-2009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan W, Hsuchou H, Jayaram B, Khan RS, Huang EY, Wu X, Chen C, Kastin AJ. Leptin action on nonneuronal cells in the CNS: potential clinical applications. Ann. N. Y. Acad. Sci. 2012;1264:64–71. doi: 10.1111/j.1749-6632.2012.06472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]