Abstract
Background
Intracerebral hemorrhage (ICH) is a well-known condition, but ICH restricted to the thalamus is less widely studied. We investigated the prognostic factors of thalamic ICHs.
Material/Methods
Seventy patients from January 2009 to November 2014 were retrospectively reviewed. Patients who demonstrated spontaneous ICH primarily affecting the thalamus on initial brain computed tomography (CT) were enrolled. Patients were categorized into 2 groups based on their Glasgow Outcome Scale (GOS) scores. Various presumptive prognostic factors were analyzed to investigate relationships between various clinical characteristics and outcomes.
Results
Of the enrolled patients, 39 showed a GOS of 4–5, and were categorized as the good outcome group, while another 31 patients showed a GOS of 1–3 and were categorized as the poor outcome group. Initial GCS score, calculated volume of hematoma, presence of intraventricular hemorrhage (IVH), coexisting complications, hydrocephalus, performance of external ventricular drainage, and modified Graeb’s scores of patients with IVH were significantly different between the 2 groups. In multivariate analysis, among the factors above, initial GCS score (P=0.002, Odds ratio [OR]=1.761, Confidence interval [CI]=1.223–2.536) and the existence of systemic complications (P=0.015, OR=0.059, CI=0.006–0.573) were independently associated with clinical outcomes. Calculated hematoma volume showed a borderline relationship with outcomes (P=0.079, OR=0.920, CI=0.839–1.010).
Conclusions
Initial GCS score and the existence of systemic complications were strong predictive factors for prognosis of thalamic ICH. Calculated hematoma volume also had predictive value for clinical outcomes.
MeSH Keywords: Glasgow Outcome Scale; Intracranial Hemorrhage, Hypertensive; Prognosis; Thalamus
Background
Spontaneous intracerebral hemorrhage (ICH) is a major public health problem with an annual incidence of 10–30 cases per 100 000 population, accounting for 2 million (10–15%) of approximately 15 million strokes, which occur worldwide each year [1–4]. It is the most devastating type of stroke, with the greatest rates of morbidity and mortality [5,6]. Most spontaneous ICHs (78–88% of cases) originate from the spontaneous rupture of small vessels damaged by chronic hypertension or amyloid angiopathy, and is termed as spontaneous ICH [2]. Secondary ICHs represent ICH that occurs in a minority of patients in association with vascular abnormalities [2]. This spontaneous ICH can affect the brain parenchyma anywhere, but more commonly affects specific locations, including the basal ganglia, thalamus, pons, and cerebellum [2,5,7]. Recently, ICHs were categorized as lobar hemorrhages and non-lobar hemorrhages based on their location. This is because their locations were found to affect the prognosis of the disease. Supratentorial non-lobar hemorrhages include ICHs located in the thalamus, basal ganglia, and internal or external capsules [8]. Among them, thalamic ICHs represent 8.3–15% of all ICHs, secondary to ICHs originating from the basal ganglia [5,9,10]. Spontaneous intracerebral hemorrhage is a relatively well-studied disease, but studies restricted to ICHs affecting mainly the thalamus are scarce. We retrospectively studied 70 cases of thalamic ICH to identify factors affecting prognosis in these patients.
Material and Methods
Patient population and inclusion criteria
We conducted a retrospective review of 653 patients who presented with ICH and who were admitted to our center from January 2009 to November 2014. The inclusion criteria were as follows: (1) spontaneous ICHs affecting primarily the thalamus; and (2) spontaneous ICHs with no antecedent disease such as arteriovenous malformations, Moyamoya disease, cerebral aneurysms, or traumatic events. Finally, 70 of the 653 patients were enrolled in the study.
Patient management
On and after making a diagnosis of spontaneous thalamic ICH, all patients were managed in a neurosurgical intensive care unit (ICU). Blood pressure was controlled to maintain systolic blood pressure below 160 mmHg, and osmotic diuretics were administrated to control increased intracranial pressure (ICP). When the patient’s condition met the indication for external ventricular drainage (EVD), EVD was performed after gaining informed consent. The indications for EVD in our institution are as follows: (1) acute hydrocephalus, measured Evans ratio >0.30 on brain CT scans (2) Evans ratio <0.30, but the patients presented neurological deterioration signs resulting from hydrocephalus or intraventricular hemorrhage (IVH). EVD side was chosen based on the patient’s condition and hemorrhagic factors observed in their initial computed tomography (CT) scan. In the acute phase of ICH, we prefer EVD to lumbar drainage to treat ventriculomegaly regardless of hydrocephalus type. There are some reasons as follows; (1) to perform continuous ICP monitoring (2) possibility of usage of urokinase through EVD catheter. Lumbar drainage is only performed as the diagnostic test of chronic hydrocephalus (communicating) for shunt operation. Thus, in this study, there was no patient with thalamic ICH who underwent a therapeutic lumbar drainage. When the patient’s condition met the indication for surgery (neuronavigation-assisted hematoma drainage or open craniotomy with hematoma removal), surgical interventions were performed after gaining informed consent. The indications for surgical intervention in our institution are as follows: (1) calculated hematoma volume >30 mL on brain CT scans, (2) 4≤ Glasgow Coma Scale (GCS) scores ≤9. Pneumatic compression stockings were applied for patients in the acute phase who required equipment for the prevention of deep vein thrombosis (DVT). Patients beyond the acute phase who did not need ICU care were transferred to a general ward, and underwent an individualized rehabilitation program.
Analysis of prognostic factors affecting clinical outcomes
Glasgow Outcome Scale (GOS) scores evaluated 1 month after admission were used to determine clinical outcomes. Patients were divided into 2 groups according to their GOS scores: the good outcome group (GOS scores of 4–5), and the poor outcome group (GOS scores of 1–3). The presumptive prognostic factors of the patients with thalamic ICH were collected and analyzed to examine their relationships with clinical outcomes. In our study, presumptive factors were divided into patient factors and hemorrhagic factors.
Patient factors included: age; sex; initial GCS scores; platelet counts; coagulopathy (prothrombin time (PT, International Normalized Ratio) >1.2, activated partial thromboplastin time (aPTT) >45 sec, or platelet count <150000 103/μL; underlying diseases, such as hypertension (previously known, patient being treated with antihypertensive medications, or blood pressure >140/90 mmHg during the non-acute phase) and diabetes mellitus (DM) (fasting glucose >7 mmol/L or patient being treated with antidiabetic medication); use of anticoagulants or antiplatelet agents; blood pressure >140/90 mmHg at visit; blood pressure >140/90 mmHg at third day of admission; and accompanying complications after admission, such as pneumonia, urinary tract infection, central nervous system infection, phlebitis, Clostridium difficile infection, or sepsis. Hemorrhagic factors included: side; co-affecting location such as basal ganglia; brain stem; existence of IVH and its volume calculated with the modified Graeb’s score system [IVH based in fourth ventricle (maximum score 4), third (maximum score 4), right and left lateral ventricles (maximum score 4 for each), right and left occipital horns (maximum score 2 for each), and the right and left temporal horns (maximum score 2 for each). An additional score of +1 is given to each compartment if it is expanded beyond normal anatomic limits attributable to clot] [11]; calculated hematoma volume with by ABC/2 formula [12]; evidence of hydrocephalus (Evans ratio >0.30); EVD, and surgical intervention for hematoma removal. Information of hemorrhagic factors was collected from each patient’s initial brain CT scan.
Statistical analysis
Presumptive prognostic factors were compared between the good and poor outcome groups with the Mann-Whitney U-test for continuous variables and Pearson’s chi-square test for categorical variables. The Mann-Whitney U-test was used to analyze modified Graeb’s score data of patients with IVH (n=50). Independent associations between prognostic factors and clinical outcomes were analyzed using binary logistic regression, and odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. The data were presented as mean ± standard deviation (SD). Analyses were performed with SPSS ver. 19.0 (IBM Corporation, Armonk, NY, USA). P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
Overall, 70 patients were enrolled in the study. The mean age was 64.36±12.31 years, and there were 32 (45%) males and 38 (55%) females. Among the 70 patients, 39 (56%) had good GOS scores (4–5) evaluated at 1 month after admission, while the other 31 (44%) patients had poor GOS scores (1–3) evaluated at 1 month after admission. The overall mortality rate was 11%. In total, eight patients (11%) died, and 6 of these died due to intractable increasing ICP. Another 2 patients died due to other causes, including sudden onset of ventricular tachycardia and respiratory arrest. EVD was performed to 20 patients, and among these patients, only 1 patient showed good outcome. A total of 5 patients underwent surgical intervention for hematoma removal. Two patients underwent open craniotomy, while 3 underwent neuronavigation-assisted hematoma removal. All 5 patients showed poor outcome. Table 1 demonstrates patient factors, while Table 2 demonstrates hemorrhagic factors. We drew a comparison between the presumptive factors of the good and poor outcome groups.
Table 1.
Patient factors of 70 patients with thalamic ICHs.
| Clinical characteristics | All patients (n=70) | Good outcome (n=39) | Poor outcome (n=31) | P-value |
|---|---|---|---|---|
| Age (years) | 64.86±12.31 | 62.54±11.91 | 67.77±12.37 | 0.094* |
| Sex (n) | 0.294** | |||
| Male | 32 | 20 | 12 | |
| Female | 38 | 19 | 19 | |
| Initial GCS score (median) | 11.11±3.89 | 13.79±1.67 | 7.74±3.21 | 0.000* |
| Hypertension (n) | 0.368** | |||
| (+) | 41 | 21 | 20 | |
| (−) | 29 | 18 | 11 | |
| Diabetes mellitus (n) | 0.051** | |||
| (+) | 17 | 6 | 11 | |
| (−) | 53 | 33 | 20 | |
| Usage of anticoagulant (n) | 0.091** | |||
| (+) | 22 | 9 | 13 | |
| (−) | 48 | 30 | 18 | |
| Platelet count (×103/mL) | 224.21±65.20 | 220.89±69.51 | 228.38±60.21 | 0.260* |
| prolonged PT/aPTT (n) | 0.140** | |||
| (+) | 12 | 9 | 3 | |
| (−) | 58 | 30 | 28 | |
| Coexisting systemic complication (n) | 0.000** | |||
| (+) | 30 | 7 | 23 | |
| (−) | 40 | 32 | 8 | |
| Initial BP (n) | 0.409** | |||
| Controlled | 18 | 9 | 9 | |
| Uncontrolled | 52 | 30 | 22 | |
| 3rd day’s BP (n) | 0.058** | |||
| Controlled | 35 | 24 | 11 | |
| Uncontrolled | 35 | 15 | 20 |
GCS – Glasgow coma scale; PT – prothrombin time; aPTT – activated partial thromboplastin time; BP – blood pressure;
Mann-Whitney U-test;
Pearson’s chi-square test.
Table 2.
Hemorrhagic factors of 70 patients with thalamic ICHs.
| Clinical characteristics | All patients (n=70) | Good outcome (n=39) | Poor outcome (n=31) | P-value |
|---|---|---|---|---|
| Side of ICH (n) | 0.470** | |||
| Right | 35 | 21 | 14 | |
| Left | 35 | 18 | 17 | |
| Volume of ICH (mL) | 17.07±15.19 | 9.56±6.24 | 26.52±17.78 | 0.000* |
| Coaffecting area (n) | 0.065** | |||
| (+) | 51 | 25 | 26 | |
| (−) | 19 | 14 | 5 | |
| IVH (n) | 0.002** | |||
| (+) | 50 | 22 | 28 | |
| (−) | 20 | 17 | 3 | |
| Modified Graeb’s score (median) | 11.62±7.67 | 7.41±5.07 | 14.93±7.81 | 0.001* |
| Hydrocephalus (n) | 0.000** | |||
| (+) | 18 | 2 | 16 | |
| (−) | 52 | 37 | 15 | |
| EVD (n) | 0.000** | |||
| (+) | 20 | 1 | 19 | |
| (−) | 50 | 38 | 12 | |
| Surgical intervention for hematoma removal (n) | 0.009** | |||
| (−) | 5 | 0 | 5 | |
| (−) | 65 | 39 | 26 |
ICH – intracerebral hemorrhage; IVH – intraventricular hemorrhage; EVD – external ventricular drainage;
Mann-Whitney U-test;
Pearson’s chi-square test.
Patient factors
Age and gender distributions were not statistically associated with clinical outcomes (P=0.094, 0.294 for each factor, Mann-Whitney U-test for age distribution and Pearson’s chi-square test for gender distribution). The initial GCS score, which was 13.79±1.67 in the good outcome group, was significantly higher than the 7.74±3.21 of the poor outcome group (P<0.001). Among the 70 patients, 22 had been taking anticoagulants or anti-platelet agents, including aspirin, clopidogrel, and warfarin. However, their distribution between the good outcome group and poor outcome group was not statistically significant (P=0.091, Pearson’s chi-square test). Laboratory data of patients who presented with abnormal bleeding profiles, including low platelet counts and prolonged PT/aPTT, were also not statistically significant (P=0.260 for platelet count, Mann-Whitney U-test, P=0.140 for pronged PT/aPTT, Pearson’s chi-square test). Forty-one (60%) patients had hypertension, but their distribution among the good and poor outcome groups was not statistically significant. However, 17 (24%) patients had DM, of which 15% were patients with good outcomes and 35% were patients with poor outcomes, and their distribution pattern showed a borderline tendency (P=0.051, Pearson’s chi-square test). The number of patients whose initial blood pressure >140/90 mmHg showed no difference between the 2 groups (P=0.409, Pearson’s chi-square test), but the number of patients whose blood pressure >140/90 mmHg on the 3rd day of admission showed a borderline tendency (P=0.058, Pearson’s chi-square test). Furthermore, complication (including pneumonia, urinary tract infections, central nervous system infections, phlebitis, C. difficile infection, and sepsis) rates during admission of the poor outcome group were significantly higher (P<0.001, Pearson’s chi-square test).
Hemorrhagic factors
The distribution of side of thalamic ICHs was not different between the 2 groups (P=0.470, Pearson’s chi-square test); however, the volume of ICH calculated in each group was significantly different (17.07±15.19 in the good outcome group and 26.52±17.78 in the poor outcome group [P<0.001, Mann-Whitney U-test]). The rate of co-affecting areas, including midbrain, basal ganglia, corona radiata, etc., was not significantly different between the 2 groups, but showed a borderline tendency (P=0.065, Pearson’s chi-square test). The rate of coexisting IVH was also significantly higher in the poor outcome group (90%) than in the good outcome group (64%) (P=0.002, Pearson’s chi-square test). In total, 50 patients presented with coexisting IVH. The volume of IVH described by modified Graeb’s scores was also significantly higher in the poor outcome group (14.93±7.81) than in the good outcome group (7.41±5.07) (n=50, P=0.001, Mann-Whitney U-test). The rate of hydrocephalus and EVD was also significantly higher in the poor outcome group (P<0.001 for both parameters, Pearson’s chi-square test). Surgical interventions such as hematoma drainage and open craniotomy were also significantly higher in the poor outcome group (P=0.009, Pearson’s chi-square test).
Evaluation of prognostic factors
Tables 3 and 4 demonstrate the results of logistic regression analysis of associations between characteristics and clinical outcomes. Among the presumptive factors, absence of IVH (OR 7.212, 95% CI 1.873–27.778), decreased modified Graeb’s scores of patients with IVH (OR 0.839, 95% CI 0.772–0.913), decreased volume of hematoma (OR 0.853, 95% CI 0.783–0.928), absence of hydrocephalus (OR 19.733, 95% CI 4.033–96.543), patient conditions did not meet the indication for EVD (OR 60.167, 95% CI 7.212–497.770), decreased initial GCS score (OR 2.209, 95% CI 1.528–3.193), absence of complications (OR 13.143, 95% CI 4.173–41.391) were statistically correlated with good outcomes of patients with thalamic ICH on univariate analysis. Among these factors, only initial GCS score and the presence of coexisting complications demonstrated an independent association with clinical outcomes (initial GCS score OR 1.761, 95% CI 1.223–2.536, P=0.002, coexisting complication OR 0.059, 95% CI 0.006–0.573, P=0.015). Volume of hematoma showed a borderline relationship (OR 0.920, 95% CI 0.839–1.010, P=0.079). Other factors, such as sex, age, platelet count, PT/aPTT, presence of IVH or hydrocephalus, EVD, surgical interventions, modified Graeb’s score of patients with IVH showed no significant associations with clinical outcomes.
Table 3.
Result of univariate analysis.
| Clinical characteristics | Univariate study | ||
|---|---|---|---|
| OR | 95% CI for OR | P value | |
| IVH | 7.212 | 1.873–27.778 | 0.004 |
| Modified Graeb’s score | 0.839 | 0.772–0.913 | <0.001 |
| Volumes of ICH | 0.853 | 0.783–0.928 | <0.001 |
| Hydrocephalus | 19.733 | 4.033–96.543 | <0.001 |
| EVD | 60.167 | 7.212–497.770 | <0.001 |
| Initial GCS score | 2.209 | 1.528–3.193 | <0.001 |
| Coexisting systemic complication | 13.143 | 4.173–41.391 | <0.001 |
IVH – intraventricular hemorrhage; ICH – intracerebral hemorrhage; EVD – external ventricular drainage; GCS – Glasgow Coma Scale; OR – odds ratio; CI – confidence interval.
Table 4.
Result of multivariate analysis.
| Clinical characteristics | Univariate study | ||
|---|---|---|---|
| OR | 95% CI for OR | P value | |
| Volumes of ICH | 0.920 | 0.839–1.010 | 0.079 |
| Initial GCS score | 1.761 | 1.223–2.536 | 0.002 |
| Coexisting systemic complication | 0.059 | 0.006–0.573 | 0.015 |
ICH – intracerebral hemorrhage; GCS – Glasgow Coma Scale; OR – odds ratio; CI – confidence interval.
Case illustrations
Case 1
A 50-year-old man with hypertension visited our hospital due to sudden right-side hemiparesis. His initial GCS score was 15. Brain CT scans demonstrated a left thalamic hemorrhage, which measured about 5 mL (Figure 1A) and CT angiography showed no vascular anomaly (Figure 1B). The patient underwent conservative therapy. He showed alert mentality and mild hemiparesis 1 month later with additional rehabilitation (GOS 4).
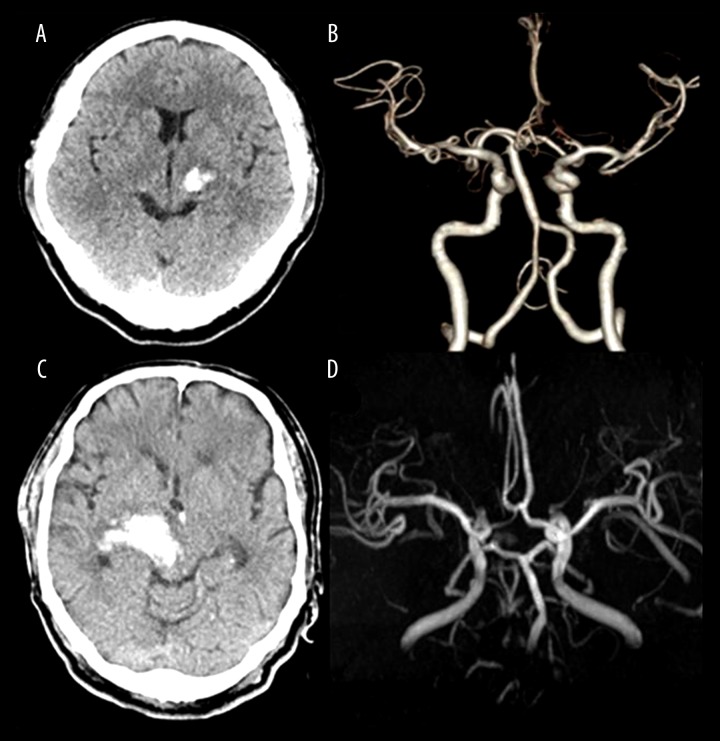
Figure 1.
(A) Initial brain computed tomography (CT) scan of case illustration 1. A 50-year-old man visited our hospital due to sudden right-side hemiparesis. 2.1×1.9×2.2 cm sized acute spontaneous ICH located on thalamus was demonstrated by brain CT scan. (B) CT angiography demonstrated no vascular abnormality. (C) Initial brain CT scan of case illustration 2. A 66-year-old man visited our hospital due to altered mentality (GCS 8) and severe right-side hemiparesis. 4.2×6.6×3.5 cm hematoma mainly affecting the thalamus was demonstrated. The hematoma extended to midbrain. (D) Angiography demonstrated no abnormality signifying a hemorrhagic event.
Case 2
A 66-year-old man with a history of hypertension, DM, chronic kidney disease (CKD) stage V, and prior history of a cerebrovascular accident (CVA, cerebral infarction at left pons) visited our institution due to sudden onset of altered mentality. He was treated for prior CVA with clopidogrel. His initial GCS score was 8. He also presented with severe hemiparesis on his left side. Initial brain CT scans demonstrated a right thalamic ICH extended to the midbrain (Figure 1C) but angiography demonstrated no vascular abnormality corresponding to a hemorrhagic event (Figure 1D). The calculated volume of hematoma was about 26 mL. His blood pressure was consistently high from admission to the third day. During admission, he had no other systemic complications. However, after 1 month, he presented a minimally conscious state, and his GOS was 2.
Discussion
We investigated the presumptive prognostic factors that might correlate to clinical outcomes of patients with thalamic ICH. In multivariate analysis of a variety of presumptive factors, only initial GCS score and the presence of systemic complications were independently correlated with clinical outcomes. Calculated initial hematoma volume showed a borderline relationship with outcomes.
Among patient factors, age and sex were important non-modifiable parameters. A number of studies have proven that age has predictive value in the clinical outcomes of patients with ICHs [5,8,13]. However, in our study, neither age nor sex was associated with clinical outcomes. We excluded ICHs located everywhere but the thalamus, so the number of enrolled patients was relatively small (n=70). Further investigation in a larger numbers of patients is probably warranted to determine any significant relationships.
We also investigated modifiable factors dependent on patients, such as hypertension, DM, and use of antiplatelet agents or anticoagulants. A history of hypertension has been consistently reported as a major risk factor of spontaneous ICHs [10], but its association with clinical prognosis has not been clearly determined [14]. In this study, history of hypertension was not associated with clinical outcomes of patients with thalamic ICHs. However, DM was not independently associated with clinical outcomes, but showed borderline tendency in univariate analysis (OR 0.331, 95% CI 0.106–1.033, P=0.057). Larger investigations are warranted to determine any associations. Drug histories, including anticoagulants and antiplatelet agents and the presence of coagulopathy, were not associated with clinical outcomes in our study.
Initial blood pressure is not considered as a risk factor or prognostic factor of ICHs [13], but there are some reports showing intensive reduction of blood pressure associated with clinical outcomes of ICHs [15–17]. In our study, initial blood pressure was not associated with clinical outcomes. In contrast, blood pressure on the 3rd day was not independently associated with clinical outcomes, but showed a borderline tendency in univariate analysis (OR 0.395, 95% CI 0.150–1.040, P=0.060). We used blood pressure estimated on the 3rd day of admission for statistical analysis, but these findings generally corresponded with those from previous studies of relationships between prognosis of ICHs and reduction of blood pressure.
Coexisting systemic complications such as pneumonia, urinary tract infections, central nervous system (CNS) infections, phlebitis, C. difficile infection, and sepsis were significantly associated with clinical outcomes of patients with thalamic ICHs. Previous studies have noted systemic complications as important predictive factors for clinical outcomes of patients with ICHs [18–20]. These complications include variable conditions such as pneumonia, urinary tract infections, thrombotic events due to immobilization, CNS infections, gastrointestinal bleeding, and renal complications. Interestingly, in our study, complications related to immobilization such as DVT or thrombotic events were not observed. In our institution, most patients in the intensive care unit undergo DVT prevention, including compression stockings or pneumatic compression stockings, and this was probably related to the lower rate of thrombotic events in our study. However, in our study, other systemic complications except thrombotic events were observed, and these complications were strongly related to prognosis of patients with thalamic ICHs (OR 0.059, 95% CI 0.006–0.573, P=0.015). This finding was constant with previous reports of relationships between prognosis of ICHs and systemic complications, except thrombotic events. Without doubt, prevention of thrombotic events is an important therapeutic strategy. In our series, 4 cases of ventriculitis were noted. All patients with ventriculitis showed poor outcomes. Recurrent episodes of EVD were considered as a common source of infection [21,22]. Four cases of ventriculitis in our study were associated with EVD. Ventriculitis without EVD was not noted in our series.
Initial GCS score was a predictive factor that demonstrated an independent association with clinical outcomes (OR 1.761, 95% CI 1.223–2.536, P=0.002) in our study. According to some previous studies about relationships between initial GCS scores and clinical outcomes, initial GCS score was strongly correlated with clinical outcomes in patients with ICHs [23,24]. Our result was consistent with these previous reports.
Hemorrhagic factors influencing the clinical outcome of patients with thalamic ICHs were side of ICH, calculated volume of hematoma, presence of co-affecting areas, presence of IVH, development of hydrocephalus, modified Graeb’s score for quantification of IVH, and performing of the EVD or hematoma removal. As in the previous study by Shah et al., side of hematoma did not relate to clinical outcomes of patients with ICHs [25]. In our study, there was no significant relationship between side of thalamic ICHs and clinical outcomes. This finding was similar to previous studies. Shah et al. also reported that clinical outcomes correlated with volume of hematoma and level of consciousness for putaminal and lobar but not for thalamic ICH [25], but in our study, there was a borderline tendency between volume of hematoma and clinical outcome in multivariate analysis (OR 0.920, 95% CI 0.839–1.010, P=0.079). As a result of univariate analysis, there was a significant relationship between the volume of hematoma and clinical outcome (OR 0.853, 95% CI 0.783–0.928, P<0.001). These findings were consistent with those of some previous studies about ICH not restricted to thalamic ICHs [8,23]. A larger study investigating thalamic ICHs is warranted.
Presence of IVHs was considered as a strong predictive factor of prognosis of ICHs, as in previous studies, and controlling the increased ICP with EVD was an important therapeutic strategy [13,26,27]. Clinical practice guidelines for ICHs in Korea also recommend that EVD for hydrocephalus can be ventricular or via the lumbar route if it is a communicating type of hydrocephalus [28]. In univariate analysis, all factors related to IVHs (presence of IVH, presence of hydrocephalus, modified Graeb’s score of patients with IVH, EVD) were associated with clinical outcomes, but these factors were not independently correlated with clinical outcomes in multivariate analysis. Surgery such as hematoma drainage and open craniotomy was not independently associated with clinical outcomes.
There were some limitations to our study. The number of patients who were enrolled in this study was relatively small. The borderline association between volume of hematoma and clinical outcome might have resulted from these limitations. Co-affecting areas and systemic complications were not subdivided due to small patient number. Finally, the 1-month follow-up period in our study was relatively short. Thus, prognostic factors of long-term outcome in thalamic ICH might be different from our results.
Conclusions
In summary, initial GCS scores and the existence of systemic complications were important prognostic factors of thalamic ICHs. On the other hand, volume of hematoma had a borderline relationship with clinical outcomes of patients with thalamic ICHs. Further investigations with a larger number of patients, a longer follow-up period, and a scrupulous set of data are required for a more accurate analysis of predictive factors of patients with thalamic ICHs.
Footnotes
Source of support: This study was supported in part by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2013R1A1A2057994), and the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C03370400)
Conflict of interest
The authors have no financial conflicts of interest.
References
- 1.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–99. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labovitz DL, Halim A, Boden-Albala B, et al. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–22. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 5.Hu YZ, Wang JW, Luo BY. Epidemiological and clinical characteristics of 266 cases of intracerebral hemorrhage in Hangzhou, China. J Zhejiang Univ Sci B. 2013;14:496–504. doi: 10.1631/jzus.B1200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandall KM, Rost NS, Sheth KN. Prognosis in intracerebral hemorrhage. Rev Neurol Dis. 2011;8:23–29. [PubMed] [Google Scholar]
- 7.Fukuda H, Munoz D, Macdonald RL. Spontaneous thalamic hemorrhage from a lateral posterior choroidal artery aneurysm. World Neurosurg. 2013;80:900e1–6. doi: 10.1016/j.wneu.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Samarasekera N, Fonville A, Lerpiniere C, et al. Influence of Intracerebral Hemorrhage Location on Incidence, Characteristics, and Outcome: Population-Based Study. Stroke. 2015;46(2):361–68. doi: 10.1161/STROKEAHA.114.007953. [DOI] [PubMed] [Google Scholar]
- 9.Gaab MR. Intracerebral hemorrhage (ICH) and intraventricular hemorrhage (IVH): improvement of bad prognosis by minimally invasive neurosurgery. World Neurosurg. 2011;75:206–8. doi: 10.1016/j.wneu.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116:e391–413. doi: 10.1161/CIRCULATIONAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 11.Morgan TC, Dawson J, Spengler D, et al. The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. 2013;44:635–41. doi: 10.1161/STROKEAHA.112.670653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 13.Chan E, Anderson CS, Wang X, et al. Significance of Intraventricular Hemorrhage in Acute Intracerebral Hemorrhage: Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial Results. Stroke. 2015;46(3):653–58. doi: 10.1161/STROKEAHA.114.008470. [DOI] [PubMed] [Google Scholar]
- 14.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Melbourne Risk Factor Study (MERFS) Group. Stroke. 1996;27:2020–25. doi: 10.1161/01.str.27.11.2020. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Shin JJ, Park SK, et al. Prognostic factors and clinical outcomes of acute intracerebral hemorrhage in patients with chronic kidney disease. J Korean Neurosurg Soc. 2013;54:296–301. doi: 10.3340/jkns.2013.54.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto Y, Koga M, Todo K, et al. Relative systolic blood pressure reduction and clinical outcomes in hyperacute intracerebral hemorrhage: the SAMURAI-ICH observational study. J Hypertens. 2015;33(5):1069–73. doi: 10.1097/HJH.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 17.Gioia LC, Kate M, Dowlatshahi D, et al. Blood pressure management in acute intracerebral hemorrhage: current evidence and ongoing controversies. Curr Opin Crit Care. 2015;21(2):99–106. doi: 10.1097/MCC.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 18.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–18. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–18. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim KD, Chang CH, Choi BY, Jung YJ. Mortality and real cause of death from the nonlesional intracerebral hemorrhage. J Korean Neurosurg Soc. 2014;55:1–4. doi: 10.3340/jkns.2014.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams TA, Leslie GD, Dobb GJ, et al. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. J Neurosurg. 2011;115:1040–46. doi: 10.3171/2011.6.JNS11167. [DOI] [PubMed] [Google Scholar]
- 22.Williamson RA, Phillips-Bute BG, McDonagh DL, et al. Predictors of extraventricular drain-associated bacterial ventriculitis. J Crit Care. 2014;29:77–82. doi: 10.1016/j.jcrc.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Cho DY, Chen CC, Lee HC, et al. Glasgow Coma Scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surg Neurol. 2008;70:628–33. doi: 10.1016/j.surneu.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang CW, Liu YJ, Lee YH, et al. Hematoma shape, hematoma size, Glasgow coma scale score and ICH score: which predicts the 30-day mortality better for intracerebral hematoma? PLoS One. 2014;9:e102326. doi: 10.1371/journal.pone.0102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SD, Kalita J, Misra UK, et al. Prognostic predictors of thalamic hemorrhage. J Clin Neurosci. 2005;12:559–61. doi: 10.1016/j.jocn.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer SA. Recombinant activated factor VII for acute intracerebral hemorrhage. Stroke. 2007;38:763–67. doi: 10.1161/01.STR.0000254499.46122.22. [DOI] [PubMed] [Google Scholar]
- 28.Kim JE, Ko SB, Kang HS, et al. Clinical practice guidelines for the medical and surgical management of primary intracerebral hemorrhage in Korea. J Korean Neurosurg Soc. 2014;56:175–87. doi: 10.3340/jkns.2014.56.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]