Abstract
Autophagy is an evolutionarily conserved catabolic process that transports cytoplasmic components to lysosomes for degradation. In addition to the canonical view of strict stress-response–induced autophagy, selectively programmed autophagy was recently reported in the context of gonad development of flies and worms, where autophagy seems to be necessary for clearance of germ plasm components. Similar functions have not been described in vertebrates. We used the medaka fish to study the role of autophagy in gonad formation and gametogenesis for the first time in a vertebrate organism for which the germ line is specified by germ plasm. Using a transgenic line deficient in the Ol-epg5 gene—a new critical component of the autophagy pathway—we show that such deficiency leads to an impaired autophagic flux, possibly attributed to compromised maturation or processing of the autophagosomes. Ol-epg5 deficiency correlates with selectively impaired spermatogenesis and low allele transmission rates of the mutant allele caused by failure of germ plasm and mitochondria clearance during the process of germ cell specification and in the adult gonads. The mouse epg-5 homolog is similarly expressed in the maturating and adult testes, suggesting an at least partially conserved function of this process during spermatogenesis in vertebrates.—Herpin, A., Englberger, E., Zehner, M., Wacker, R., Gessler, M., Schartl, M. Defective autophagy through epg5 mutation results in failure to reduce germ plasm and mitochondria.
Keywords: gonad development, medaka, primordial germ cell
Autophagy is an evolutionarily conserved intracellular catabolic process involving the formation of a double-membrane structure—the autophagosome—that engulfs portions of the cytosol and delivers them to lysosomes for subsequent degradation (1, 2). The process was initially described as a cellular survival pathway that recycles intracellular components to compensate for nutrient depletion and ensures the appropriate degradation of organelles such as mitochondria and endoplasmic reticulum (3). Among the autophagosomal substrates are also cytosolic proteins and ribosomes (4), as well as bacteria and viruses (5). This variety of substrates explains the close link that has been noticed between autophagy defects and pathologies such as neurodegeneration (6), cardiomyopathy (7), and tumor progression (8), as well as developmental disorders (9).
Besides a canonical view of a strict stress-response–induced autophagy, also referred to as macroautophagy, peculiar programmed autophagy processes are now reported in the context of gonad formation and gametogenesis in Drosophila and Caenorhabditis elegans (9–13). In particular, during early embryogenesis, autophagy seems to be required for specific degradation and clearance of germ plasm components (also called P-granules) in somatic cells, which guarantee an exclusive distribution of P-granules to germline precursor cells. A similar function has, however, so far not been described in vertebrates (13, 14). In the worm C. elegans autophagy is also contributing to prevent mitochondrial heteroplasmy(15–17). Although such mechanisms involving autophagy for proper clearance of P-granules/germ plasm and mitochondrial components during gametogenesis are well described in C. elegans (13, 15, 17) and Drosophila (16), it is not known whether these processes are functionally conserved across metazoans and particularly in vertebrates.
A specialized type of maternally contributed protein/RNA aggregates that are suggested to carry germ cell determinants and are segregated from oocytes into germ cell precursor cells, has been described in many organisms ranging from protostomes (18, 19) to vertebrates (20), including medaka (21). These aggregates, called P-granules in C. elegans, germ granules, germ plasm or nuage in other animals are found at every stage of germ cell development, except in mature sperm (18, 22, 23). One of the phylogenetically most conserved constitutive protein components of the germ plasm is Vasa, the prototype member of the Drosophila Vasa DEAD-box helicase family (24, 25). In C. elegans, restriction of P-granules/germ plasm to germline blastomeres is achieved through a combination of events, including migration toward the cytoplasm inherited from germ blastomeres and degradation and disassembly of P-granules/germ plasm from the cytoplasm of somatic daughter cells during early embryonic divisions (14, 26).
In C. elegans, 4 new genes involved in autophagy—epg-2, -3, -4 and -5 (ectopic PGL granules)—have recently been identified (27). epg-2 mediates recognition of P-granule proteins for delivery to autophagosomes and appears to be specific to nematodes. epg-3, -4, and -5 are conserved from worms to mammals, but lack homologs in yeast and are necessary for starvation-induced autophagy (27). In particular, worm embryos carrying mutations in the epg-5 gene accumulate autolysosomes that fail to degrade somatic P-granules (27). epg-3, -4, and -5 mutations also cause defects associated with the loss of autophagy, such as reduced survival of newly hatched larvae in the absence of food. Epistasis analysis showing suppression of the epg-5 phenotype in other autophagic mutants (i.e., atg-3, atg-13, or atg-5) suggests that epg-5 acts downstream of genes that regulate autophagosome formation (27). Consistently, silencing the epg-5 mammalian homolog mEPG-5 in cell culture leads to the persistence and accumulation of autolysosomes that fail to degrade their contents (27). Although a recent study demonstrated that autophagy is necessary for clearing the components of P-granules from somatic cells in developing C. elegans embryos (13), the underlying mechanisms regulating germ plasm depletion in somatic cells or during spermatogenesis are not yet known in protostomes.
In this study, we used medaka to study a possible role for autophagy in gonad formation and gametogenesis for the first time in a vertebrate organism for which the germ line is specified by germ plasm. We show that epg-5 is not essential for oogenesis. However, proper autophagy is absolutely mandatory for correct early and late spermatogenesis after meiosis. Defective autophagy impairs spermatogenesis and germ plasm clearance, as well as possibly paternal mitochondria degradation during the course of spermatogenesis and just after fertilization. In the mouse, epg-5 is specifically expressed in spermatogonia of the maturating and adult testes, suggesting a common conserved function in spermatogenesis in vertebrates.
MATERIALS AND METHODS
Insertion mapping and determination of allele-specific expression
For transgene insertion mapping, a genomic DNA library was constructed in λ-DASHII-EcoRI phages (cat. no. 247714; Stratagene, La Jolla, CA, USA) with a pool from 5 mutant fish as the source of DNA. High-molecular-weight genomic DNA was extracted from testes with the genomic-tip kit from Qiagen (cat. no. 10262; Hildene, Germany). A total of 1.6 × 106 independent clones were recovered. After amplification, 2.5 × 105 recombinant λ-DASH phages were plated at 5 × 104 per dish, adsorbed to nitrocellulose, and screened at high stringency using the full-length GFP gene as a probe. GFP probes were labeled radioactively by the hot PCR method, with 32P-labeled deoxycytidine triphosphate (dCTP) and the pCS2 + GFP plasmid used as a template. Positive clones were purified and end sequenced. Sequencing revealed a unique insertion of the green fluorescent protein transgene (GFP) 1455 bp downstream of the stop codon in the 3′-UTR of the epg-5 medaka gene shortly before the polyadenylation signal (Supplemental Fig. 1B).
Monitoring allele-specific expression of wild-type and mutant forms of Ol-epg5 transcripts was possible by means of the resulting lack of polyadenylation for the mutant form of Ol-epg5 after insertion. Hence, for real-time PCR quantification, from the same mRNA samples, 2 pools of cDNA were produced each time: one pool was reverse transcribed with oligo-dT primers and the other with random primers. This method resulted in 2 pools of cDNAs containing either the wild type only or wild-type and mutant forms of the Ol-epg5 transcripts.
Immunohistochemistry and histology
Immunohistochemistry was performed as described elsewhere (28). Ovaries or testes from juvenile and adult fish were fixed with 4% paraformaldehyde/balanced salt solution [(BSS); 111 mM NaCl, 5.37 mM KCl, 1 mM CaCl2·H2O, 0.6 mM MgSO4·7H2O, and 5 mM HEPES (pH 7.3)] for 30 min on ice. After fixation, the samples were washed 3 times for 10 min each time with maleic acid buffer containing Tween 20 [MABT;100 mM maleic acid, 150 mM NaCl (pH 7.5), 0.1% Triton X-100] and subsequently twice for 30 min with MABDT buffer [MABT buffer complemented with 1% bovine serum albumin (BSA) and 1% DMSO]. After blocking in MABDT blocking buffer (MABDT buffer supplemented with 2% lamb or sheep serum), samples were incubated in MABDT blocking buffer together with the primary antibody (1:150 dilution) overnight at 4°C (anti-ATG5 antibody, cat. no. NB110-53818, or anti-LC3, cat. no. NB100-2220; Novus Biologicals, Littleton, CO, USA). The samples were then washed 3 times for 5 min each time in MABDT buffer and washed again 4 times for 30 min each time in MABDT-blocking buffer on ice. Thereafter, they were incubated overnight at 4°C, with the secondary antibody diluted at 1:600 in MABDT-blocking buffer. Finally, the specimens were washed in phosphate-buffered saline (PBS), stained with Hoechst solution for 3 h at 4°C, mounted, and imaged with a Nikon C1 confocal microscope.
For immunofluorescence detection, tissue sections were prepared and rehydrated (29). Endogenous peroxidase activity was blocked by incubating the slides in 3% H2O2 in PBS. After the sections were washed with PBS 3 times for 15 min each time and blocked in 3% BSA, anti-ATG5 antibody (cat. no. NB110-53818; Novus Biologicals) was added (1:20 dilution for 1 h at room temperature). Slides were then washed with PBS (3 times, 15 min each time). Nuclear staining was performed with Hoechst 33342 (1:10000 dilution; 15 min at room temperature). Sections were then mounted in Mowiol medium (Roth, Karlsruhe, Germany).
Mitochondrial quantification, staining, and monitoring of heteroplasmy
Quantification of mitochondria was performed by RT-PCR using the following primer sets: Mf-CytB-F(forward)1: 5′-gatgttaactacggctgactaatccg-3′, Mf-CytB-R(reverse)1: 5′-gtctgaattgaggccggttgggttgt-3′; Mf-Cox1-Fw01: 5′-gcgcatgccttcgtaataat-3′, Mf-Cox1-Rv01: 5′-acgccagaggaagctaacaa-3′; and [Mf-ND1-Fw01: 5′-agtacgaccctccacctcct-3′, Mf-ND1-Rv01: 5′-gggcgtattttgagttggaa-3′, targeting 3 different genes.
For staining of active mitochondria in living cells, testes were dissected and immediately incubated in L-15 culture medium together with 1 µM MitoTracker for 1 h at 27°C. A primary stock of 1 mM MitoTracker Deep Red FM (cat. no. M22426; Life Technologies-Molecular Probes, Eugene, OR, USA) was prepared in anhydrous DMSO and stored frozen. After incubation, testes were briefly washed in L-15 medium and subsequently fixed for 30 min in 4% paraformaldehyde in 2× PBS buffer. Samples were then mounted for confocal microscopy analysis.
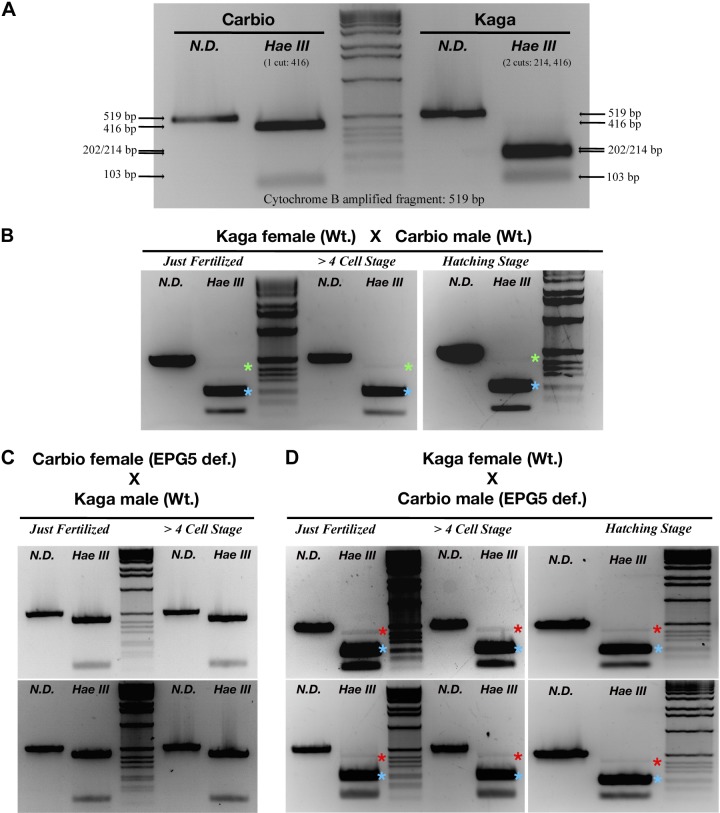
Heteroplasmy was monitored by means of the occurrence of a single nucleotide polymorphism in the mitochondrial cytochrome b gene between the Kaga and Carbio medaka strains. A 519 bp fragment of the cytochrome b gene was amplified by PCR ([CytB Fw01, CATCTGCCGGGATGTTAACTACGG; CytB Rv01, GAAATTAAGGCTACTAGCAAGGCAGC) and subjected to restriction digestion by the HaeIII enzyme to detect the polymorphism (20 U enzyme, incubated overnight at 37°C). This method resulted in either 2 bands for the Carbio strain (103 and 416 bp) or 3 bands (103, 202, and 214 bp) for the Kaga strain (see Fig. 7).
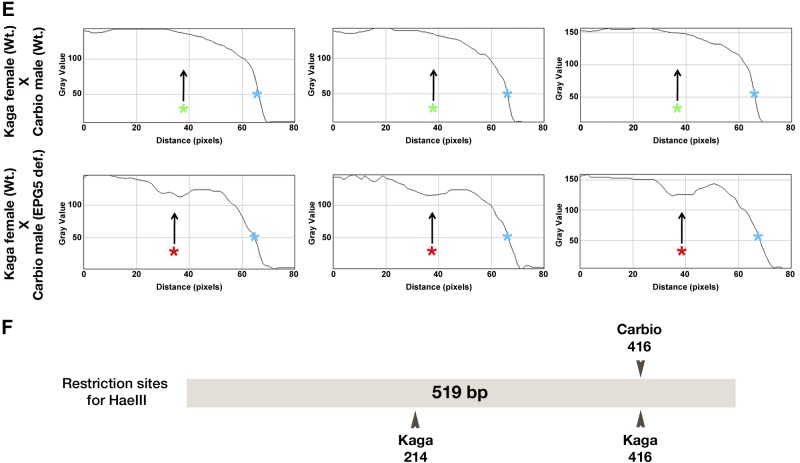
Figure 7.
PCR-based monitoring of heteroplasmy in offspring of Ol-epg5-deficient vs. wild-type fish. PCR monitoring of paternal and maternal mitochondrial DNA in offspring resulting from crosses of Carbio Ol-epg5-deficient males with Kaga wild-type females revealed higher levels of heteroplasmy through paternal mitochondria transmission. A) Heteroplasmy was monitored by means of the occurrence of a single nucleotide polymorphism in the mitochondrial cytochrome b gene between the Kaga and Carbio medaka strains. A 519 bp fragment of the cytochrome b gene, was amplified by PCR and subjected to restriction digestion with the HaeIII enzyme to detect the polymorphism. This method revealed either 2 bands for the Carbio strain (103 and 416 bp) or 3 bands for the Kaga strain (103, 202, and 214 bp). B–D) Whereas in crossings of Kaga wild-type females with Ol-epg5-deficient Carbio wild-type males, or of females with Kaga wild-type males, paternal mitochondrial DNA was almost undetectable (B, C), crossing Kaga wild-type females with Carbio males deficient for Ol-epg5 resulted in a clearly visible paternal mitochondria contribution to the offspring that persisted during development (red stars in D compared with green stars in B). E) Profile plot analyses of gray-scale images (Image J, NIH, Bethesda, MD, USA) of the gels for the 2 crossings presented in (B, top row) and (D, bottom row), confirmed a higher polymorphism (presence of a band at 416 bp, red star, appearing as a lower gray-scale value) in the progeny when the father was deficient in Ol-epg5. For references, the maternal bands at 202/214 bp were taken (gray-scale value = 0) and the gel background values set to 150. F) Restriction and single-nucleotide polymorphism map of the amplified fragment used for monitoring heteroplasmy.
Bacterial artificial chromosome recombination
A bacterial artificial chromosome (BAC) clone encompassing medaka epg-5 (ola1-177J01) genomic region was obtained from NRBP Medaka (http://www.shigen.nig.ac.jp/medaka/). A BAC transgenic method using homologous recombination was used to generate the reporter constructs (30, 31). The following primers were used to amplify mCherry fragments for homologous recombination into the BAC clone: MDP-BAC-F, GCAGCAGGTTGGTGTGACGGCATTTTACCAAGTGGTCTCCTTCGTGTGTGAGGACCCACCGGTCGCCACCATGGT; and MDP-BAC-R, CAATGTGGGGAGATTGTGGTGCTTACCTGTCCCAGTATCTCCACACAGGAAGTCGACCAGTTGGTGATTTTG. After homologous recombination the generated fragment was inserted into the BAC clone in-frame of the 14th exon of the epg-5 medaka gene (Supplemental Fig. 1C).
Generation of an epg-5 BAC transgenic medaka line and imaging analyses
The Carbio (Carolina Biological Supply cat. no. 2674) strain of medaka (Oryzias latipes) was used for the establishment of the epg-5 transgenic line. Microinjection of DNA was performed as described elsewhere (32), with BAC clone DNA at a concentration of 50–100 ng/ml. G0 fish larvae were then screened for fluorescence (mCherry), and positive individuals were raised to adulthood. Siblings from positive G0 fish were mated to each other and offspring were again sorted for fluorescence. The result was the creation of a truncated EPG5 fusion protein reporter transgenic line only, because the fluorescent reporter was inserted in frame into the EPG5 gene (Supplemental Fig. 1). In this line, the EPG5-mCherry fusion protein is not functional (insertion into the 14th exon out of 30), but it is incorporated into the autophagosomes, enabling us to follow the dynamics of expression. The Sox9b, Dmrt1a, Dmrt1bY, and Olvas transgenic lines were described earlier (22, 30, 33).
For imaging embryos, hatchlings or tissues were mounted with 1.2% low-melting-temperature agarose. Confocal pictures and image stacks were acquired with a Nikon C1 (eclipse Ti) confocal laser scanning microscope and NIS elements AR software (Nikon, Tokyo, Japan). For histology (see Fig. 3F–I), sections were stained with hematoxylin and eosin according to a standard protocol.
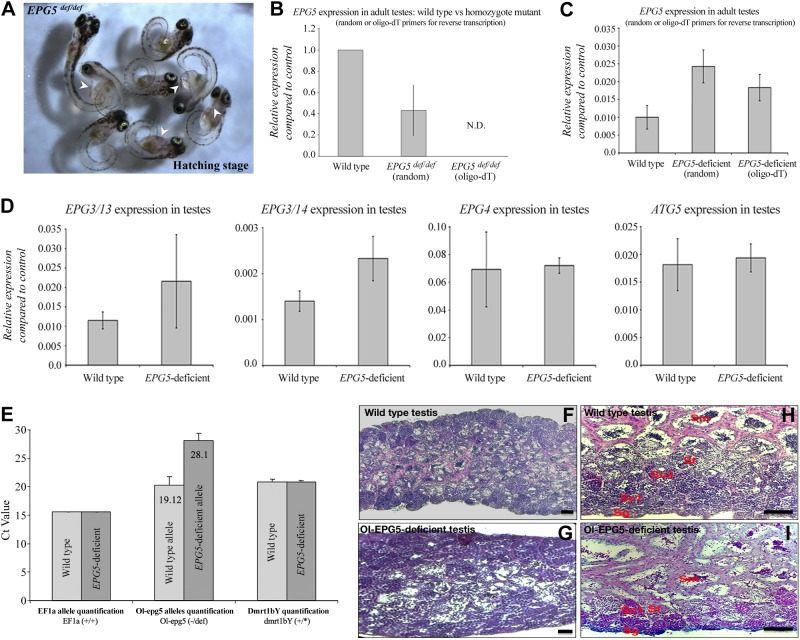
Figure 3.
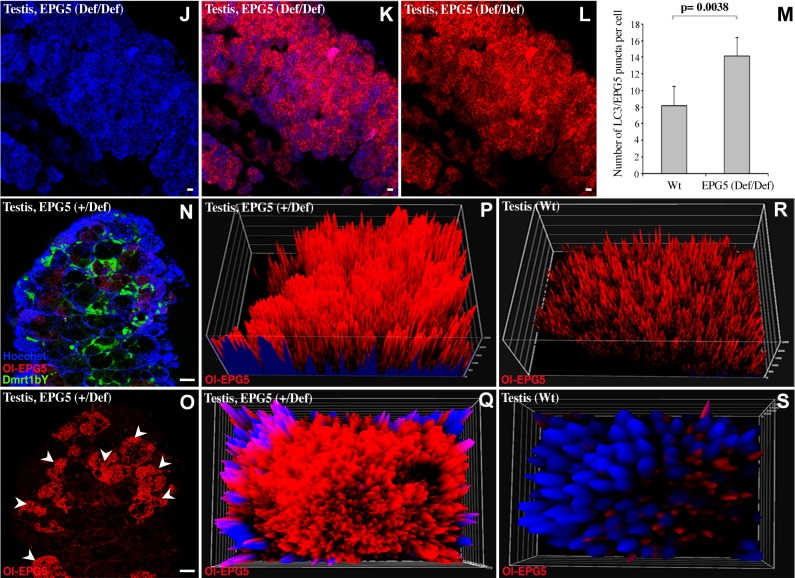
Characterization of epg5-deficient medaka testes. A) Ol-epg5 mRNA quantification in young testes (EPG5def/def), with either oligo-dT or random primers used for reverse transcription to discriminate mutant and wild-type alleles, showed a reduction of ∼60% of Ol-epg5 expression/stability for the homozygous mutants when compared with the wild-type allele. Mutant Ol-epg5 mRNAs were consistently not detected when reverse transcription was performed with an oligo-dT primer. B) As phenotypes, Ol-epg5 homozygous mutants display, in addition to a developmental delay, a curly shape (tail curled down) and pericardial edema (arrowhead). C) Ol-epg5 mRNA quantification in adult testes (EPG5+/def), with either oligo-dT or random primers used for reverse transcription to discriminate both mutant and wild-type alleles, showed reduced Ol-epg5 expression/stability for the mutant allele when compared with the wild-type allele. D) Expression analysis of other genes involved in the same autophagy pathway revealed Ol-epg3 genes of medaka to be up-regulated in mutant fish, whereas Ol-epg4 and Ol-atg5 expression levels remain unaffected. E) To quantify the relative abundance of the Ol-epg5 wild-type vs. the mutated allele, real-time PCR quantifications were performed with sperm DNA. Although quantification of homozygous (EF1a gene) or heterozygous (dmrt1bY gene) alleles did not display any variation between wild-type and Ol-epg5-deficient fish sperm DNAs, quantification of the Ol-epg5 mutant vs. wild-type allele in total sperm DNA showed that the allele encompassing the mutant Ol-epg5 was barely represented in sperm DNA of mutant fish. F– I) Histology of testis structures (hematoxylin and eosin staining) of (F, H) wild-type and (G, I) Ol-epg5-deficient testes. G, I) Ol-epg5-deficient testes were smaller and displayed a disorganized structure. G) In Ol-epg5-deficient testes, the interstitial epithelium and tubules were not completely developed and the cyst structure of the testis was not preserved (vs. F). I) Ol-epg5-deficient testis clearly showed an accumulation of spermatogonia and the fully differentiated spermatocytes were underrepresented when compared with the wild type (H). J–L, N–S) Reporter monitoring for Ol-epg5 (Ol-epg5-mCherry) expression showed a clear relocalization and accumulation of the fusion protein within the (N–Q) Ol-epg5-deficient testes. Such accumulation was even more pronounced in Ol-epg5(def/def) testes (J–L) compared with controls (Fig. 2A, E). M) An accumulation of LC3/EPG5-positive puncta was observed when there was a deficiency of Ol-epg5 alleles. N, O) Testicular localization of Ol-epg5 in a triple-transgenic fish (Ol-epg5-deficient/Ol-epg5:mCherry/Ol-dmrt1bY:GFP). In Ol-epg5-deficient testes, a clear accumulation of the Ol-epg5:mCherry fusion protein was apparent in the germ cells of certain cysts (O, arrowheads), but not in the (N) somatic Sertoli cells, specifically marked by dmrt1bY (37, 38). P–S) Intensity surface projections show the accumulation of Ol-epg5:mCherry fusion protein in (P) Ol-epg5-deficient testis and cysts (Q) compared with wild type (R, S, respectively). Scale bars, 10 (F–I) and 5 µm (J–L, N, O).
In situ hybridization
In situ hybridization of paraffin-embedded sections, whole embryos, or mouse tissues was performed (29, 34, 35). In brief, materials were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned at 7–10 µm. Hybridization was performed with digoxigenin-labeled antisense riboprobes (Mm-Lamin C2 or Mm-EPG5), and the slides were washed extensively before treatment with RNAse-A (Qiagen). Signals were visualized with an anti-Dig-AP antibody (cat. no. 11093274910; Roche Diagnostics, Mannheim, Germany) and BM purple substrate (cat. no. 11442074001; Roche Diagnostics). Slides were then mounted in Kaiser’s glycerol gelatin medium (cat. no. 1092420100; Merck, Darmstadt, Germany).
Quantitative real-time RT-PCR
For real-time quantitative (q)RT-PCR, 2 µg RNA were reverse transcribed with the Revert Aid First-Strand cDNA synthesis kit (cat. no. K1622; Thermo Scientific, Dreieich, Germany) with either oligo(dT) or random primers. Real-time qRT-PCR was performed with an iCycler iQ5 Real-Time PCR Detection System (BioRad, Hercules, CA, USA). Reactions contained 1:50 of the cDNA reaction, and PCR was performed with an annealing temperature of 60°C and quantification of SybrGreen (Life Technologies, Darmstadt, Germany) incorporation. PCR products were confirmed by melting curve analysis and agarose gel electrophoresis. Housekeeping genes (HPRT for mouse or Ef1a for medaka) were used for normalizing expression levels. The following primers were used: Ol-ef1a F01: GCCCCTGGACACAGAGACTTCATCA, Ol-ef1a R01: AAGGGGGCTCGGTGGAGTCCAT; Ol-epg-3/13 Fw01: ACTGTGAACAGCCTCAGAGACGG, Ol-epg-3/13 Rv01: CAGTACATAAGACCAGGAGTAA; Ol-epg-3/14 Fw01: CCGGTCAGACGGCGGTCGCAATTG, Ol-epg-3/14 Rv01: GGACGAGGAGGATCAGCAAGCC; Ol-epg-4 Fw01: CAGTGCAAATGAAGCAAGGA, Ol-epg-4 Rv01: GGAGATGTGGGATGAGGAGA; Ol-atg-5 Fw01: ACAAACGACAGACCGTTCATCC, Ol-atg-5 Rv01: TCTCCAGCAGAGGTTCGATTC; Mm-epg-5 Fw01: GGAGGCTCAGCTCGTTGACGAGC, Mm-epg5 Rv01: CAGCTTCACTATCAGTTCCGAGGAACAG; and Mm-HPRT Fw01: TGTTGTTGGATATGCCCTTG, Mm-HPRT Rv01: ACTGGCAACATCAACAGGACT. All measurements were performed at least twice, and mean values were calculated.
RESULTS
Ol-epg5 gene structure and expression
The medaka Ol-epg5 gene, encompassing 30 exons, encodes a unique transcript of 4.779 kb. The predicted protein is 1592 amino acids in length (Supplemental Fig. 1A). epg5 is a single-copy gene in the medaka, similar to all bilaterian genomes analyzed so far. In birds (chicken, turkey, and zebrafinch), the epg5 gene is located on the Z chromosome.
During development Ol-epg5, mRNA is maternally supplied. After midblastula transition and the start of zygotic transcription, it is only moderately expressed during the whole embryonic development up to hatching (Fig. 1A, C). In adult organs, Ol-epg5 transcripts are mainly present in spleen, brain, eyes, gills, and gonads of both sexes. Whereas the expression levels of Ol-epg5 are generally similar between male and female organs, including gonads, a significantly higher expression of Ol-epg5 (more than 5-fold) was detected in the spleens of the males (Fig. 1B).
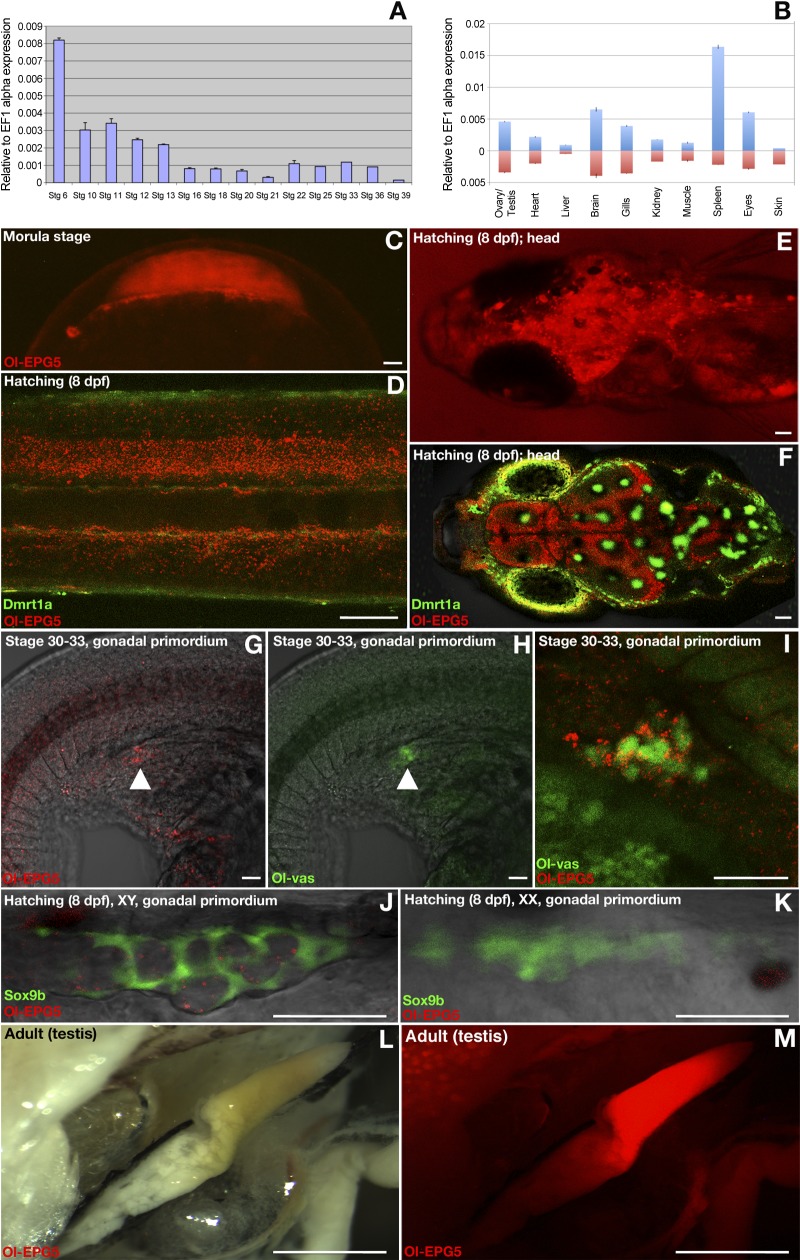
Figure 1.
Expression of Ol-epg5 during early development and in gonads of medaka. A) Expression of Ol-epg5 during early stages of medaka larval development. B) Expression of Ol-epg5 in adult tissues (male: upturned bars; female, downturned bars). C) BAC reporter expression of Ol-epg5 was detected as early as the morula stage. D–I) During medaka larval development the background expression of Ol-epg5 progressively became restricted to interneurons and sensory neurons on the respective dorsal and ventral sides of the notochord (D), brain (fore-, mid- and hind brain) (E, F), and PGCs (G–I) of the embryos. For better contrast, green fluorescence can be observed in the skin and eyes (dmrt1a green fluorescence) (D, F) and in pigment cells (F, auto-fluorescence). G–I) At stages 30–33, Ol-epg5 was specifically coexpressed with Ol-vas (a specific marker of the germ plasm) in the germ cells (arrowheads). J, K) At the hatching stage, Ol-epg5 was expressed in the male germ cells of the primordial gonad (J) but was absent in female germ cells (K). Sox9b fluorescence (green) allows visualization of the somatic presupporting cells surrounding the germ cells of the gonadal primordium (30). L–M) Ol-epg5 was highly expressed in the testes of adult medaka. Scale bars, 110 (C–F), 7.5 (G–K), and 3000 µm (L, M).
For analyzing the spatial expression pattern of Ol-epg5 a transgenic reporter line was established from a recombined BAC encompassing the whole genomic region of epg5 and carrying a fluorescent (mCherry) reporter fused to the first 684 amino acids of the Ol-epg5 protein (Materials and Methods and Supplemental Fig. 1C). Such reporter lines have shown optimal spatial resolution and high reliability of gene expression (28, 31). The Ol-epg5-mCherry fusion protein line (nonfunctional for Ol-epg5, because Ol-epg5 is truncated) allows the monitoring of Ol-epg5 expression at both the transcriptional and the protein localization levels. Being consistent with RT-PCR data (Fig. 1A), analysis of the Ol-epg5 BAC transgenic line revealed that the Ol-epg5-mCherry fusion protein is expressed as early as the morula stage before zygotic transcription starts, implying active transcription during oocyte development, and accumulation of maternal mRNA (Fig. 1A, C). During the whole period of embryonic development, a ubiquitous background level expression was detected (Fig. 1A). This background expression became progressively restricted to the brain (fore-, mid- and hindbrain) and the interneurons and sensory neurons on the respective dorsal and ventral sides of the notochord of the hatchlings (Fig. 1D–F). As early as stages 30–33, when the germ cells reached the undifferentiated mesodermal gonadal primordium, specific colocalization of Ol-epg5 and Ol-vas, a germ plasm marker, was observed in the primordial germ cells (PGCs) of both sexes (Fig. 1G–I). Three days later, at the hatching stage, sexually dimorphic male-specific expression of Ol-epg5 was evident in the germ cells (Fig. 1J, K). High Ol-epg5 reporter expression was also noted in adult testes (Fig. 1L, M).
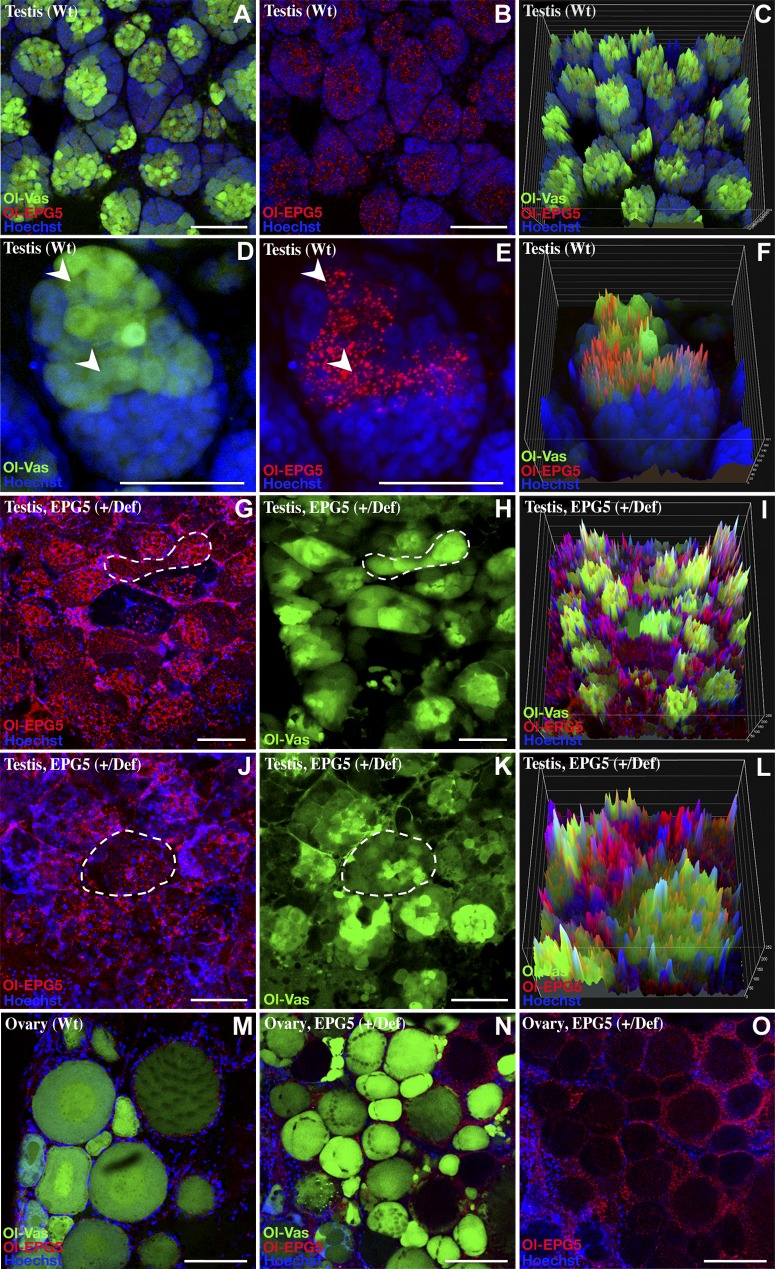
To find out whether Ol-epg5 is involved in gametogenesis, expression and protein localization of Ol-epg5 were investigated at cellular resolution in gonads of both sexes (see Figs. 2 and 4). In adult testis, Ol-epg5 expression was restricted to germ and spermatogonial cells (Fig. 2A–D). Analysis of double-transgenic Ol-epg5/sox9b fish revealed the absence of Ol-epg5 expression in the sox9b-positive cells. Sox9b is a specific marker of the Sertoli cells (30), indicating the absence of Ol-epg5 expression in the somatic part of the male gonad (Fig. 2C, D). However, the Ol-epg5:mCherry fusion protein always appeared as granules or puncta dispersed in the cytoplasm of the germ cells (Fig. 2 in general; 2H in particular).
Figure 2.
Colocalization of medaka Ol-epg5 in adult testes, with specific markers of the autophagy pathway (ATG5 and LC3). A, B) Medaka Ol-epg5 (Ol-epg5-mCherry reporter only) was expressed in the germ cells of the adult testis. B) Within the adult testis cysts, Ol-epg5 expression was restricted to only a subset of cells. C, D) Within each cyst of the testis, Ol-epg5 expression was specifically detected in the germ cells (spermatogonia). Only background expression of Ol-epg5 was detected in the somatic part of the testis, as illustrated in testes of double-transgenic fish for Ol-epg5 and Ol-Sox9b. E–J) Immunochemistry with an ATG5 antibody, a specific marker of the canonical autophagy pathway, revealed colocalization of ATG5 with Ol-epg5 in the germ cells of the testis cysts. G, J) At subcellular resolution, the colocalization was no more apparent between the cytoplasmic expression of ATG5 and a more puncta-restricted expression of Ol-epg5 (J). K–N) Immunohistochemistry with an LC3 antibody, a specific marker of the late steps of autophagy (autophagosomes), revealed both cellular-type and subcellular colocalization together with Ol-epg5-positive puncta. Scale bars, 5 µm.
Figure 4.
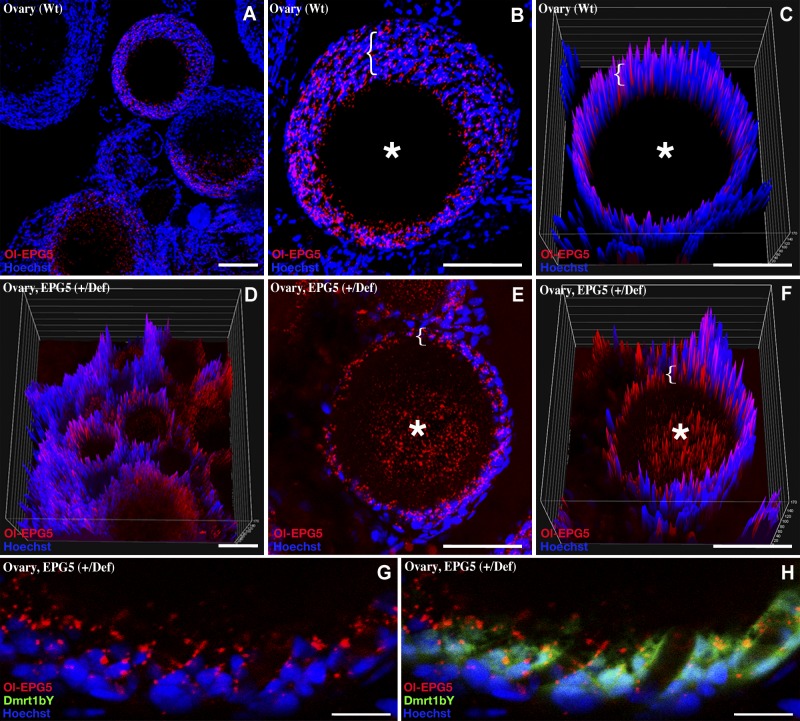
Ovarian localization of Ol-epg5 in wild-type and Ol-epg5-deficient fish. A–C) In the ovary, Medaka Ol-epg5 was expressed in the surrounding supporting cells (brackets) of the oocytes (asterisks). Ol-epg5 progressively accumulates in the supporting layer while the oocytes maturate (brackets in B and C). D–H) Ovarian localization of Ol-epg5 in a triple-transgenic fish (Ol-epg5-deficient/Ol-epg5:mCherry/Ol-dmrt1bY:GFP). In Ol-epg5-deficient ovaries, a clear accumulation of the Ol-epg5:mCherry fusion protein was apparent in the cell layer surrounding the oocytes (brackets) and in the oocytes (asterisks). C, D, F) Intensity surface projections show accumulation of epg5:mCherry fusion protein in the cytoplasm of oocytes (asterisk) of (D, F) Ol-epg5-deficient ovaries compared with wild type (C). G, H) In the surrounding layer of the oocytes, Ol-epg5 precisely colocalized with Ol-dmrt1bY, a marker of the granulosa cells (37, 38). Scale bars, 10 (A–H) and 40 µm (I, J).
Immunochemistry using an ATG5 antibody, a specific marker of the early steps of the canonical autophagy pathway, revealed coexpression of Ol-epg5 and ATG5 in the spermatogonial cells of the testis (Fig. 2E–J). Nevertheless, at subcellular resolution, the colocalization was not apparent between a cytoplasmic expression for ATG5 and a more puncta-restricted expression of Ol-epg5 (Fig. 2H, I vs. J). Immunohistochemistry with an LC3 antibody, a specific marker of the late steps of autophagy, marking the autophagosomes (36), revealed both cell type and subcellular colocalization with Ol-epg5 puncta (Fig. 2K–M vs. 2N). In ovaries, Ol-epg5 mainly localizes to the somatic cell layer surrounding the oocytes (brackets) but not in the oocytes themselves (asterisks in Fig. 4A–C).
Characterization of EPG5-deficient transgenic medaka
In a screening of transgenic fluorescent reporter medaka, we isolated one line that displayed strong transgene transmission distortion (Supplemental Table 1). We noted that the allele carrying the inserted transgene was transmitted to the progeny at varying rates, ranging from 0 to the expected mendelian ratio of 50% in the offspring of heterozygotes crossed to wild-type fish and that transgene segregation distortion was much more pronounced and significant in males than in females. Homozygous mutant fish displaying transgene segregation distortion were obtained exceptionally rarely (<1/100), indicating very high prehatching lethality of such individuals. Although the heterozygous fish (Ol-epg5+/def) did not exhibit any apparent phenotype, homozygous embryos (Ol-epg5def/def) were massively affected (Fig. 3A). In addition to a developmental delay, a curly shape (tail curled down) and pericardial edema was observed (Fig. 3A; Table 1). Molecular analysis revealed a single transgene insertion within the 3′UTR of the Ol-epg5 gene (Supplemental Fig. 1B). Hence, the mutant Ol-epg5 transcripts lack part of their 3′UTR, including the wild-type polyadenylation signal (Supplemental Fig. 1B and Materials and Methods).
TABLE 1.
Comparison of the symptoms and phenotypes caused by EPG5 deficiency in human, mouse, worm, and medaka
| Symptoms and observations | Organisms deficient in EPG5 |
|||
|---|---|---|---|---|
| Human (VICI syndrome) | Mouse | C. elegans | Medaka | |
| General | Postnatal growth retardation, developmental delay and severe psychomotor delay. Facial dysmorphism (cleft lip and palate and micrognathia). Cataract. Hypopigmentation (skin, hair, retina). Immunodeficiency.a | Grow normally into sexually mature adults. Neural symptoms at 4 mo of age and then gradual hind limb paralysis.a | Viable | Viable. Developmental delay; curly shape (tail curled down, Fig.3B); pericardial edema.a |
| Life span | Die before the age of 3 y. | Eventually die by 10–12 mo. | Normal | Normal. Die after a few weeks of development; rarely reach sexual maturity. |
| Myopathic abnormalities | Increased variability in fiber size. Fiber atrophy and prominent central nuclei atrophic fibers. Abnormal glycogen deposits in subsarcolemmal locations. | Variation in fiber size. Scattered and clustered atrophic fibers. Increased number of myofibers with centrally located nuclei. Higher glycogen content. Accumulation of abnormally enlarged mitochondria. Reinnervation of denervated muscle fibers by sprouting from normal axons. | ND | ND |
| Mitochondria | Accumulation of mitochondria in subsarcolemmal locations. | Accumulation of abnormally enlarged mitochondria in myofibers. | ND | Failure in reducing the number of mitochondria during the course of spermatogenesis. |
| Corpus callosum | Agenesis of the corpus callosum (failure to develop the large bundle of fibers that connect the cerebral hemispheres). Variable penetrance of this phenotype. | Corpus callosum significantly thinner or completely absent. | NA | NA |
| Germ cells and spermatogenesis | NA | High expression of epg5 in spermatogonia cells, but a priori not symptomatic. | Failure in somatic P-granule clearance. | Impaired spermatogenesis. Failure of germ plasm and mitochondria clearance during spermatogenesis. Do not reproduce. |
Abbreviations for medaka: normal, heterozygote; italic, homozygote. Symptoms for mutations in autophagy-related genes in mice: ATG5/ATG7/Ei24, neurologic deficit at the age of 2–3 wk and death at the age of 3–4 mo. Extensive neuron loss in various cerebral and cerebellar regions. ATG16I or ATG4b: reduction of autophagy but no obvious histopathologic alterations. aAutophagy flux is impaired and aggregates accumulate.
To analyze whether this results in lower mRNA stability and lower transcript copy numbers, we performed RT-PCR quantifications of both the wild-type and mutant forms (Fig. 3B, C). Ol-epg5 mRNA quantification in testes of young males (EPG5def/def), with either oligo-dT or random primers used for reverse transcription to discriminate both mutant and wild-type alleles, showed a reduction of ∼60% of Ol-epg5 expression/stability for the homozygous mutants when compared with wild type (Fig. 3B). Consistently, mutant Ol-epg5 mRNAs are not detected when the reverse transcription was performed with an oligo-dT primer. In the heterozygous context, Ol-epg5 mRNA quantification in adult testes, with either oligo-dT or random primers used for reverse transcription to discriminate both alleles (see Materials and Methods for the strategy), showed a reduction of Ol-epg5 expression for the mutant allele of about two-thirds compared with the wild-type allele. However, a general, but slight, up-regulation of the expression of the wild-type Ol-epg5 allele was noted (Fig. 3C). Although it was expressed in the postmeiotic cells, lower transcript copy numbers of the Ol-epg5 mutant allele were expected in these cells, as observed for the homozygous mutant. Also the 2 Ol-epg3 genes of medaka showed up-regulation in mutant fish (Fig. 3D). In contrast, Ol-epg4 and Ol-atg5 expression levels remained unaffected.
Quantification of the mutant vs. wild-type allele in total sperm DNA revealed that the allele encompassing the mutant Ol-epg5 is barely represented within sperm of mutant fish (Fig. 3E). To find out whether the underrepresentation of mutant sperm cells is linked to a failure during spermatogenesis, testis structures of wild-type and Ol-epg5-deficient testes were compared (Fig. 3F–I). Mutant testes were smaller and had a slightly disorganized structure. It seems that the interstitial epithelium and tubules were not completely developed, and the cyst structures of the testis were not preserved (Fig. 3F vs. G). Ol-epg5-deficient testes also showed accumulation of spermatogonia, whereas fully differentiated spermatocytes were underrepresented (Fig. 3H vs. I).
Next, Ol-epg5 quantification and localization were examined in gonads of Ol-epg5 mutants (Fig. 3J–M). Because the dmrt1bY gene is a specific marker of the somatic gonad in medaka, a triple transgenic line (Ol-epg5-deficient, Ol-epg5:mCherry and Dmrt1bY:GFP) was established (Figs. 3N, O; 4D–H). In gonads of such fish, an apparent accumulation of Ol-epg5:mCherry fusion protein was observed in both sexes (Fig. 3J–L, N, O vs. 2A–D; 4D–F vs. A–C). Ol-epg5-deficient testes displayed higher amounts of Ol-epg5-mCherry fusion protein in spermatogonia and in type 1 and 2 spermatocytes (Fig. 3J–L, N–O vs. 2A–D), whereas no particular accumulation was apparent in the somatic lineage (Fig. 3N, O). Since a perfect colocalization between Ol-EPG5 and LC3-positive puncta was observed in testes (Fig. 2N), a quantification of these puncta was performed in wild-type vs. Ol-EPG5def/def testis (Fig. 3M). Similar to the Ol-EPG5-mCherry reporter fusion protein, within the same genetic background (Fig. 3J, K, N–Q), an apparent accumulation of LC3/EPG5-positive puncta was observed when Ol-EPG5 was deficient (Fig. 3M). Ol-epg5-deficient ovaries displayed an accumulation of the Ol-epg5 fusion protein in the oocytes (asterisks in Fig. 4E, F vs. B, C) and in the supporting somatic cells (brackets in Fig. 4E vs. B, G, H). This visual accumulation of Ol-epg5 observed in mutant compared with wild-type gonads was confirmed by intensity surface projection plots (Figs. 3P, Q vs. R, S; 4C vs. F).
Failure to eliminate a germ plasm component during spermatogenesis in Ol-epg5-deficient fish
The key processes of fish germ cell specification, differentiation, and survival are the formation, germ cell restriction, and degradation of germ plasm components (such as vasa, nanos, piwi, and tudor) during the course of spermatogenesis [see (39) for review]. Because the worm epg5 homolog has been shown to be involved in the autophagic process of degradation of P-granules in somatic cells during embryogenesis (27), the kinetics of germ plasm degradation was monitored in wild-type (Fig. 5A–F, M) and Ol-epg5-deficient fish gonads (Fig. 5G–L, N, O). In wild-type testis, colocalization of the germ plasm marker Ol-vas (22) and Ol-epg5 was observed in spermatogonia and type 1 spermatocytes (Fig. 5A–F). Whereas high Ol-vas and low Ol-epg5 expression was seen in spermatogonia, low Ol-vas and higher Ol-epg5 expression was apparent in type 1 spermatocytes (Fig. 5D, F). In contrast, in Ol-epg5-deficient testes Ol-vas was more widely distributed over the entire cysts, and its expression was not restricted to spermatogonia and type 1 spermatocytes (Fig. 5G–L). In parallel, an accumulation of Ol-epg5-positive granules was observed (Fig. 5G, J).
Figure 5.
Colocalization of Medaka Ol-epg5 with Ol-vas, a specific marker of the germ plasm in adult gonads, and failure of Ol-epg5-deficient fish testes to eliminate Ol-vas during the course of gametogenesis. A–F) Testes of double-transgenic (Ol-vas:GFP and Ol-epg5:mCherry) fish displaying perfect colocalization of Ol-epg5 and Ol-vas (a specific marker of the germ plasm) during the course of spermatogenesis in spermatogonia and type 1 spermatocytes (arrowheads). G–L) In Ol-epg5-deficient testes, Ol-vas expression was not restricted to spermatogonia but was present in the whole cyst (dotted outlines). Intensity surface projections showed accumulation of Ol-epg5:mCherry fusion protein and Ol-vas in Ol-epg5-deficient (I) testes and(L) cysts compared with wild type (C, F, respectively). In addition, a disorganized structure of the cysts was obvious (G, H, vs. A, B). M–O) Although Ol-epg5 accumulated significantly in the granulosa cells of the (N, O) Ol-epg5-deficient ovaries compared with wild type (M), Ol-vas expression distribution remained apparently identical to the wild-type situation (M vs. N). Scale bars, 5 (A–E, G, H, J, K) and 10 µm (M–O).
In mutant females, although an accumulation of Ol-epg5 was noted in Ol-epg5-deficient ovaries, no significant changes were apparent in Ol-vas distribution in oocytes over the different maturation stages compared with that in wild type (Fig. 5N, O vs. M).
Failure of Ol-epg5-deficient fish to reduce the number of mitochondria during the course of spermatogenesis favors heteroplasmy
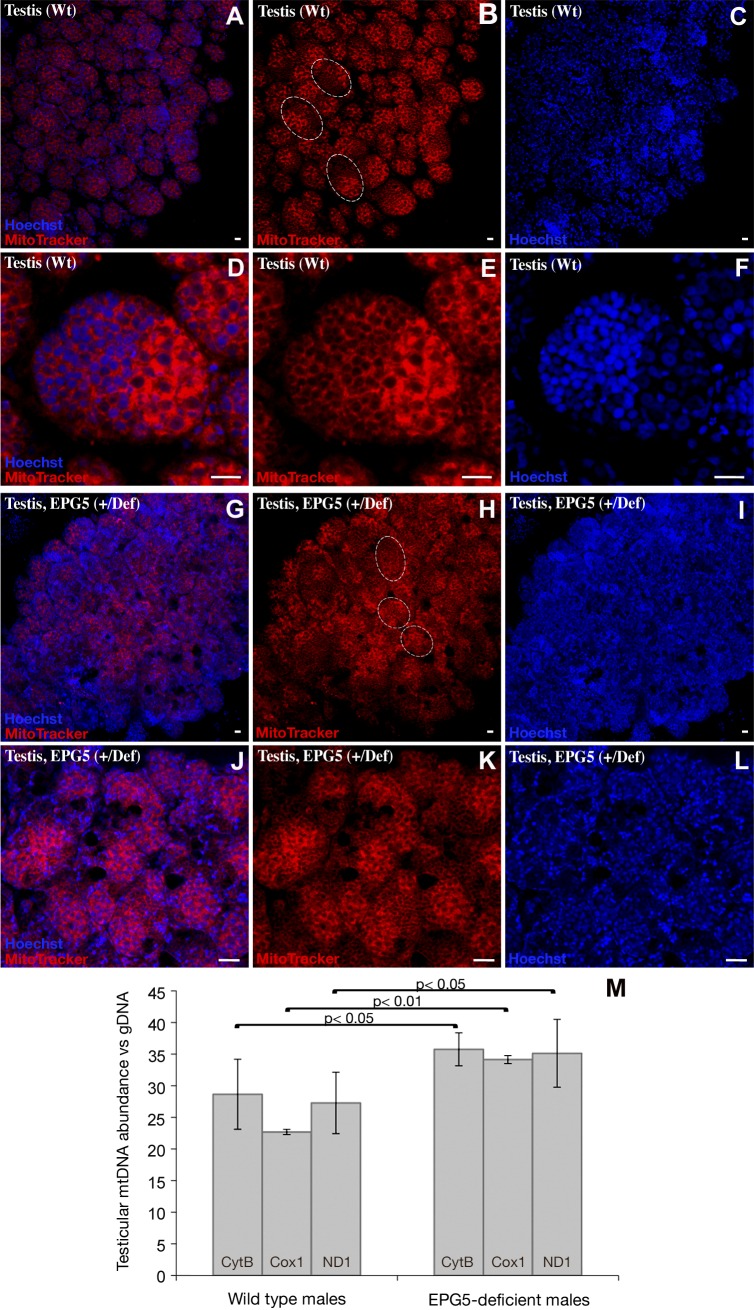
It has been shown that prevention of heteroplasmy (the presence of a mixture of both maternal and paternal mitochondrial genomes within an individual) in medaka is achieved by a gradual reduction of the number of mitochondria during the course of spermatogenesis (40). To determine whether this phenomenon of mitochondrial reduction is linked to autophagy, we used the MitoTracker stain (Life Technologies-Molecular Probes) to label in vivo and quantify the number of mitochondria during spermatogenesis in a wild type or Ol-epg5-deficient genetic background (Fig. 6). Although strong staining was detected in the spermatogonia of wild-type testes, a progressive decrease in signal intensity was observed during spermatogenesis (Fig. 6A–F). In contrast, Ol-epg5-deficient testes exhibited a sustained signal through all stages of spermatogenesis (Fig. 6G–L). Quantification of mitochondrial DNA by RT- PCR consistently revealed a significantly higher mitochondrial DNA content in Ol-epg5-deficient than in wild-type testes (Fig. 6M). Further, a PCR and restriction nuclease digest–based method for monitoring heteroplasmy (see Materials and Methods) suggested that this higher rate of paternal mitochondria retention during spermatogenesis is linked to an apparent higher heteroplasmy in the progeny. Besides, a background heteroplasmy was noted, even in crosses with wild-type fish (Fig. 7).
Figure 6.
Increase of mitochondria in Ol-epg5-deficient testes. A–L) In addition to an obvious disorganized structure (see also Fig. 3E, G), (G–L) Ol-epg5-deficient testes stained with MitoTracker (Life Technologies-Molecular Probes) showed an increase in mitochondria compared with wild type (A–F). M) Real-time PCR quantification of mitochondrial DNA revealed a significantly higher DNA content in Ol-epg5-deficient testes than in wild types. Dotted lines: cysts in the testis. Scale bars, 5 µm.
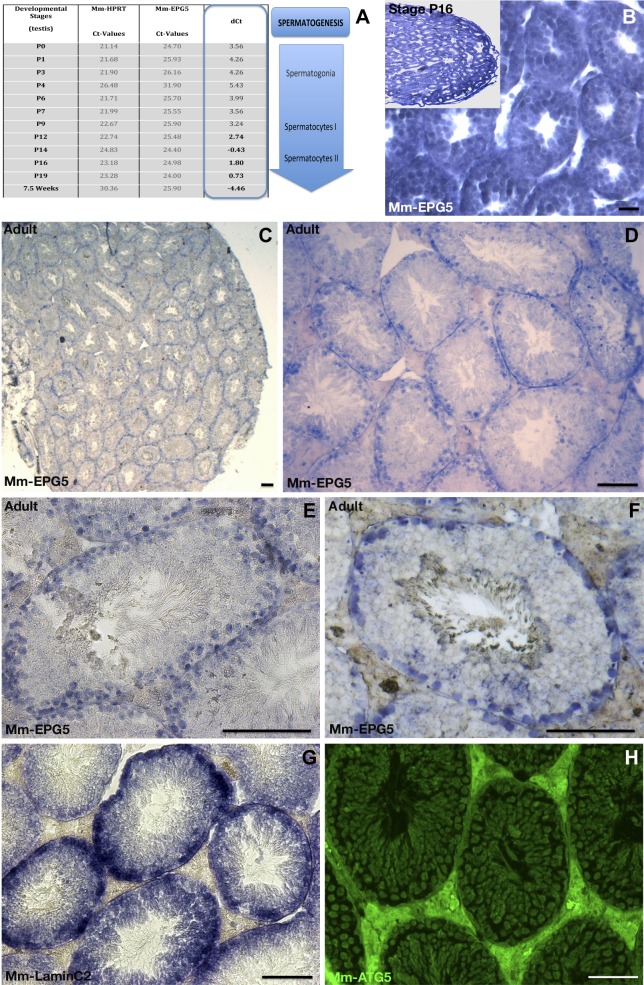
Divergent expression patterns of Mm-epg5 in spermatogonia compared with the ATG-5 autophagy marker in mouse testis
In contrast to C. elegans and medaka, mammals lack P-granules or germ plasm (41), but also undergo a reduction in the number of mitochondria during the course of spermatogenesis. To study a possible role of epg5 in mammalian spermatogenesis, expression of epg5 was investigated during mouse testis development (Fig. 8). During early mouse development, testicular expression of Mm-epg5 gradually increased from stage P0 on and reached maximum expression at stage 16 (Fig. 8A). At stage 16, Mm-epg5 was specifically expressed in meiotic germ cells and type 1 spermatocytes (Fig. 8B). In adult testes, Mm-epg5 was exclusively expressed in round cells at the periphery of the seminiferous tubules (Fig. 8E, F). Costaining with lamin C2 (LC2) (42) confirmed the specific expression of Mm-epg5 in testicular meiotic germ cells only (Fig. 8F vs. G). In contrast to the situation observed in medaka testes, where Ol-epg5 and Ol-atg5 strictly colocalize within germ cells, expression of Mm-atg5 is not only restricted to spermatogonia but also more widely distributed in different mouse testicular cell types, including spermatogonia cells (Fig. 8H). This finding indicates a specific spermatogonia-only autophagy process in which Mm-epg5 is involved, likely different from the one involving Mm-atg5.
Figure 8.
Testicular expression of Mm-epg5 the mouse homolog of epg5. A) qRT-PCR showed a gradually increasing expression of Mm-epg5 during mouse testicular development from stage P0 up to 7.5 wk. B–F) In situ hybridization of Mm-epg5 at different stages of testis development (blue). B) At stage 16, Mm-epg5 was expressed in spermatogonia/spermatocytes. C–F) In male gonads of adult mice Mm-epg5 expression was restricted to spermatogonia and localized identically to the spermatogonial marker LC2 (F vs. G). F, H) In the adult mouse testis, Mm-epg5 did not colocalize with ATG5. H) In contrast to medaka testis, immunochemistry using an ATG5 antibody revealed that the expression of Mm-ATG5 was not restricted to germ cells, but was more widely and ubiquitously expressed in mouse testis. F vs. H) Mm-epg5 and Mm-ATG5 do not particularly colocalize in spermatogonia cells. Scale bars, 100 µm.
DISCUSSION
The epg5 gene was recently described in the worm C. elegans (27), mouse (43), and human (44) for its functionally conserved role in the autophagy/lysosomal degradation pathway. Although always resulting in an accumulation of nondegradative autophagic vacuoles, the above-mentioned organisms deficient in Epg5 nevertheless demonstrated diverse and selective defects and multisymptomatic pathologies summarized in Table 1. Hence, although certainly at the origin of a profound derailment of autophagy, the specific physiologic outcomes inherent in the loss of function of EPG5 from worm to human during diverse developmental processes or tissue homeostasis remain ambiguous. In a fish model deficient in EPG5, our study provides evidence that Ol-epg5, like its worm, murine, and human homologs, also regulates the final degradation steps of the autophagy pathway. We found a role for Ol-epg5 function during gonad formation and gametogenesis of vertebrates, being absolutely mandatory for correct early and late spermatogenesis after meiosis.
The expression pattern of Ol-epg5 in medaka correlates with the multisymptomatic pathology of the EPG5 mutation-induced Vici syndrome in humans, resulting in immunodeficiency, cataract, defects in CNS development and cardiomyopathic disorders as consequences of defective autophagosomal clearance [see (44), and Table 1]. Besides being maternally deposited and moderately expressed during embryonic development, Ol-epg5 is particularly highly expressed in spleen, eyes, brain, and heart. Furthermore, high expression of Ol-epg5 in gills and gonads suggests additional roles for the fish Ol-epg5 in these tissues. The gonadal expression of Ol-epg5 in medaka germ cells and type 1 spermatocytes correlates with the specific function of epg5 in C. elegans for germ plasm clearance during the process of germ cell specification (see (27) and Table 1). The presence of such a function is supported by the fact that, in both worm and medaka, deficiency in epg5 leads to an inability to clear germ plasm components. In the case of medaka, this failure further results in impaired spermatogenesis.
Worm embryos with a mutation in epg5 accumulate autolysosomes in which there is no degradation of the contents (27). The same phenomenon of autophagic granule accumulation was also noticed after EPG5 mutation in human and mice [(43, 44), see Table 1]. Knockdown of EPG5 in mammalians cells (27) or mutation in EPG5, causing the Vici syndrome (44), both result in defective autophagy because of accumulation of nondegradable lysosomes. In the testes of medaka, although expressed in the same cell types (germ cells and spermatogonia), but in contrast to mice (43), Ol-ATG-5 and Ol-EPG-5 do not strictly colocalize in the very same subcellular structures of the cells. ATG-5 is expressed during the early steps of autophagosome formation, whereas EPG-5 appears to have a more restricted role during the late steps of autolysosome degradation (27). Only immunohistochemistry with a marker (LC3) of late autolysosome degradation revealed a perfect colocalization of Ol-LC3- and Ol-EPG-5-positive puncta. Consistently, medaka fish deficient in Ol-epg5 (Ol-epg-5+/def and Ol-epg-5def/def) also display accumulation of autolysosomes as monitored by the simultaneous increase of truncated reporter Ol-epg5:mcherry fusion protein and LC3-positives granules compared with the wild-type situation. It is now clear that, similar to its worm’s counterpart, Ol-EPG-5 is likely late-acting within the same autophagy pathway (27). Hence, our results showing accumulation of autophagosomes in Ol-epg5-deficient fish are consistent with an evolutionarily conserved function of epg5 in regulating the final degradation step of the autophagy pathway at least between worms and medaka.
Results show that Ol-epg5-deficiency correlates with impaired spermatogenesis and low allele transmission rates of the mutant allele. The variable penetrance of the phenotype and allele transmission rates may be ascribable to a reduced Ol-epg5 expression rather than a complete knockout, together with heterozygosity at many loci due to a mixed genetic background. Such pleiotropisms have also been noticed for knockouts of epg5 in mice (43) and defective alleles in humans (44) (see also Table 1). Whereas diploid cells heterozygous for Ol-epg5 mutation apparently do not display any phenotype, only germ cells, haploid postmeiotic germ cells, and type 1 spermatocytes exhibit the observed Ol-epg5 mutant phenotype. In all cases, fish or human, the observations similarly suggest a derailment of autophagy on multiple regulatory levels, including transcriptional regulation (44). Our study provides evidence that Ol-epg5, as is true of its worm, murine, and human homologs, regulates the final degradation steps of the autophagy pathway.
We found that autophagy is an essential process during spermatogenesis of medaka. It is well known that the spermatogenic process goes along with a drastic reduction in organelles (17, 40, 45). We have shown that this process requires Ol-epg5 function and infer that an effective autophagic process is necessary for reduction of mitochondria. Indeed, a defective autophagy would lead to a failure in organelle reduction, particularly germ plasm and mitochondria. The phenotype of the Ol-epg5 medaka mutant indicates that this specifically occurs in germ cells and type 1 spermatocytes.
In the testes of the Ol-epg5 mutant medaka, abnormal mitochondrial distribution, as in mice and humans deficient in epg5, was observed, (43, 44), see Table 1). As a consequence, the deficiency of Ol-epg5 in testes of medaka correlated with a higher rate of paternal retention of mitochondria during spermatogenesis and thus could explain an apparent higher heteroplasmy in the progeny. Because a slight heteroplasmy background was observed in the crosses of wild-type fish compared with an apparent higher heteroplasmy in Ol-epg-5-deficient crosses, our results indicate that autophagy, although probably not solely responsible, is at least partially involved in the process of paternal mitochondrial reduction during spermatogenesis and after fertilization in medaka. Such an exclusive role of autophagy during that specific process has been seriously questioned in mice (46, 47).
Expression of epg5 in both fish and worms strictly colocalizes with LC3, a specific marker of the late phases of autophagy (48), implying signaling through the same pathway as well as similar roles in the final degradation step of the autophagy pathway. The expression patterns of epg-5 and atg-5, although redundant in the early stages of spermatogenesis in mice and medaka, become quite divergent later in the process, as spermatogenesis progresses. Although no gonad-specific phenotypes were studied in detail in Mm-Epg5 homozygous mice (Epg5−/−), the expression of Mm-epg5 gradually increases during mouse testis development and in meiotic germ cells of the adult testis. This conservation of expression patterns suggests evolutionarily conserved functions during spermatogenesis-associated organelle reduction. The absence of a gonad phenotype in mice may be a consequence of genetic redundancy or functional redundancy of parallel pathways of autophagy. Hence, the distinct expression patterns of Mm-epg5 being meiotic germ-cell–restricted in mouse testis and widely expressed Mm-ATG5 may not strictly link the germ line epg5 pathway with the mammalian somatic canonical autophagy pathway to which ATG5 belongs. Whereas the evolutionarily conserved expression patterns of epg5 homologs in male germ cells of worm, vertebrates, and mammals suggest common fundamental roles during spermatogenesis, the functions of EPG5 may differ slightly in mammals.
Whereas medaka Ol-epg5 and worm epg5 (27) are probably involved in the autophagic process of germ plasm resorption and degradation during the course of spermatogenesis, such germ line P-granules or germ plasm organelles are absent in mammals (41). Hence, instead of targeting germ plasm organelles, the mammalian EPG5-driven autophagic process may directly act on germ-cell–specific mRNAs or proteins independently recruited via other mechanisms not involving the formation of germ plasm. Moreover, it was recently shown in mice that despite the fact that some autophagy-related proteins, such as SQSTM1 and LC3 localize close to sperm mitochondria before the 2-cell stage, autophagy does not participate in the elimination of sperm mitochondria and mtDNA (46, 47). On the other hand, such a clearance process may not be as essential in mammals as in fish and worms, because neither mice nor humans deficient in epg5 seem to display any apparent spermatogenic defects (43, 44).
Acknowledgments
The authors thank the National Institute for Basic Biology, Japan (NRBP Medaka) for providing genomic resources and BAC clones; the fish facilities at the University of Würzburg and at the INRA Rennes (F. Borel, C. Duret); and S. Bertho for technical assistance in performing the immunohistochemistry. This work was supported in part by the Deutsche Forschungsgemeinschaft (DFG) through Grants HE 7135/2-1 (to A.H.), SCHA 408/12-1 (to M.S.), and SFB 688 (TP A16) (to M.G.); the DFG Graduate Training Program Grant GK 1048, “Molecular basis of organ development in vertebrates”; and U.S. National Institutes of Health Grant 1R01GM085318-01A2, subaward No. 212791A, “Mechanisms of sex determination in zebrafish.” A.H. and M.S designed the experiments and wrote the manuscript. A.H., E.E., M.Z., and R.W. performed the experiments. A.H., M.G., and M.S. discussed/commented on the results and edited the manuscript. All authors approved the manuscript. The authors declare no conflicts of interest.
Glossary
- ATG-5
autophagy protein 5
- BAC
bacterial artificial chromosome
- LC@
lamin C2
- MABT
maleic acid buffer containing Tween 20
- MABDT
MABT buffer with DMSO
- Ol-EPG-5
Oryzias latipes ectopic PGL granules-5
- PGC
primordial germ cell
- P-granules
perinuclear granules
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Mizushima N. (2007) Autophagy: process and function. Genes Dev. 21, 2861–2873 [DOI] [PubMed] [Google Scholar]
- 2.Yang Z., Klionsky D. J. (2009) An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 335, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Z., Klionsky D. J. (2007) Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 9, 1102–1109 [DOI] [PubMed] [Google Scholar]
- 4.Ding W. X., Yin X. M. (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393, 547–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yordy, B., Iwasaki, A. (2011) Autophagy in the control and pathogenesis of viral infection. Curr. Opin. Virol. 1, 196–203 [DOI] [PMC free article] [PubMed]
- 6.Johnson, C. W., Melia, T. J., Yamamoto, A. (2012) Modulating macroautophagy: a neuronal perspective. Future Med. Chem. 4, 1715–1731 [DOI] [PMC free article] [PubMed]
- 7.Gottlieb R. A., Jr M. R. (2013) Autophagy: an affair of the heart. Heart Fail. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carew, J. S., Kelly, K. R., Nawrocki, S. T. (2012) Autophagy as a target for cancer therapy: new developments. Cancer Manag. Res. 4, 357–365 [DOI] [PMC free article] [PubMed]
- 9.Di Bartolomeo S., Nazio F., Cecconi F. (2010) The role of autophagy during development in higher eukaryotes. Traffic 11, 1280–1289 [DOI] [PubMed] [Google Scholar]
- 10.Barth J. M., Szabad J., Hafen E., Köhler K. (2011) Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 18, 915–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nezis I. P., Shravage B. V., Sagona A. P., Lamark T., Bjørkøy G., Johansen T., Rusten T. E., Brech A., Baehrecke E. H., Stenmark H. (2010) Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J. Cell Biol. 190, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomé R. G., Santos H. B., Arantes F. P., Domingos F. F., Bazzoli N., Rizzo E. (2009) Dual roles for autophagy during follicular atresia in fish ovary. Autophagy 5, 117–119 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Yan L., Zhou Z., Yang P., Tian E., Zhang K., Zhao Y., Li Z., Song B., Han J., Miao L., Zhang H. (2009) SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell 136, 308–321 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Tian E., Zhang H. (2009) Selective autophagic degradation of maternally-loaded germline P granule components in somatic cells during C. elegans embryogenesis. Autophagy 5, 717–719 [DOI] [PubMed] [Google Scholar]
- 15.Al Rawi S., Louvet-Vallée S., Djeddi A., Sachse M., Culetto E., Hajjar C., Boyd L., Legouis R., Galy V. (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334, 1144–1147 [DOI] [PubMed] [Google Scholar]
- 16.Politi Y., Gal L., Kalifa Y., Ravid L., Elazar Z., Arama E. (2014) Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila. Dev. Cell 29, 305–320 [DOI] [PubMed] [Google Scholar]
- 17.Sato M., Sato K. (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 334, 1141–1144 [DOI] [PubMed] [Google Scholar]
- 18.Strome S., Lehmann R. (2007) Germ versus soma decisions: lessons from flies and worms. Science 316, 392–393 [DOI] [PubMed] [Google Scholar]
- 19.Voronina E. (2013) The diverse functions of germline P-granules in Caenorhabditis elegans. Mol. Reprod. Dev. 80, 624–631 [DOI] [PubMed] [Google Scholar]
- 20.Johnson A. D., Richardson E., Bachvarova R. F., Crother B. I. (2011) Evolution of the germ line-soma relationship in vertebrate embryos. Reproduction 141, 291–300 [DOI] [PubMed] [Google Scholar]
- 21.Herpin A., Rohr S., Riedel D., Kluever N., Raz E., Schartl M. (2007) Specification of primordial germ cells in medaka (Oryzias latipes). BMC Dev. Biol. 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M., Kinoshita M., Kobayashi D., Nagahama Y. (2001) Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: a useful model to monitor germ cells in a live vertebrate. Proc. Natl. Acad. Sci. USA 98, 2544–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf N., Priess J., Hirsh D. (1983) Segregation of germline granules in early embryos of Caenorhabditis elegans: an electron microscopic analysis. J. Embryol. Exp. Morphol. 73, 297–306 [PubMed] [Google Scholar]
- 24.Hay B., Jan L. Y., Jan Y. N. (1988) A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell 55, 577–587 [DOI] [PubMed] [Google Scholar]
- 25.Lasko P. F., Ashburner M. (1988) The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335, 611–617 [DOI] [PubMed] [Google Scholar]
- 26.Berekelia L. A., Ponomarev M. B., Mikriukov A. A., Luchinskaia N. N., Beliavskiĭ A. V. (2005) [Molecular mechanisms of germ cell line determination in animals]. Mol. Biol. (Mosk.) 39, 664–677 [PubMed] [Google Scholar]
- 27.Tian Y., Li Z., Hu W., Ren H., Tian E., Zhao Y., Lu Q., Huang X., Yang P., Li X., Wang X., Kovács A. L., Yu L., Zhang H. (2010) C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell 141, 1042–1055 [DOI] [PubMed] [Google Scholar]
- 28.Herpin A., Adolfi M. C., Nicol B., Hinzmann M., Schmidt C., Klughammer J., Engel M., Tanaka M., Guiguen Y., Schartl M. (2013) Divergent expression regulation of gonad development genes in medaka shows incomplete conservation of the downstream regulatory network of vertebrate sex determination. Mol. Biol. Evol. 30, 2328–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leimeister C., Bach A., Gessler M. (1998) Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev. 75, 29–42 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura S., Aoki Y., Saito D., Kuroki Y., Fujiyama A., Naruse K., Tanaka M. (2008) Sox9b/sox9a2-EGFP transgenic medaka reveals the morphological reorganization of the gonads and a common precursor of both the female and male supporting cells. Mol. Reprod. Dev. 75, 472–476 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura S., Saito D., Tanaka M. (2008) Generation of transgenic medaka using modified bacterial artificial chromosome. Dev. Growth Differ. 50, 415–419 [DOI] [PubMed] [Google Scholar]
- 32.Herpin A., Nakamura S., Wagner T. U., Tanaka M., Schartl M. (2009) A highly conserved cis-regulatory motif directs differential gonadal synexpression of Dmrt1 transcripts during gonad development. Nucleic Acids Res. 37, 1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herpin A., Braasch I., Kraeussling M., Schmidt C., Thoma E. C., Nakamura S., Tanaka M., Schartl M. (2010) Transcriptional rewiring of the sex determining dmrt1 gene duplicate by transposable elements. PLoS Genet. 6, e1000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herpin A., Fischer P., Liedtke D., Kluever N., Neuner C., Raz E., Schartl M. (2008) Sequential SDF1a and b-induced mobility guides Medaka PGC migration. Dev. Biol. 320, 319–327 [DOI] [PubMed] [Google Scholar]
- 35.Klüver N., Herpin A., Braasch I., Driessle J., Schartl M. (2009) Regulatory back-up circuit of medaka Wt1 co-orthologs ensures PGC maintenance. Dev. Biol. 325, 179–188 [DOI] [PubMed] [Google Scholar]
- 36.Klionsky, D. J., Abdalla, F. C., Abeliovich, H., Abraham, R. T., Acevedo-Arozena, A., Adeli, K., Agholme, L., Agnello, M., Agostinis, P., Aguirre-Ghiso, J. A., et al. Denton, D., Deretic, V., Desai, S. D., Devenish, R. J., Di Gioacchino, Zuckerbraun, B. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8, 445–544 [DOI] [PMC free article] [PubMed]
- 37.Herpin A., Schindler D., Kraiss A., Hornung U., Winkler C., Schartl M. (2007) Inhibition of primordial germ cell proliferation by the medaka male determining gene Dmrt I bY. BMC Dev. Biol. 7, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hornung U., Herpin A., Schartl M. (2007) Expression of the male determining gene dmrt1bY and its autosomal coorthologue dmrt1a in medaka. Sex. Dev. 1, 197–206 [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y., King M. L. (2004) Sending RNAs into the future: RNA localization and germ cell fate. IUBMB Life 56, 19–27 [DOI] [PubMed] [Google Scholar]
- 40.Nishimura Y., Yoshinari T., Naruse K., Yamada T., Sumi K., Mitani H., Higashiyama T., Kuroiwa T. (2006) Active digestion of sperm mitochondrial DNA in single living sperm revealed by optical tweezers. Proc. Natl. Acad. Sci. USA 103, 1382–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Extavour C. G., Akam M. (2003) Mechanisms of germ cell specification across the metazoans: epigenesis and preformation. Development 130, 5869–5884 [DOI] [PubMed] [Google Scholar]
- 42.Furukawa K., Inagaki H., Hotta Y. (1994) Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp. Cell Res. 212, 426–430 [DOI] [PubMed] [Google Scholar]
- 43.Zhao H., Zhao Y. G., Wang X., Xu L., Miao L., Feng D., Chen Q., Kovács A. L., Fan D., Zhang H. (2013) Mice deficient in Epg5 exhibit selective neuronal vulnerability to degeneration. J. Cell Biol. 200, 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullup T., Kho A. L., Dionisi-Vici C., Brandmeier B., Smith F., Urry Z., Simpson M. A., Yau S., Bertini E., McClelland V., Al-Owain M., Koelker S., Koerner C., Hoffmann G. F., Wijburg F. A., ten Hoedt A. E., Rogers R. C., Manchester D., Miyata R., Hayashi M., Said E., Soler D., Kroisel P. M., Windpassinger C., Filloux F. M., Al-Kaabi S., Hertecant J., Del Campo M., Buk S., Bodi I., Goebel H. H., Sewry C. A., Abbs S., Mohammed S., Josifova D., Gautel M., Jungbluth H. (2013) Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat. Genet. 45, 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q., Li H., Xue D. (2011) Elimination of paternal mitochondria through the lysosomal degradation pathway in C. elegans. Cell Res. 21, 1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo S. M., Ge Z. J., Wang Z. W., Jiang Z. Z., Wang Z. B., Ouyang Y. C., Hou Y., Schatten H., Sun Q. Y. (2013) Unique insights into maternal mitochondrial inheritance in mice. Proc. Natl. Acad. Sci. USA 110, 13038–13043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo S. M., Sun Q. Y. (2013) Autophagy is not involved in the degradation of sperm mitochondria after fertilization in mice. Autophagy 9, 2156–2157 [DOI] [PubMed] [Google Scholar]
- 48.Yang Z., Klionsky D. J. (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]