Abstract
INTRODUCTION
(RAIR) Recto-anal inhibitory reflex is an integral part of normal defecation. The physiological characteristics of RAIR along anal length and anterior-posterior axis are unknown.
AIM
To perform topographic and vector-graphic evaluation of RAIR along anal canal using high definition manometry (HDM), and examine the role of various muscle components.
METHODS
Anorectal topography was assessed in 10 healthy volunteers using HDM probe with 256 sensors. RAIR data were analyzed every mm along the length of anal canal for topographic, baseline, residual, and plateau pressures during 5 mean volumes of balloon inflation (15cc, 40cc, 71cc, 101cc, 177cc), and in 3D by dividing anal canal into 4×2.1 mm grids.
RESULTS
Relaxation pressure progressively increases along anal canal with increasing balloon volume up to 71 cc and thereafter plateaus. In 3-D, RAIR is maximally seen at the middle and upper portions of anal canal (levels 1.2-3.2 cm) and posteriorly. Peak residual pressure was seen at proximal anal canal.
CONCLUSION
RAIR is characterized by differential anal relaxation along Anterior-Posterior axis, longitudinally along the length of anal canal, and it depends on the rectal distention volume. It is maximally seen at internal anal sphincter pressure zone. Multidimensional analyses indicate that external anal sphincter provides bulk of anal residual pressure. Our findings emphasize importance of sensor location and orientation; as anterior and more distal location may miss RAIR.
Introduction
The recto anal inhibitory reflex or RAIR is an anal reflex response characterized by a transient relaxation of the anal canal following distention of the rectum. RAIR was first described by Gowers (1) in 1877 and later by Denny-Brown (2) in 1935. Physiologically, the RAIR is hypothesized to allow for the sampling and discrimination of rectal contents.(3) As such, the RAIR is thought to be a major component in maintaining health in continence and an abnormal response has been implicated in defecation disorders.(4). The RAIR is described manometrically by the relaxation pressure or loss of anal canal pressure during rectal balloon distention, and the residual pressure or the lowest anal canal pressure point of the reflex, see figure 1. The amplitude and duration of the RAIR has been shown to be dependent on the frequency and volume of rectal distention.(5-6) With increasing rectal volumes, the RAIR residual pressure will decrease and the duration of time of the RAIR will increase.
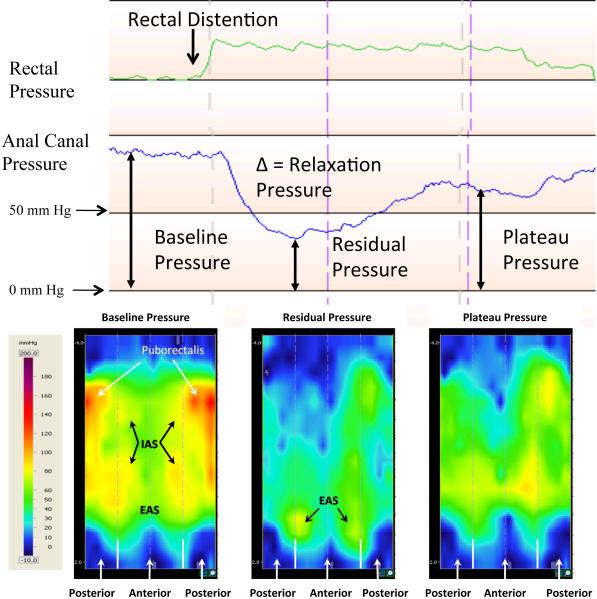
Figure 1.
The top panel shows a continuous recording of the rectal and anal manometric pressure sequences after a 70 cc rectal distention induces RAIR in one subject. The lower panel shows the topographic pressure map of the anal canal during the baseline, residual, and plateau pressures. The top panel exhibits the rise in rectal pressure with distention, the reflexive relaxation of the anal canal, and its partial recovery of pressure. The puborectalis, IAS, and EAS high pressure zones are marked in the baseline pressure map in the lower panel. The IAS provides pressure in the anterior and posterior superior anal canal, while the puborectalis in just the superior posterior anal canal. The pressure map at the residual pressure indicates a decrease in pressure at the puborectalis and IAS zones, with the EAS zone still providing anal canal pressure. The plateau topographic pressure map indicates a partial return to the baseline pressures in the IAS and puborectalis regions.
The anal canal is in constant communication with the rectum and central nervous system. (7) RAIR is mediated by a complex intramural neuronal plexus(4), specifically nonadrenergic, non-cholinergic inhibitory neurons. Several neurotransmitters have been hypothesized to mediate the relaxation, such as VIP and ATP (8), but most recent evidence implicates nitric oxide.(9) The modulation of the RAIR is processed in supra spinal and parasympathetic centers.(10)
Much data exist on the normative ranges of anorectal manometry both at rest and during squeeze maneuvers (11-14), but there has been little characterization of the pressure changes during RAIR. Zbar et al mapped out the RAIR at three sphincter locations in healthy, constipated, and incontinent patients. They found different relaxation pressures based on location and between the study populations. (15) Other studies have examined the RAIR's clinical value in general, such as in patients with constipation and incontinence, and have found some differences. (6, 16-18) Indeed there are several conditions known to have a complete lack of RAIR, including dysganglionosis, post circular myotomy, and lower anterior resections. (4, 8, 19-22) Evaluation of the RAIR can be used to facilitate a diagnosis of dyganglionosis, such as Hirschsprung's disease.(4)
The advent of HDM provides a unique opportunity to examine the anorectum. Briefly, the probe has 256 pressure sensors that allow for a very high resolution pressure map along the anal canal both longitudinally and circumferentially. This level of detail allows for manometric and anatomic characterization, as recently shown. (23) Previous studies have examined the relationships of manometric data to anatomical imaging, both with ultrasound (24) and MRI (25-26). One study also used an ultrasound probe as water was injected into the rectum to examine the RAIR.(27) However, these studies contributions were of low fidelity and could not simultaneously compare or tease out the activity of the individual muscles that make up the anal sphincter complex or examine changes in 3-D.
We tested the hypothesis that RAIR is characterized by a differential anal pressure response that varies both circumferentially and in the posterior axis. Therefore, our aim was to perform anal manometry (in healthy subjects) using 3-D high definition manometry, and to examine the anal pressure changes at every mm along the length of the anal canal and in the circumferential axis.
Materials and Methods
Subjects
Ten healthy subjects (6 males, mean age 35 years ± 4 yrs) were evaluated. All subjects filled out a bowel symptom questionnaire addressing GI health and anorectal function. Healthy subjects reported no constipation or incontinence symptoms, were not taking any medications besides oral contraceptive pill and multivitamins, and had no history of GI surgery other than appendectomy. All subjects had a normal physical examination. The study was approved by the Institutional Review Board of the University of Iowa, and all participants gave written informed consent.
Study Protocol
After an overnight fast, subjects attended the motility lab. No routine bowel preparation was used. High Definition 3-DManometry was performed with the subject in the left lateral position. The probe measures 6.4 cm in length and has an outer diameter of 10.75 mm. It has 256 pressure sensors that are arranged in 16 rows, and each row has 16 circumferentially-oriented sensors. The probe has a central lumen for inflation and a Luer lock at one end through which a balloon is attached. The balloon is composed of a non-latex clear thermoplastic elastomer, 3.3 cm long, with a 400 cc capacity. The probe is attached to an amplifier and recorder system and the manometric and topographic images are displayed on a computer monitor using specialized software (Motility Visualization System v.2.2, Sierra Scientific, Los Angeles, CA). See Cheeney et al for a detailed technical discussion of the HDM probe. (23) A digital rectal exam was performed before placement of the probe and saline enema placed if stool was detected. The lubricated probe was inserted such that a panel of pressure sensors lay across the anal canal, and some extending above and just below the anal canal. The probe was oriented with the back corresponding to the dorsal aspect of the subject. This allowed for the detailed reading of the rectum and anal canal with respect to orientation. The probe was held in place manually during the study.
After probe placement, a rest period of 7 minutes followed to allow the resting sphincter to baseline. Next, a hand held syringe connected to the HDM probe was used to inflate the balloon in the rectum in a stepwise, graded fashion using intermittent balloon distention technique. (11, 28) The subjects were given a sensation chart and asked to describe their sensations with each distention. The sensory thresholds at which subjects reported their first sensation, constant sensation, desire to defecate, urge to defecate, and the maximum tolerable volume were recorded. The starting rectal distention volume used was 10 cc, with a 10 cc stepwise increase until the subject experienced the first sensation. After that a 30 cc stepwise distention volume was used until maximum tolerable volume or a total volume of 320 cc was reached. Each distention was held for at least 30 seconds, and after a deflation and rest period of 2 minutes was re-inflated to the next volume. The subject was unaware of the timing or the volume of balloon distention.
Data Analysis
The HDM recordings were analyzed for the presence of RAIR. Figure 1 displays the RAIR manometrically. Immediately following rectal distention there is a drop in anal canal pressure till it reaches the nadir or residual pressure in the anal canal at a particular level. This change in resting pressure is calculated as the relaxation pressure. From the residual pressure the anal canal pressure usually recover completely, raise to a plateau pressure some level between the baseline and lowest pressure, or remain at the residual pressure. Figure 1 shows a RAIR with the various pressure responses. Once the rectal balloon distention is deflated, the rectal pressure returns to its baseline pressure and simultaneously the anal canal pressure also returns to its baseline pressure. Four points in the progression of the RAIR were analyzed, the baseline, residual, and plateau pressure, Fig 1, and from these measurements the relaxation pressure was calculated. The baseline pressure was taken shortly before rectal distention (within 5-10 seconds) and the plateau pressure was taken after the upward phase of the RAIR had stabilized, but before the rectal distention was released. Distention volumes were grouped based on the first 4 rectal distention volumes that elicited a RAIR per subject, without any overlap in distention volume between the groups. The range for each group was 10cc-20cc, 30-50cc, 60-80cc, 90-110cc, and the final volume before the subjects reported maximum intolerable volume. The average volumes were 15 cc, 40 cc, 71 cc, 101 cc, and 177 cc respectively, see table 1.
Table 1.
RAIR distention volume groupings
| Group | Average ± SEM (cc) | Range (cc) |
|---|---|---|
| 1 | 15 ± 1.7 | 10–20 |
| 2 | 40 ± 2.1 | 30–50 |
| 3 | 71 ± 1.8 | 60–80 |
| 4 | 101 ± 1.8 | 90–110 |
| 5 | 177 ± 18.7 | 130–280 |
RAIR, recto-anal inhibitory reflex.
Pressure data were examined at every millimeter distance along the anal canal simultaneously. Additionally recordings were made on 3D topographic pressure maps (figure 1). The outer edges of the map show the pressure changes in the posterior portion of the anal canal and the middle or the inner portion of the image show the pressure changes in the anterior portion of the anal canal. The topographic view was used to set the zero point or the anal verge, which was the point where the continuous pressure recording sharply dropped to atmospheric pressure. The pressure map was further divided into 4-mm by 2.1-mm grids and an average pressure per grid was taken to give both longitudinal and circumferential pressure data of the anal canal during RAIR. The high pressure zone of the three muscular structures, puborectalis, internal anal sphincter (IAS), and external anal sphincter (EAS), of the anal canal is shown.
Baseline pressures were analyzed across the length of the anal canal every millimeter and in the anterior and posterior vectors by combining the anterior grids and posterior grids every 4 millimeters along the canal. This achieved an anterior and posterior specific longitudinal pressure at a 4 mm resolution. Similar analysis was performed for the residual and plateau pressures. The relaxation pressure along the anal canal and in the anterior and posterior axes was calculated by subtracting the residual pressure from the baseline pressure. Pressures from the 10 subjects were then averaged for each rectal distention pressure.
Results
All subjects exhibited the RAIR at multiple rectal distention volumes, ranging from 10cc to 280cc. The average minimal rectal distention volume required to elicit the RAIR was 15cc ± 1.7. Rectal sensory thresholds; In rectal distention volume group 1 (10cc to 20cc), 8 subjects experienced first sensation and 2 felt nothing. In group 2 (30cc to 50cc), 1 subject reported first sensation, 1 had no sensation, 7 reported constant sensation, and 1 reported desire to defecate. In group 3 (60cc to 80cc), 6 subjects reported constant sensations and 4 a desire to defecate. In group 4 (90cc to 110cc), 6 subjects reported a constant sensation, 2 desire to defecate, and 2 an urge to defecate. In the highest rectal distention group (130cc to 280cc), 4 subjects reported a desire to defecate, 4 others an urge to defecate, and 1 reported a maximum tolerable volume.
Anal Pressure Profiles
All baseline anal pressure profiles showed a peak pressure in the range of 70-80 mmHg at a level of 1.6cm from the anal verge, which corresponds to the level of the IAS. Additionally, there was a hump in the posterior pressure from 2.4cm to 4cm corresponding to the puborectalis muscle. This is consistent with previous analysis of baseline anorectal pressure in healthy subjects. (23)
RAIR
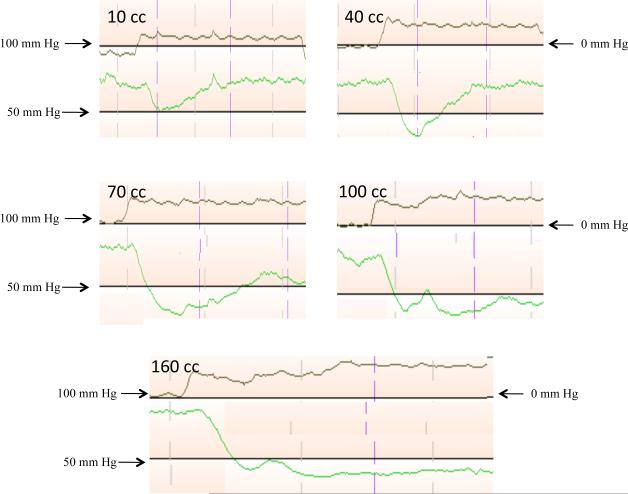
Figure 2 shows the morphologic changes in the anal pressure projected during the RAIR in one subject at rectal distention volumes of 10 cc, 40 cc, 70 cc, 100 cc, and 160 cc in a manometric display. From this figure, the morphology of the RAIR at different rectal distention volumes may be appreciated. At 10 cc distention volume the RAIR is shallow and the pressure decrease quickly recovers to baseline pressure. At 40 cc, the RAIR is deeper and the pressure does not recover fully to the baseline pressure, which is a plateau pressure. At 70 cc, the RAIR maximally deepens and the plateau pressure is now significantly lower than the baseline pressure. At 100 cc, the RAIR remains deep and the plateau pressure is just greater than the residual pressure. Finally, at very large rectal distention volumes, like 160 cc, the RAIR remains at the residual pressure for the entire duration of rectal distention. Furthermore rectal distention volumes of 100 cc and 160 cc both show transient spike in pressure during the down stroke of the RAIR and this is due to the sensorimotor response. (29)
Figure 2.
Each panel represents a single continuous recording of the rectal and anal manometric pressure sequences during RAIR at 10cc, 40cc, 70cc, 100cc, and 160cc in a single subject. The panels show the progressive nature of the RAIR with increasing rectal distention volumes. The nadir of the RAIR is progressively lower till 70cc rectal distention and the plateau pressure also gradually decreases with larger rectal distention volumes. The pressure scale on the left corresponds to the anal manometric pressure and the right to the rectal manometric pressure.
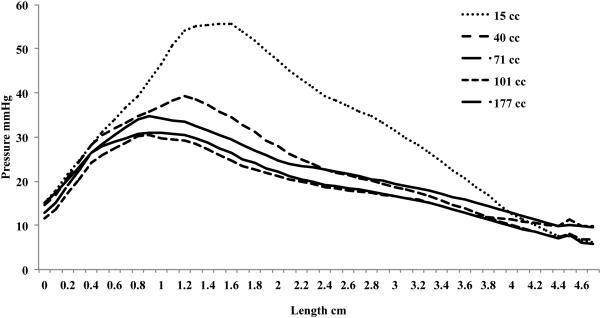
Relaxation Pressure
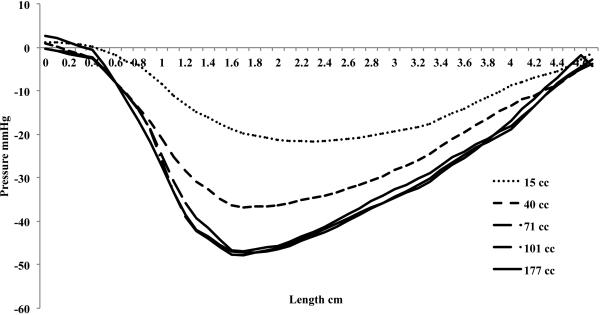
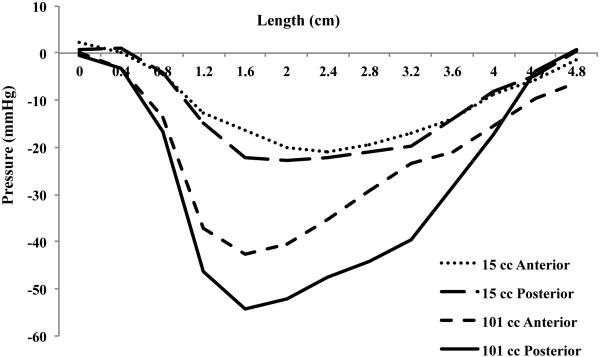
The relaxation pressure is a function of rectal distention volume, with the relaxation pressure increasing until a mean rectal distention volume of 71 cc, figure 3. The maximal peak relaxation pressure is 47.3 mmHg ± 2.6. The relaxation pressure is also dependent on the location along the anal canal. Figure 3 shows the mean relaxation pressure for the different rectal distention volumes, with the peak relaxation pressure at 1.7 cm from the anal verge in all groups. After the initial peak, there is a gradual decline in the relaxation pressure along the anal canal. Figure 4 shows the mean relaxation pressure at 15 cc and 101 cc rectal distention volumes along the anterior and posterior vectors. At a mean 15 cc distention, the relaxation is the same circumferentially. There is a constant 20 mmHg relaxation ranging from 1.2 cm to 3.6 cm from the anal verge occurring in both the anterior and posterior half of the anal canal. At a mean volume of 101 cc rectal distention, however, there is an obvious increase in relaxation pressure in the posterior vector, with both anterior and posterior relaxation pressure peaking again at 1.7 cm from the anal verge.
Figure 3.
Mean relaxation pressure from all subjects at every millimeter along the length of the anal canal. The nadir of the pressure for each rectal distention group is progressively lower till an average 71 cc rectal distention, at which the anal canal cannot relax any further. The peak relaxation pressure is at 1.7 cm from the anal verge for 40 cc, 71 cc, 101 cc, and 177 cc mean rectal distention and at 2.3 cm from the anal verge for the 15 cc mean rectal distention group.
Figure 4.
Mean relaxation pressure from all subjects every 4 millimeters along the anterior and posterior anal canal at a mean 15 cc and 101 cc rectal distention volume. The mean 15 cc rectal distention volume shows a constant 20 mmHg relaxation pressure ranging from 1.2 cm to 3.6 cm in the anterior and posterior anal canal, while the 101 cc rectal distention volume shows a peak at 1.7 cm from the anal verge and a larger pressure change in the superior-posterior anal canal region compared to the superior-anterior region, indicating both the IAS and puborectalis are relaxing.
Residual Pressure
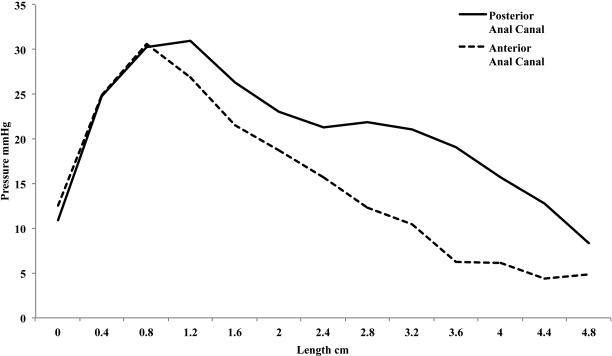
As reflected in the relaxation pressure, the residual pressure decreases to a minimum peak pressure with increasing rectal distention volumes to 31.9 ± 1.9 mmHg starting at the mean rectal distention volume of 71 cc, figure 5. Additionally, as the rectal distention volume increases the peak residual pressure shifts towards the anal verge. In figure 5, the location of the peak residual pressure for the mean 15 cc distention group is similar to the baseline peak at 1.7 cm from the anal verge. The peak of the residual pressure starting at a mean 71 cc rectal distention volume has shifted to 0.9 cm from the verge. Figure 6 displays the mean residual pressure from the 101 cc rectal distention group in the anterior and posterior half of the anal canal. There is a peak at 0.9 cm in the anterior and posterior vectors and an additional hump in the posterior only vector ranging from 2.4 cm to 4.4 cm, corresponding to the external anal sphincter and puborectalis muscle respectively. (23, 30) Additionally, on visual inspection of the topographic pressure map at residual pressure, lower image of figure 1, the majority of the pressure appears to be originating from the EAS high pressure zone.
Figure 5.
Mean residual pressure from all subjects at every millimeter along the length of the anal canal. The peak residual pressure for the mean 15 cc rectal distention volume is at 1.6 cm from the anal verge. The peak shifts distally with larger rectal distention volumes, moving to 0.9 cm from the verge at 71 cc distention volume. This indicates the increasing relaxation of the IAS.
Figure 6.
Mean residual pressure from all subjects at a mean 101 cc rectal distention volume along the anterior and posterior half of the anal canal every 4 millimeters. The residual pressure in the anterior and posterior vectors is at 0.9 cm from the anal verge. This corresponds to the EAS and indicates this sphincter provides anal canal pressure during the residual pressure phase of the RAIR.
Plateau Pressure
Much like the residual pressure, the plateau pressure also has a progressive, linear decline in pressure with increased rectal distention. Starting at mean 40 cc rectal distention, the plateau pressure begins to fall below baseline pressure, with the plateau pressure progressively decreasing till it merges with the residual pressure at very high rectal distention volumes, see the 160 cc rectal distention in figure 2. There is a similar proximal shift in the location of the peak plateau pressure with increasing rectal distention volumes, with the mean peak pressure in the 177 cc rectal distention group having moved distally to 1.3 cm from the anal verge.
Discussion
This study provides detailed characterization of the anal pressure charges in the circumferential and anterior-posterior axis at rest and during the RAIR. We found that increased volume of rectal distension induces a progressively larger amplitude and greater duration of RAIR. However, beyond 71 cc of balloon distention there is no further decrease in anal pressure. This confirms previous observation [16] and provides significant new information. The use of continuous pressure recording both along the anal canal and circumferentially, has provided new and more accurate measurements to the changes. Additionally, the HDM provided real time data for each component of the anal muscular structures, figure 1.
The exact role of the different anal sphincters during the RAIR has not been fully evaluated due to the limitations of current manometric techniques. Anal canal pressure is maintained by the internal anal sphincter (IAS), external anal sphincter (EAS), and the U shaped puborectalis sling in the superior aspect of the canal.(31) The peak pressure of the IAS is seen at a level of 1.6cm from the anal verge, which corresponds to the peak relaxation pressure. Here, we have independently confirmed that the major component that contributes to the relaxation during the RAIR is the IAS. Secondly, the EAS is the only muscular structure located from 0.5 cm to 1 cm from the anal verge. (30-31) As the relaxation pressure increases due to increasing IAS relaxation, the EAS becomes the prominent muscular structure that maintains continence as evidenced by the distal location of the peak residual pressure. Finally the posterior superior location of the puborectalis from 2.4 cm to 4.0 cm from the anal canal (23) allows for the interpretation of puborectalis muscle activity. This pressure zone can be seen in the residual pressure in figure 5, indicating that the puborectalis contributes to the residual pressure, as well as the EAS, even at high rectal distention volumes. The changing peak pressure of the residual pressure may be explained by its relative contribution to the tone of the three anal canal structures.
The residual pressure and plateau pressure have a separate graded response that matches rectal distention. The level of residual pressure has been shown to be related to distention volume, (32-33). However, we found that the plateau level is also linearly related to the volume of distention. Hence the control mechanisms between the rectum and anal canal that govern the RAIR must mediate both an initial relaxation and a later plateau period of relaxation. Although this study shows a RAIR with rectal distention, it has been shown that electrical stimulation of the rectal mucosa can elicit anal canal relaxation as well. (34) These observations warrant further investigation into how rectal innervations differentially regulate both the residual and plateau pressures specific to the same rectal sensory perception.
Our findings further elucidate the mechanisms of defecation and attempt to explain its voluntary nature. With higher rectal distention volumes, the anal canal tone during the RAIR becomes progressively dependent on the voluntary EAS and the puborectalis muscle. Physiologically, this is reflected by the voluntary effort that maintains continence with larger rectal contents. The physiologic sensation may also be related to RAIR parameters. With very low rectal distention volumes, the pressure recovers fully to baseline sphincter pressure, and no subject sensed a constant sensation. It is not until the plateau pressure fails to fully recover that subjects begin to feel a constant sensation. At very high rectal distentions when the plateau pressure merges with the residual pressure, all subjects report varying needs to defecate. These observations portray an updated model of the RAIR and how it relates to the mechanism(s) of perception and defecation.
The dynamic nature of anal canal reflexes underscores the importance for a standardized and reproducible method for sensor placement when performing anorectal manometry (ARM) and high resolution anorectal manometry (HRM). The current study allowed for the setting of the zero point, or anal verge, by reading the topographic pressures. This post-procedure, individualized method is not available in the routinely used ARM. Furthermore, we showed that pressure does not just change continuously along the anal canal, but also in the Anterior-Posterior (A-P) vector. At larger distention volumes, the difference between the A-P pressures can be nearly double, figure 4. Thus, proper probe placement are critical with ARM and HRM techniques both along the anal canal and its orientation in the A-P vector are critical with an anterior and more distal location being less likely to detect RAIR.
RAIR plays a prominent role in normal defecation and sensation; consequently a dysfunction of RAIR leads to anorectal disorders. Enteric dysganglionoses such as Hirschsprung's disease are classified by a lack of enteric nervous system at some level above the IAS. (35) The diagnostic utility of the absence of RAIR in Hirschsprung's Disease has been reported to have a sensitivity of 91% and specificity of 94% in one systematic review.(36) Although others have reported lower numbers, the variability may in fact be due to how the test is performed, and in particular where the sensors are located or at what level the balloon is placed. HDM's ability to identify specific sphincters and its flexibility with probe placement may improve the utility of manometry in detecting this common pediatric GI disorder. Perhaps more generally, conditions such as incontinence and constipation may also benefit from a very detailed evaluation of the reflexes and sensori-motor function of the anorectum.
Acknowledgments
Grant Support: This research was supported in part by grant R01DK 57100-03 National Institute of Health and Gregory Cheeney is a recipient of a Clinical Research Fellowship from the Doris Duke Charitable Foundation. This work was presented at Digestive Disease Week, New Orleans, LO, 2010.
Footnotes
Guarantor: Satish SC Rao– Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, critical revision, important intellectual content and final approval.
Contributing Authors:
Gregory Cheeney - Study concept and design, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, and final approval.
Michelle Nguyen - Data acquisition, data collection, data interpretation
Jessica Valestin - Data acquisition, data collection, study recruitment
Competing Interests: the authors have no competing interests.
References
- 1.Gowers WR. The automatic action of the sphincter ani. Proc R Soc Lond [Biol] 1877;26:77–84. [Google Scholar]
- 2.Denny-Brown D, Robertson EG. An investigation of the nervous control of defaecation. Brain. 1935;58:256–310. doi: 10.1111/j.1463-1318.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- 3.Duthie HL, Bennett RC. The relation of sensation in the anal canal to the functional anal sphincter: a possible factor in anal continence. Gut. 1963;4:179–182. doi: 10.1136/gut.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangwan YP, Solla JA. Internal anal sphincter: advances and insights. Dis Colon Rectum. 1998;41:1297–1311. doi: 10.1007/BF02258232. [DOI] [PubMed] [Google Scholar]
- 5.Sun WM, Read NW, Prior A, Daly JA, Cheah SK, Grundy D. Sensory and motor responses to rectal distention vary according to rate and pattern of balloon inflation. Gastroenterology. 1990;99:1008–1015. doi: 10.1016/0016-5085(90)90620-g. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro FJ, Regadas FS, Murad-Regadas SM, Rodrigues LV, Leal VM. Comparative evaluation of the effect of sustained inflation and rapid inflation/deflation of the intrarectal balloon upon rectoanal inhibitory reflex parameters in asymptomatic subjects. Tech Coloproctol. 2007;11:323–326. doi: 10.1007/s10151-007-0374-6. [DOI] [PubMed] [Google Scholar]
- 7.Hobday DI, Aziz Q, Thacker N, Hollander I, Jackson A, Thompson DG. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–368. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 8.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 9.Stebbing JF, Brading AF, Mortensen NJ. Nitric oxide and the rectoanal inhibitory reflex: retrograde neuronal tracing reveals a descending nitrergic rectoanal pathway in a guinea-pig model. Br J Surg. 1996;83:493–498. doi: 10.1002/bjs.1800830417. [DOI] [PubMed] [Google Scholar]
- 10.Beuret-Blanquart F, Weber J, Gouverneur JP, Demangeon S, Denis P. Colonic transit time and anorectal manometric anomalies in 19 patients with complete transection of the spinal cord. J Auton Nerv Syst. 1990;30:199–207. doi: 10.1016/0165-1838(90)90251-d. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaliha C, Sultan AH, Emmanuel AV. Normal ranges for anorectal manometry and sensation in women of reproductive age. Colorectal Dis. 2007;9:839–844. doi: 10.1111/j.1463-1318.2007.01212.x. [DOI] [PubMed] [Google Scholar]
- 13.Cali RL, Blatchford GJ, Perry RE, Pitsch RM, Thorson AG, Christensen MA. Normal variation in anorectal manometry. Dis Colon Rectum. 1992;35:1161–1164. doi: 10.1007/BF02251969. [DOI] [PubMed] [Google Scholar]
- 14.Raza N, Bielefeldt K. Discriminative value of anorectal manometry in clinical practice. Dig Dis Sci. 2009;54:2503–2511. doi: 10.1007/s10620-008-0631-1. [DOI] [PubMed] [Google Scholar]
- 15.Zbar AP, Aslam M, Gold DM, Gatzen C, Gosling A, Kmiot WA. Parameters of the rectoanal inhibitory reflex in patients with idiopathic fecal incontinence and chronic constipation. Dis Colon Rectum. 1998;41:200–208. doi: 10.1007/BF02238249. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Pasricha PJ, Sallam HS, Ma L, Chen JD. Clinical significance of quantitative assessment of rectoanal inhibitory reflex (RAIR) in patients with constipation. J Clin Gastroenterol. 2008;42:692–698. doi: 10.1097/MCG.0b013e31814927ba. [DOI] [PubMed] [Google Scholar]
- 17.Kaur G, Gardiner A, Duthie GS. Rectoanal reflex parameters in incontinence and constipation. Dis Colon Rectum. 2002;45:928–933. doi: 10.1007/s10350-004-6331-9. [DOI] [PubMed] [Google Scholar]
- 18.Feinberg L, Mahajan L, Steffen R. The constipated child: is there a correlation between symptoms and manometric findings? J Pediatr Gastroenterol Nutr. 2008;47:607–611. doi: 10.1097/mpg.0b013e3181684c94. [DOI] [PubMed] [Google Scholar]
- 19.Penninckx F, Lestar B, Kerremans R. The internal anal sphincter: mechanisms of control and its role in maintaining anal continence. Baillieres Clin Gastroenterol. 1992;6:193–214. doi: 10.1016/0950-3528(92)90027-c. [DOI] [PubMed] [Google Scholar]
- 20.Sun WM, Read NW, Miner PB, Kerrigan DD, Donnelly TC. The role of transient internal sphincter relaxation in faecal incontinence? Int J Colorectal Dis. 1990;5:31–36. doi: 10.1007/BF00496147. [DOI] [PubMed] [Google Scholar]
- 21.Cortesini C, Pucciani F, Carassale GL, Paparozzi C. Anorectal physiology after anterior resection and pull-through operation. Eur Surg Res. 1983;15:176–183. doi: 10.1159/000128350. [DOI] [PubMed] [Google Scholar]
- 22.Shimotake T, Kubota Y, Inoue K, Yanagihara J, Iwai N. Absence of rectoanal inhibitory reflex in a child with multiple endocrine neoplasia type 2B. J Pediatr Surg. 1998;33:1268–1271. doi: 10.1016/s0022-3468(98)90166-x. [DOI] [PubMed] [Google Scholar]
- 23.Cheeney G, Remes-Troche JM, Attaluri A, Rao SS. Investigation of Anal Motor Characteristics of the Sensori-Motor Response (SMR) using 3-D Anorectal Pressure Topography. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gantke B, Schafer A, Enck P, Lubke HJ. Sonographic, manometric, and myographic evaluation of the anal sphincters morphology and function. Dis Colon Rectum. 1993;36:1037–1041. doi: 10.1007/BF02047296. [DOI] [PubMed] [Google Scholar]
- 25.Cornella JL, Hibner M, Fenner DE, Kriegshauser JS, Hentz J, Magrina JF. Three-dimensional reconstruction of magnetic resonance images of the anal sphincter and correlation between sphincter volume and pressure. Am J Obstet Gynecol. 2003;189:130–135. doi: 10.1067/mob.2003.545. [DOI] [PubMed] [Google Scholar]
- 26.Fenner DE, Kriegshauser JS, Lee HH, Beart RW, Weaver A, Cornella JL. Anatomic and physiologic measurements of the internal and external anal sphincters in normal females. Obstet Gynecol. 1998;91:369–374. doi: 10.1016/s0029-7844(97)00678-9. [DOI] [PubMed] [Google Scholar]
- 27.Orno AK, Lovkvist H, Marsal K, von Steyern KV, Arnbjornsson E. Sonographic visualization of the rectoanal inhibitory reflex in children suspected of having Hirschsprung disease: a pilot study. J Ultrasound Med. 2008;27:1165–1169. doi: 10.7863/jum.2008.27.8.1165. [DOI] [PubMed] [Google Scholar]
- 28.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 29.De Ocampo S, Remes-Troche JM, Miller MJ, Rao SSC. Rectoanal sensorimotor response in humans during rectal distension. Diseases of the Colon & Rectum. 2007;50:1639–1646. doi: 10.1007/s10350-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol. 2006;101:1092–1097. doi: 10.1111/j.1572-0241.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 31.Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am. 2008;37:493–509. vii. doi: 10.1016/j.gtc.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortesini C, Paparozzi C, Carassale G, Bechi P. Rectoanal reflex in healthy subject. Boll Soc Ital Biol Sper. 1979;55:877–883. [PubMed] [Google Scholar]
- 33.Meunier P, Mollard P. Control of the internal anal sphincter (manometric study with human subjects). Pflugers Arch. 1977;370:233–239. doi: 10.1007/BF00585532. [DOI] [PubMed] [Google Scholar]
- 34.Kamm MA, Lennard-Jones JE, Nicholls RJ. Evaluation of the intrinsic innervation of the internal anal sphincter using electrical stimulation. Gut. 1989;30:935–938. doi: 10.1136/gut.30.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gariepy CE. Developmental disorders of the enteric nervous system: genetic and molecular bases. J Pediatr Gastroenterol Nutr. 2004;39:5–11. doi: 10.1097/00005176-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 36.de Lorijn F, Kremer LC, Reitsma JB, Benninga MA. Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr. 2006;42:496–505. doi: 10.1097/01.mpg.0000214164.90939.92. [DOI] [PubMed] [Google Scholar]