Abstract
Electrophilic compounds are a newly recognized class of redox-active neuroprotective compounds with electron deficient, electrophilic carbon centers that react with specific cysteine residues on targeted proteins via thiol (S-)alkylation. Although plants produce a variety of physiologically active electrophilic compounds, the detailed mechanism of action of these compounds remains unknown. Catechol ring-containing compounds have attracted attention because they become electrophilic quinones upon oxidation, although they are not themselves electrophilic. In this study, we focused on the neuroprotective effects of one such compound, carnosic acid (CA), found in the herb rosemary obtained from Rosmarinus officinalis. We found that CA activates the Keap1/Nrf2 transcriptional pathway by binding to specific Keap1 cysteine residues, thus protecting neurons from oxidative stress and excitotoxicity. In cerebrocortical cultures, CA-biotin accumulates in non-neuronal cells at low concentrations and in neurons at higher concentrations. We present evidence that both the neuronal and non-neuronal distribution of CA may contribute to its neuroprotective effect. Furthermore, CA translocates into the brain, increases the level of reduced glutathione in vivo, and protects the brain against middle cerebral artery ischemia/reperfusion, suggesting that CA may represent a new type of neuroprotective electrophilic compound.
Keywords: carnosic acid, cysteine thiol, Keap1, neurite outgrowth-promoting prostaglandin 11, Nrf2, S-alkylation
Introduction
Glutamate, the major excitatory amino acid in the brain, exerts various actions on neurons, affecting development, plasticity, and survival (Nakanishi 2005; Barco et al. 2006). Under physiological conditions, glutamate plays a major role in learning and memory in part via NMDA receptor mediated pathways (Nakanishi 2005; Barco et al. 2006). However, under pathological conditions, glutamate can induce neuronal cell death, termed ‘excitotoxicity,’ predominantly by excessive activation of the NMDA receptor (Choi 1992; Ankacrona et al. 1995; Hara and Snyder 2007). In immature neurons, which have not yet expressed functional NMDA receptors, high concentrations of glutamate induce a novel type of neuronal death mediated by depletion of GSH, termed ‘oxidative glutamate toxicity’ (Murphy et al. 1989, 1990; Dargusch and Schubert 2002). Although these two types of neuronal death induced by glutamate are distinct from each other, oxidative stress is involved in both types (Murphy et al. 1989; Coyle and Puttfarcken 1993; Dugan et al. 1995). For this reason, several antioxidant molecules have been reported to protect neurons against both excitotoxicity and oxidative glutamate toxicity (Murphy et al. 1990; Ankacrona et al. 1995; Dargusch and Schubert 2002). Thus, one possible strategy for the development of neuroprotective drugs is to search for low-molecular-weight compounds that can regulate redox state and thereby counter oxidative damage (Satoh and Lipton 2007).
Recently, our group, in addition to those of Johnson and Murphy, reported that a series of compounds not necessarily possessing antioxidative activity themselves could nonetheless transcriptionally induce antioxidative enzymes to afford neuroprotection (Kraft et al. 2004; Shih et al. 2005; Satoh et al. 2006). Such electrophilic compounds have an advantage over antioxidant molecules because their action is more sustained and amplified by transcription-mediated signaling pathways (Satoh and Lipton 2007). Electrophiles induce the expression of a set of antioxidant enzymes, called ‘phase 2 enzymes,’ including heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), and c-glutamyl cysteine ligase (c-GCL), all of which provide efficient cytoprotection by regulating the intracellular redox state (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006). Representing a specific transcriptional element located in the 5′ upstream promoter region of genes that encode phase 2 enzymes, the antioxidant-responsive element (ARE) plays a central role in the induction of such enzymes (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006). A key cascade involved is termed the Keap1/Nrf2 pathway, which is comprised of Keap1, a regulator protein, and Nrf2, a transcription factor that binds to the ARE. Keap1 is an adapter protein that facilitates ubiquitination of Nrf2 and thus drives constitutive degradation of this transcription factor. When electrophiles react with critical cysteine residues on the Keap1 protein to form an adduct, they perturb this system, thereby stabilizing Nrf2 and allowing it to be translocated from the cytoplasm into the nucleus, where it binds to AREs and stimulates the transcription of phase 2 genes (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006).
Neuroprotective electrophilic compounds reported previously may be divided into two major groups, catechol- and enone-types, as shown in Fig. 1 (Kraft et al. 2004; Satoh et al. 2006; Satoh and Lipton 2007). These two types of compounds manifest distinctive features. One difference lies in their degree of electrophilicity. Enone-type electrophilic compounds, including the enone-type curcumin (Yazawa et al. 2006) and dienone-type neurite outgrowth-promoting prostaglandin (NEPP11) (Satoh et al. 2006), are themselves electrophilic. In contrast, catechol-type compounds are not themselves electrophilic but become electrophilic by oxidative conversion to a quinone (Nakamura et al. 2003). Thus, these catechol-type compounds function as prodrugs, which require conversion from catechol to quinone to exert their neuroprotective effect (Lipton 2004; Satoh and Lipton 2007). Another distinctive difference between the catechol-type and quinone-type is their distribution in neuronal cultures. Tertbutyl hydroquinone (TBHQ), a catechol-type neuroprotective electrophilic compound, reportedly acts preferentially in astrocytes and protects neurons by a paracrine mechanism (Ahlgren-Beckendorf et al. 1999; Lee et al. 2003b; Kraft et al. 2004). In contrast, NEPP11, an enone-type neuroprotective electrophilic compound, accumulates in neurons to induce HO-1, thereby exerting a direct protective action on neurons (Satoh et al. 2006).
Figure 1.
Core chemical structures of neuroprotective electrophilic compounds of the catechol and enone types.
As plants produce a variety of electrophilic compounds, we looked for naturally occurring electrophilic compounds in plants of the catechol-type that might protect neurons through transcriptional activation. Carnosic acid (CA; Fig. 2a) is a naturally occurring catechol-type poly-phenolic diterpene obtained from Rosmarinus officinalis (rosemary) and comprises about 5% of the dry weight of rosemary leaves (Kosaka and Yokoi 2003). CA reportedly has various biological actions, possibly effected through phosphatidylinositol 3-kinase (Martin et al. 2004), peroxisome proliferator- activated receptor c (Rau et al. 2006), cyclin A/B1 (Visanji et al. 2006), and free radical-scavenging activity (Aruoma et al. 1992). In the present study, we investigated the actions of CA to answer the following questions:
Figure 2.
Thiols from GSH and BSA as targets for CA binding. (a) Chemical reactions leading to GS-CA adduct formation as proposed from the results of NMR analysis. The catechol form of CA slowly oxidizes to the quinone derivative, an electrophilic compound, which serves as a target for nucleophilic attack by GSH or other protein thiols. The asterisk (*) indicates the electrophilic carbon in the quinone form of CA. The chemical structure of the GS-CA adduct was confirmed by 1H-NMR, 13C-NMR, and nuclear overhauser effect analysis in deuteriumized DMSO. Nuclear overhauser effects, highlighted by red double-headed arrows, yielded evidence that C(14) was subjected to nucleophilic attack by GSH. Note: The quinone-ring of CA returns to a catechol-type ring in response to adduct formation because the thiol of GSH donates an electron pair to the ring. Rectangular box: Time course of GS-CA adduct formation. GSH (18 mmol/L) and CA (6 mmol/L) were incubated and slowly mixed in 50% ethanol-PBS, pH 7.4, at 37°C for 7 h. GS-CA formation was then quantified by HPLC under the following conditions: column, μBondasphere C18; temperature, 40°C; HPLC system, Shimadzu (Kyoto, Japan) LC10Avp; detector, UV 230 nm; running solvent (i) 2% acetic acid and (ii) acetonitrile, 30% B isocratic at 1 mL/min. (b) Chemical structure of CAB. Biotin was conjugated at the carbonic acid site of CA with a chemical linker. (c) Dose-dependent BSA-CA adduct formation. BSA (1 μg/lane) was incubated with various concentrations of CAB (1–100 μmol/L) for 5 h at 23°C. The BSA/CAB samples were subjected to electrophoresis and probed with peroxidase-conjugated streptavidin (upper panel). A duplicate gel was stained with Coomassie brilliant blue as a control to assess loading of BSA. (d) Inhibition of BSA-CA adduct formation by NEM. BSA (1 μg/lane) was incubated with various concentrations of NEM (10–1000 μmol/L) for 30 min at 23°C. Vehicle (lane 1) or CAB (10 μmol/L, lanes 2–7) was then added, and the samples were incubated for 5 h at 23°C prior to blotting and probing with peroxidase-conjugated streptavidin (upper panel). A duplicate gel was stained with Coomassie brilliant blue (lower panel).
Is CA neuroprotective both in vitro and in vivo?
Does CA activate the Keap1/Nrf2 pathway?
Is CA distributed in neurons or in non-neuronal cells?
Materials and methods
Chemicals
Fraction V bovine serum albumin (BSA), 4′,6-diamino-2-phenylindole, dimethyl sulfoxide (DMSO), fluorescein diacetate, glutamate, Hoechst 33,258, GSH, GSSG, 3-(4,5- dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide, N-ethylmaleimide (NEM), NMDA, propidium iodide, rotenone, and 2, 3, 5-triphenyltetrazolium chloride (TTC) were purchased from WAKO Chemicals Inc. (Tokyo, Japan) or Sigma (St Louis, MO, USA).
Carnosic acid and biotinylated carnosic acid
Carnosic acid was extracted from rosemary leaves as previously described (Kosaka and Yokoi 2003). Biotinylated carnosic acid (CAB) was synthesized according to Kosaka and Yokoi (2003) by the following method: CA (90 mg), acetonitrile (2 mL), and dicyclohexylcarbodiimide (67 mg; Tokyo Kasei Kyogyo Co., Tokyo, Japan) were mixed for 3 min on ice. Then, 5-(biotinamido) pentylamine (41 mg; Pierce, Rockford, IL, USA), dissolved in 80% acetonitrile, was added to the mixture, which was subsequently incubated for 10 min on ice. Thereafter, hydrochloric acid was added to terminate the reaction. The reaction product was separated by preparative liquid chromatography (ODS column consisting of ODS-S-50c; Maruzen Co., Tokyo, Japan) with acetonitrile and hydrochloric acid as solvent. Both CA and CAB were prepared as 10 mmol/L stock solutions in DMSO.
Western blotting for biotinylated carnosic acid
Bovine serum albumin with or without CAB was separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then electrophoretically transferred to a nitrocellulose membrane (Amersham Life Science, Piscataway, NJ, USA). Next, the membrane was blocked for 1 h at 23°C in Ca2+, Mg2+())-phosphate-buffered saline containing 0.1% Tween 20 (PBS-T) and 5% non-fat dry milk and then incubated for 1 h with horseradish peroxidase-conjugated streptavidin. After the membrane had been washed three times with PBS-T, signals were detected using ECL western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Antibodies
Anti-Nrf2 and anti-Keap1 monoclonal mouse IgGs were generated by Ken Itoh (Hirosaki University, Aomori, Japan). Other antibodies used were FITC-conjugated anti-mouse IgG, rhodamine-conjugated anti-mouse IgG, rhodamine-conjugated anti-rabbit IgG, peroxidase conjugated anti-rabbit IgG, rhodamine-conjugated streptavidin (Jackson Immuno Research Laboratories, Westgrove, PA, USA), anti-S100b monoclonal antibody, peroxidase-conjugated streptavidin, streptavidin immobilized on 4%-beaded agarose (Pierce), anti-haemagglutinin monoclonal antibody, and anti microtubule associated protein (MAP)2, and anti-NeuN monoclonal mouse IgGs, and protein A immobilized on Sepharose CL-4B (Sigma).
Plasmid constructs
We used single-stranded oligonucleotide containing the GSTYa ARE core sequence (Wasserman and Fahl 1997): sense, 5′ CGCGTTAGCTTGGAAATGACATTGCTAATGGTGACAAAGCAACTTTA- 3′ and antisense, 5′-GATCTAAAGTTGCTTTGTCACCATTAGCAATGTCATTTCCAAGCTAA- 3′. The dsDNA thus obtained was inserted into the MluI and BglII sites of the pGL3- promoter vector (Promega Co., Madison, WI, USA). pEF6-Nrf2 (Nrf2WT), pEF6-Nrf2 (Nrf2DN), and pEF6-Keap1 vectors Nrf2 wild-type (WT) and Nrf2 dominant-negative (DN) were generated by PCR amplification of mouse cDNA with the following oligonucleotides: sense for Nrf2WT 5′-GCCATGATGGACTTGGAGTTGCCACCGCCA- 3′, sense for Nrf2DN 5′-GCCATGGGTGAATCCCAATGTGAAAATACA- 3′, and common antisense 5′-GTTTTTCTTTGTATCTGGCTTCTTGCTTTT-3′ (Alam et al. 1999). To obtain a Keap1 expression vector, we generated Keap1cDNA using sense 5′-CCACCATGCAGCCCGAACCCAAGCTTAGC- 3′ and antisense 5′ AAGCAAATTGATCAACAAAACTGTACCTGC- 3′. The amplification products were cloned into the pEF6 vector (Invitrogen, Carlsbad, CA, USA).
Expression vectors for HA-tagged Keap1 and Keap1 deletion mutants
These constructs were obtained from Dr Akira Kobayashi of Tohoku University (Miyagi, Japan) (Hosoya et al. 2005; Kobayashi et al. 2006).
PC12h and COS7 cell cultures
Cell lines were cultured and analyzed by cell death assays as previously described (Satoh et al. 2000, 2001, 2003). For generation of stable transformants, PC12h cells were seeded onto 100-mm petri dishes in Dulbecco’s Modified Eagle’s medium supplemented with 8% fetal calf serum and 8% horse serum (Invitrogen). The cells were then transfected with pEF6-Nrf2WT or pEF6-Nrf2DN using TransFast (Promega Co.). The following day, the medium was replaced with fresh medium containing 30 lg/mL blastcidine. After 2 weeks, five colonies of each type of transfectant were isolated. We screened for high (PC12hW1B) and low (PC12hD5D)-expressing c-GCL clones by RT-PCR. We found that ARE activity was high in PC12hW1B and low in PC12hD5D cells, suggesting that the levels of c-GCL and ARE activity were well correlated. Expression of the Nrf2WT and Nrf2DN constructs was confirmed by RT-PCR.
RT-PCR
In order to examine phase 2 gene induction by CA in PC12h cells and brain lysates, RT-PCR of total RNA from cortical cultures was performed using the following primer pairs (Hosoya et al. 2005; Kobayashi et al. 2006): cyclophilin A (89bp), 5′ ACAGGTCCTGGCATCTTGTC- 3′ (sense) and 5′-AGCCACTCAGTCTTGGCAGT- 3′ (antisense); HO-1 (284 bp), 5′-CAGTCGCCTCCAGAGTTTCC- 3′ (sense) and 5′-TACAAGGAGGCCATCACC AGC-3′ (antisense); GCL-M (280 bp), 5′-CTGCTAAACTGTTCATTGTAGG- 3′ (sense) and 5′-CTATTGGGTTTTACCTGTG-3′ (antisense); GCL-C (213 bp), 5′-GTCTTCAGGTGAACATTCCAAGC- 3′ (sense) and 5′-TGTTCTTCAGGGGCTCCAGTC-3′ (antisense); and NQO1 (212 bp), 5′-GTGTACAGCATTGGCCACAC- 3′ (sense).
Primary cortical cultures and assays for two types of glutamate toxicity
Cerebrocortical neurons have been used as an in vitro system in order to investigate the cellular mechanism of neuronal death caused by glutamate. The level of expression of functional NMDA receptors is key in determining whether excitotoxicity or oxidative glutamate toxicity predominates. With increasing days in vitro (DIV), the expression level of these receptors in cortical cultures increases. In these immature cortical cultures, which do not yet express functional NMDA receptors, oxidative glutamate toxicity is dominant; and high concentrations of glutamate (2 mmol/L) induce cell death via oxidative stress (Murphy et al. 1990; Lee et al. 2003a). In light of this background information, in the present study, we prepared cerebrocortical cultures from embryonic day 17 (E17) Sprague–Dawley rats and examined them at DIV2 as an experimental system for oxidative glutamate toxicity (Murphy et al. 1990; Lee et al. 2003a). Rotenone, a complex I inhibitor of the mitochondrial electron-transport chain, also induces oxidative stress in immature cortical cultures (Murphy et al. 1990; Lee et al. 2003a).
In addition, we used mature cortical cultures (E17, DIV21) to assess excitotoxicity because functional NMDA receptors are expressed on these neurons. Exposure of mature cortical cultures to relatively mild insults, such as low concentrations of NMDA (50 lmol/L) for short durations (15 min) has been shown to cause delayed and predominantly apoptotic neuronal cell death in this system; in contrast, higher concentrations of NMDA for longer exposure times result in necrosis (Bonfoco et al. 1995; Budd et al. 2000). To induce predominantly neuronal apoptosis (rather than necrosis), we exposed cortical cultures to 50 lmol/L NMDA for 15 min [plus co-agonist 5 lmol/L glycine in 1.8 mmol/L CaCl2, nominally Mg2+-free Earle’s balanced salt solution to prevent magnesium-mediated block of NMDA receptor-operated channels] (Bonfoco et al. 1995). After exposure to NMDA, the cultures were returned to normal medium containing vehicle or CA, and then incubated for 20 h prior to analyzing them for cell survival. CA or vehicle was present 1 h before the addition of NMDA and remained throughout the experiments. To assess the ability of CA to block NMDA-induced neuronal apoptosis, we identified apoptotic neurons by double immunofluorescence labeling with anti-NeuN and anti- MAP2 to specifically label neurons and with Hoechst staining for nuclear morphology to detect apoptosis. The percentage of neurons and non-neuronal cells in these mature cortical cultures (E17, DIV21) was 33.6 ± 4.9% and 66.4 ± 4.4%, respectively, as determined by use of specific markers (Satoh et al. 2006). In the immature cortical cultures (E17, DIV2), the percentage of neuronal cells was over 90% (Satoh et al. 2001).
Immunocytochemistry
Cultures were fixed with 3% p-formaldehyde at 23°C for 20 min. After three washes in PBS, the cells were permeabilized with 0.3% Triton X-100 for 5 min. After three additional washes in PBS, the cells were incubated at 4°C overnight with primary antibodies. They were then washed three times in PBS containing 0.2% Tween 20 (PBS-T), and next incubated with secondary antibodies for 1 h at 23°C. Thereafter, the cells were again washed, and their nuclei stained with Hoechst 33,258 (5 lg/mL) or with 4′,6-diamino-2-phenylindole (1 lg/mL) for 5 min. Stained preparations were mounted and examined by epifluorescence microscopy. The following primary antibodies were used: anti-Nrf2 antibody, anti-MAP2 and anti-NeuN antibodies to identify neurons (dendrites and nuclei, respectively), and anti-S100b antibody to identify astrocytes. As secondary antibodies, we used FITC-conjugated anti-mouse IgG, rhodamine conjugated anti-mouse IgG, or rhodamine-conjugated streptavidin.
Transient transfection and measurement of luciferase activity
PC12h cells or cerebrocortical cultures (E17, DIV21) were incubated for 5 h in Earle’s balanced salt solution containing 1 lg of a plasmid DNA plus Lipofectamine 2000 (Invitrogen). Transfection efficiency was normalized to b-galactosidase activity expressed by co-transfection with pSV-b-gal (Promega Co.). For reporter gene assays, cells were transfected with 1 lg of the reporter construct [ARE(GSTYa)-luciferase] and 0.2 lg pSV-b-gal for 1 h. The cells were then washed in PBS alone and incubated in the culture medium for another 24 h with or without CA. Firefly luciferase activity and b-galactosidase activity in cell lysates were measured using a Luciferase System and b Galactosidase Enzyme Assay System, respectively (Promega Co.).
Immunoprecipitation with agarose-immobilized streptavidin or antibody
PC12h cells (CAB- or vehicle-treated) were lysed in radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail. The lysates were centrifuged at 20 000 g for 10 min at 4°C, after which the supernatants were incubated with agarose-immobilized streptavidin at 4°C for 1 h. Alternatively, for immunoprecipitation with anti-Keap1, the cell lysates were incubated with the antibody at 4°C overnight, after which protein A immobilized on Sepharose CL-4B was added and incubation continued at 4°C for 1 h. Then, the complexes were washed three times with rinse buffer [150 mmol/L NaCl, 50 mmol/L Tris–HCl (pH 7.5), 0.1% Nonidet P-40, 1 mmol/L EDTA, and 0.02% NaN3). SDS sample buffer was added, the mixture was boiled for 5 min, and the supernatants were subjected to SDS–polyacrylamide gel electrophoresis. Immunoblotting was then performed as described above.
Tanslocation of CA into the brain detected by HPLC
For experiments testing penetration of drug into the CNS, we used adult male C57BL/6 mice (Charles River Japan, Shizuoka, Japan) weighing 22–26 g. The mice were kept in cages with ad libitum access to food and water under standardized housing conditions (natural light–dark cycle, temperature of 23 ± 3°C, relative humidity of 50 ± 10%). After a 7-day adaptation to laboratory conditions, the animals were randomly assigned to each experimental group (n = 10 mice each). Four C57BL/6 mice, fastened for 18 h, were each orally administered 3 mg of CA in 0.3 mL olive oil. One and 3 h after the injection, under ether anesthesia, serum and brain were isolated for chemical analysis. CA was extracted from tissue with acetonitrile and ethanol. CA levels were obtained by HPLC as described in the legend to Fig. 2.
Measurement of brain GSH
After exsanguination via heart puncture under ether anesthesia, mouse brains were removed and quickly frozen in liquid nitrogen. We ruled out GSH as a contaminant from blood vessels by HPLC analysis of blood components in the brain lysates. Along these lines, we observed a major unknown peak (retention time 12.5 min, probably representing a degradation product of CA) in the serum of CA-injected mice; however, this peak was not detected in the brain lysates, indicating that contamination from the blood did not occur under our conditions.
The frozen brains were lysed with 1% sulfosalicylic acid on ice for 10 min, and total glutathione (GSH and GSSG) was determined as described previously (Sagara et al. 2002). Briefly, after the lysates had been incubated on ice for 10 min, the supernatants were collected after centrifugation at 20 000 g in Eppendorf microfuge tubes (Eppendorf Japan, Tokyo, Japan). Upon neutralization of each supernatant with triethanolamine, the total glutathione (reduced and oxidized) concentration was determined. Pure GSH was used to obtain the standard curve. For the determination of oxidized GSH, the method of Griffith using 2-vinylpyridine was used to deplete the reduced GSH.
Focal cerebral ischemia and reperfusion
All animal procedures were approved by the Animal Welfare Committee of the Burnham Institute for Medical Research and met both state and federal guidelines. CA (1 mg/kg) was injected intraperitoneally in a vehicle of 10% DMSO in PBS (10 lL/g body weight); controls received vehicle alone. The investigator was blinded to the treatment group. The intraluminal filament model of middle cerebral artery occlusion (MCAO)/reperfusion was utilized, as previously described (Wang et al. 1998; Gu et al. 2002, 2005; Satoh et al. 2006). Male C57BL/6 mice, aged 6–8 weeks and weighing 20–30 g, were housed in a 12-h light/12-h dark cycle and permitted food and water intake ad libitum. After an overnight fast, the animals were anesthetized with an isoflurane and 70% nitrous oxide/30% oxygen mixture delivered through a nose cone. Anesthesia was maintained for the duration of the surgical procedure, which typically lasted 10 min in our hands. To ensure successful placement of the intraluminal suture for occlusion and subsequent reperfusion, we monitored regional cerebral blood flow (rCBF) in the area of the right middle cerebral artery in all animals. A laser Doppler flowmeter (Perimed, North Royalton, OH, USA) with the probe fixed on the skull surface (3 mm lateral to midline and 2 mm posterior to the bregma), located at the distal arterial supply of the middle cerebral artery, measured rCBF, as described previously (Wang et al. 1998; Gu et al. 2002, 2005). All mice subjected to a 2-h right MCAO met the criteria that the rCBF was reduced to < 25% of the baseline during ischemia and recovered to > 50% of the baseline within 2 h after the onset of reperfusion.
Core temperature was maintained at 37 ± 1°C with a servocontrolled heating blanket. We monitored other physiological variables, including arterial blood pressure, blood gases, and glucose, and these parameters did not differ significantly between vehicle- and CA-treated mice. The right femoral artery was cannulated to monitor blood pressure and sample arterial blood gases and glucose. Blood pressure was continually recorded before ischemia, during ischemia, and at reperfusion with a blood-pressure transducer, a bridge amplifier, and a computerized data acquisition system (MacLabs 8s; ADInstruments, Castle Hill, NSW, Australia). Arterial blood gases and glucose were measured before ischemia and 15 min after reperfusion with a blood gas and glucose analyzer (Stat Profile Ultra C; Nova Biomedical, Waltham, MA, USA).
After mice underwent 2-h MCAO followed by 24-h reperfusion, they were killed and their brains sliced into 1-mm thick sections. Each slice was incubated for 10 min in a 2.5% solution of TTC at 37°C and then fixed in 4% buffered formaldehyde solution for storage. To minimize the effect of brain edema, infarct volume was determined by subtracting the volume of the contralateral noninfarcted hemisphere (left) from the ipsilateral hemisphere (right). Infarction occurred in the right MCA territory and was quantified with a computerized image analysis system (NIH image, version 1.62; Bethesda, MD, USA), as described previously (Wang et al. 1998; Gu et al. 2002, 2005).
Ethical approval
We obtained approval of the ethical committees of Nagase Co., Ltd. (Hyogo, Japan) the Burnham Institute for Medical Research, and Iwate University regarding the DNA experiments, primary cortical cultures, and animal experiments.
Statistical analysis
Each experiment was repeated at least three times in quadruplicate. Data are presented as mean ± SEM. Statistical significance was determined by ANOVA followed by a post hoc Schéffe’s test.
Results
Carnosic acid binds to GSH and protein thiol
Carnosic acid has been proposed to donate protons and electrons to oxygen and other oxygen radicals, as suggested in the case of TBHQ (Nakamura et al. 2003). Simultaneously, CA is oxidized to a quinone, as shown in Fig. 2a. Using NMR, we concluded that carbon (14) of CA is the single target of nucleophilic attack by GSH thiol. Facile oxidation of the catechol ring of CA results in conversion to its quinone derivative (Fig. 2a). Thiol-containing compounds such as GSH can induce a nucleophilic attack on the electrophilic carbon and thus form a GS-CA adduct. The resulting chemical structure of GS-CA is shown in Fig. 2a, based on the following detailed evidence: (i) 1H-NMR showed that the proton signal (6.332 ppm) assigned at C(14) of CA was lost upon adduct formation of GS-CA; (ii) 13CNMR showed that the 13C signal assigned at C(14) of CA was shifted to a lower magnetic field by GS-CA adduct formation (from 119.3 to 147.1 ppm); and (iii) 1H-NMR of GS-CA showed that nuclear overhauser effects were observed between C(15) and C(21), C(15) and C(22), C(17) and C(21), and C(21) and C(22), indicating that these hydrogen atoms were very close to each other (red double headed arrows in Fig. 2a).
Next, we assessed the rate of GS-CA adduct formation (rectangular box in Fig. 2a). As the GS-CA adduct is highly stable, oxidation from catechol to quinone has been proposed to be the rate-limiting step of these chemical reactions. Although the reaction solution contained a molar excess of CA and GSH, the GS-CA adduct appeared very slowly. Even after an 18-h incubation, only 17.5% of the CA had reacted to form the adduct. This result suggests that the conversion (oxidation) of the catechol to the quinone form of CA is a very slow process in the cell-free system.
Next, we assessed the binding of CA to other thiols, in this case to BSA. Because of its single free thiol group on cysteine 34, BSA has been used for in vitro demonstration of adduct formation with electrophilic compounds (Satoh et al. 2006). In order to monitor this reaction, we synthesized CAB, the chemical structure of which is shown in Fig. 2b. With peroxidase-conjugated streptavidin as a probe, we could detect adduct formation of BSA with CAB (Fig. 2c). For this purpose, BSA was mixed with vehicle or CAB in PBS at 23°C for 5 h, electrophoresed, and then probed with streptavidin. After exposure to CAB, a single band (68 kDa) corresponding to the complex of BSA and CAB was detected (lanes 2–8). In this manner, we demonstrated that CAB could form an adduct with BSA in a dose-dependent manner.
Additionally, we examined whether cysteine thiol was essential for formation of the complex. We reasoned that if carbon #14 of CA binds to the free cysteine of BSA, then pre-treatment with NEM, an irreversible thiol alkylating agent should abolish this binding. We found that pretreatment with NEM depressed the streptavidin signal in a dose-dependent manner, while the total protein remained virtually the same, as judged from Coomassie brilliant bluestaining of the gel (Fig. 2d). These results suggest that cysteine thiols are a target of CA.
Carnosic acid activates the Keap1/Nrf2/ARE pathway
Neuroprotective effects of electrophilic compounds are often manifest via activation of the Keap1/Nrf2 pathway (Kraft et al. 2004; Shih et al. 2005; Satoh et al. 2006). The initial reaction in this activation cascade is the binding of an electrophilic compound to specific cysteines on Keap1 protein (Zhang et al. 2004; Eggler et al. 2005; Hong et al. 2005). Such binding initiates a cellular transcription pathway leading to induction of phase 2 enzymes (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006). Therefore, we examined the domains of Keap1 (designated BTB, IVR, and DGR) to determine those essential for binding to CA (Fig. 3a). Accordingly, for co-immunoprecipitation experiments, we transfected COS7 cells with DNA expressing either HA-tagged WT Keap1 protein (HA-WT Keap1) or various deletion mutants of tagged Keap1 protein (HADBTB Keap1, HA-DIVR Keap1, and HA-DDGR Keap1; Fig. 3b). The transfected COS7 cells were then treated with vehicle or 10 lmol/L CAB and lysed. We quantified the expression level of Keap1 protein in total cell lysates with anti-HA antibody (left-hand panel, Fig. 3b). Each Keap1 mutant protein was expressed at a similar level of the predicted molecular weight (57 kDa for HA-DBTB Keap1, 53 kDa for HA-DIVR Keap1, and 37 kDa for HA-DDGR Keap1). Next, in order to detect Keap1/CAB complexes, we immunoprecipitated cell lysates with streptavidin and probed with anti-HA antibody (right-hand panel, Fig. 3b). A 73-kDa protein, corresponding to Keap1-WT bound to CAB, was observed in cells treated with CAB (lane 2). DDGRKeap1 also manifested strong binding to CAB (lane 5). In contrast, DIVR Keap1 showed very little binding to CAB (lane 4), whereas DBTB Keap1 displayed no binding at all (lane 3). Taken together, these results are consistent with the notion that maximal CA binding requires the BTB and IVR domains of Keap1 protein (Hosoya et al. 2005; Kobayashi et al. 2006). The requirement of the BTB domain for CA binding is in accord with prior results (Zhang et al. 2004; Eggler et al. 2005; Hong et al. 2005). After binding of the electrophilic quinone-form of CA to Keap1 protein, activation of the Keap1/Nrf2 pathway requires nuclear translocation of Nrf2 (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006). Thus, we next examined the intracellular distribution of Nrf2 protein in COS7 cells by immunofluorescence (Fig. 3c). Under basal conditions, Nrf2 protein was predominantly localized in the cytoplasm (upper panels), but was translocated into the nucleus upon exposure to 10 lmol/L CA (lower panels), suggesting that Nrf2 was translocated in response to the binding of CA to Keap1 protein. Similar to COS7 cells, neural PC12h cells transfected with Keap1 (non-HA-tagged) expression vector and exposed to CA formed CA/Keap1 complexes (Fig. 3d).
Figure 3.
Activation of the Keap1/Nrf2 pathway by CA in COS7 and PC12h cells. (a) Schematic representation of Keap1 deletion mutants. Deletion mutants for the major Keap1 domains (BTB, IVR, and DGR) and HA (N-terminal)-tagged wild-type Keap1(HA-WT Keap1) were constructed. Numbers represent the position of cysteine residues in Keap1. Red-colored cysteines (151, 273, and 288) were previously reported to be electrophilic targets (Zhang et al. 2004; Eggler et al. 2005; Hong et al. 2005; Hosoya et al. 2005; Kobayashi et al. 2006). (b) CA binding to BTB or IVR domains of Keap1 protein. COS-7 cells were transfected with WT Keap1 or deletion mutant expression vectors and cultured for 48 h. Then the cells were treated with CAB (10 μmol/L) for 1 h. Cell lysates were blotted with anti-HA antibody (INPUT) or immunoprecipitated with streptavidin–agarose beads and blotted by anti-HA antibody (IP). (c) Nuclear translocation of Nrf2 following treatment with CA. COS7 cells were either not treated (NT) or treated with (CA) for 48 h and then stained with anti-Nrf2 monoclonal antibody (green) and 4′,6-diamino-2-phenylindole (blue). Arrows indicate nuclei. (d) CA binding to Keap1 in PC12h cells. PC12h cells were transfected with pEF6-Keap1, a Keap1 expression vector without an HA tag, and cultured for 48 h. Cell lysates were blotted with anti-Keap1 antibody (INPUT) or immunoprecipitated with streptavidin–agarose beads and probed with anti-Keap1 antibody (IP). (e) CA induces phase 2 gene expression in PC12h cells. PC12h cells were incubated with CA (10 μmol/L) for various times, and total RNA was extracted and subjected to RT-PCR. (f) CA activates the ARE in PC12h cells. PC12h cells were transfected with an ARE(GSTYa)-luciferase reporter gene construct or empty vector plus Nrf2DN- or Keap 1-expression vector. (g) CA activates the ARE in PC12h cells. PC12h cells were transfected with an ARE(GSTYa)-luciferase reporter gene construct plus either vehicle, 10 μmol/L CA, 10 μmol/L CAB, or 20 μmol/L CAB.
Phase 2 enzymes represent an important effector activated by electrophilic-induction of the Keap1/Nrf2 pathway. In order to detect induction of the phase 2 enzymes c-GCL light chain, c-GCL heavy chain, HO-1, and NQO1, we performed RT-PCR using mRNA of PC12h cells pre-treated with 10 lmol/L CA (Fig. 3e). The cyclophilin A gene was used as an internal positive control. All of the phase 2 genes were induced by CA after 6–24 h incubation. These results are consistent with the notion that CA induced a set of phase 2 genes, possibly through activation of the Keap1/Nrf2 pathway, as previously shown for other electrophiles.
Next, we examined the involvement of the Keap1/Nrf2 pathway more precisely by studying activation of the ARE. This transcription element responds to the Keap1/Nrf2 pathway; we monitored activation of the ARE by performing luciferase reporter gene assays using PC12h cells transfected with ARE(GSTYa)-luciferase (Alam et al. 1999) in the presence or absence of an Nrf2DN or Keap1 expression vector (Fig. 3f). Based on luciferase activity, CA (10 lmol/L) stimulated expression of ARE-based transcription > 10- fold. In contrast, CA-stimulated activation of the ARE was significantly repressed by co transfection with Nrf2DN or Keap1. This series of experiments suggests that CA activates the ARE via the Keap1/Nrf2 pathway.
Biotinylated carnosic acid also significantly activated the ARE, but its potency was substantially lower than that of CA (Fig. 3g). For example, 20 lmol/L CAB activated the ARE to a much lesser extent than 10 lmol/L CA. Similarly, a greater level of CAB than CA was required to protect PC12h cells against oxidative glutamate toxicity (data not shown). Thus, it appears that several times the dose of CAB than CA is required to activate the Keap1/Nrf2 pathway.
Carnosic acid protects PC12h cells by activating the Keap1/Nrf2 pathway
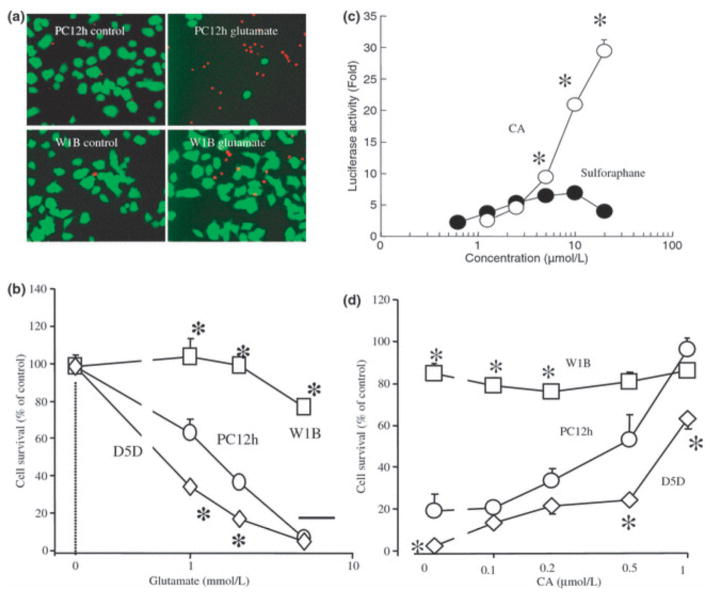
As many phase 2 enzymes are involved in the redox regulation of cells, induction of these enzymes often affords resistance to oxidative stress (Talalay 2000; Itoh et al. 2004; Padmanabhan et al. 2006). Thus, we examined whether CA could protect PC12h cells from such insults. We prepared naïve PC12h cells, Nrf2WT-expressing PC12h cells (PC12hW1B), and Nrf2DN-expressing PC12h cells (PC12hD5D). In order to examine the effects of the Keap1/Nrf2 pathway on cell survival in the face of oxidative stress, we exposed the cells to a high concentration of glutamate. In PC12h cells, high (millimolar) concentrations of glutamate induce oxidative cell death primarily by depleting intracellular GSH because of inhibition of cystine influx (Pereira and Oliveira 1997). To visualize surviving and dead cells, we stained cultures with fluorescein diacetate and propidium iodide, respectively (Fig. 4a). Additionally, cell survival was quantified by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Fig. 4b). We found that glutamate (1–10 mmol/L) induced death of PC12h cells within 20 h whereas PC12hW1B cells, over-expressing Nrf2WT, were highly resistant to this form of oxidative stress. PC12hD5D cells, expressing Nrf2DN, exhibited increased susceptibility to cell death. These results suggest not only that Nrf2 protein can protect cells but that endogenous Nrf2 is involved in the cytoprotective response against oxidative stress.
Figure 4.
Carnosic acid (CA) protects PC12h cells via Nrf2. (a) Nrf2 inhibits cell death. PC12h cells and PC12hW1B cells (expressing Nrf2WT) were exposed to 5 mmol/L glutamate for 20 h and then incubated in a solution of fluorescein diacetate (10 μmol/L) and propidium iodide (1 μg/mL). Viable cells stained with fluorescein (green) and dead cells with propidium iodide (red). (b) Nrf2DN increases cell death. PC12h cells, PC12hW1B cells, and PC12hD5D cells (expressing Nrf2DN) were incubated with various concentrations of glutamate for 20 h. Cell survival was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. *Significantly different (p < 0.01) from PC12h cells by anova. (c) Dose-dependent activation of the ARE by CA and sulforaphane. PC12h cells were tranfected with an ARE-luciferase reporter gene plasmid, and then incubated for 20 h with various concentrations of CA or sulforaphane. *Significantly different (p < 0.01) between CA and sulforaphane by anova. (d) CA protects PC12h cells in an Nrf2-dependent manner. Various concentrations of CA were added to PC12, PC12hW1B, or PC12hD5D cells 1 h prior to exposure to 5 mmol/L glutamate for 20 h. Viability was then assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. *Significantly different (p < 0.01) from PC12h cells by anova.
Next, we examined activation of the ARE by CA compared with sulforaphane, an electrophilic compound produced by plants and previously shown to potently activate the Keap1/Nrf2 pathway (Kraft et al. 2004; Hong et al. 2005). Surprisingly, on an equimolar basis CA activated the ARE to a much greater extent than sulforaphane (Fig. 4c). This difference in ARE activation appears to be critical for cell survival because sulforaphane did not protect PC12h cells against oxidative glutamate toxicity in our assays (data not shown). This result is also consistent with the notion that the ability of CA to activate the ARE coincides with its neuroprotective effect, as observed below.
We next tested the hypothesis that CA-induced neuroprotection is mediated by the Keap1/Nrf2 pathway. We found that 0.1–1 lmol/L CA dose-dependently protected PC12h cells against oxidative glutamate toxicity (Fig. 4d). However, the protective effects afforded by CA were significantly suppressed in PC12hD5D (Fig. 4d). In contrast, PC12hWIB manifested less cell death after an oxidative glutamate insult. At 2 lmol/L, CA exhibited virtually complete protection of each cell type tested, including PC12h, PC12hW1B, and PC12hD5D (data not shown). These results suggest that CA protected PC12h cells against oxidative stress in an Nrf2-dependent manner.
Carnosic acid protects cortical neurons via activation of the Keap1/Nrf2 pathway
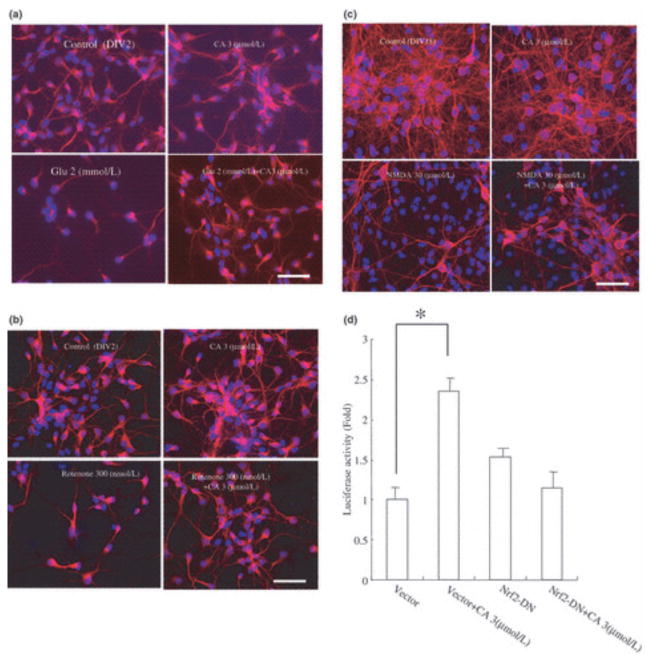
We also assessed whether CA could protect immature cortical neurons in primary culture from an oxidative glutamate insult; these cells do not yet express functional glutamate/NMDA receptors and consequently succumb to non-receptor-mediated oxidative cell death because of the inhibition of cystine influx, similar to PC12h cells. We found that CA protected these cortical neurons from exposure to glutamate (Fig. 5a) or rotenone (Fig. 5b). Glutamate (2 mmol/L) and rotenone (300 nmol/L) decreased the number of MAP2 and NeuN-positive cells to 30.8 ± 2.5% and 25.8 ± 1.9% of the control value, respectively. In contrast, CA (3 lmol/L) increased cell survival in the face of these insults to 73.8 ± 3.7% and 80.4 ± 3.4%, respectively. These results suggest that CA protects primary CNS neurons against oxidative stress.
Figure 5.
Carnosic acid (CA) protects cortical neurons via Nrf2. (a and b) CA inhibits oxidative glutamate toxicity. CA (3 μmol/L) or vehicle was added to cerebrocortical cultures (E17, DIV2) 60 min prior to exposure to glutamate or rotenone. The cultures were then incubated for 20 h and stained with anti-MAP2 and anti-NeuN (red) as well as with Hoechst dye (blue). (c) CA inhibits excitotoxicity. CA (3 μmol/L) or vehicle was added to cerebrocortical cultures (E17, DIV21) 60 min prior to exposure to NMDA (50 μmol/L for 15 min). The cultures were then incubated for 20 h and subsequently stained with anti-MAP2 and anti-NeuN (red) as well as with Hoechst dye (blue). (d) CA activates the ARE in an Nrf2-dependent manner. Cortical cultures (E17, DIV21) were transfected with ARE-luciferase reporter gene DNA (1 μg/well) and co-transfected with pEF6 or pEFNrf2DN. CA (3 μmol/L) or vehicle was then added to the cultures. After a 24-h incubation, cell lysates were used for luciferase reporter gene assays. Values are mean ± SEM; *p < 0.01 by anova.
Next, we examined the actions of CA on excitotoxic (NMDA receptor-mediated) neuronal cell death. In this case, we used more mature cerebrocortical neurons in primary culture that expressed NMDA receptors. NMDA reduced the number of viable cells to 15 ± 1.6%, whereas 3 lmol/L CA increased the survival to 36 ± 2.3% (Fig. 5c). Taken together, these results suggest that CA protected cultured primary neurons from NMDA receptor-mediated excitotoxicity as well as against non-receptor mediated oxidative stress.
To confirm that CA activated the ARE in these cortical cultures, we performed luciferase reporter gene assays. ARE mediated transcription increased 2.3-fold in the presence of 3 lmol/L CA, and this effect was abrogated by Nrf2DN, suggesting that CA activated the ARE in an Nrf2 dependent manner.
Carnosic acid accumulates both in neurons and in non-neuronal cells
In order to determine the site(s) of action of CA, we treated mixed neuronal/glial cortical cultures (E17, DIV21) with CAB, and then stained them with rhodamine-conjugated streptavidin after fixation. Concurrently, the cultures were stained with antibody against the neuronal marker MAP2 (Fig. 6a, c, and e) or the non-neuronal cell marker S100b (Fig. 6b, d, and f). At low concentrations of CAB (e.g. 3 lmol/L), neurons were not strongly labeled, whereas non-neuronal cells were strongly positive for CAB-streptavidin, indicating that CAB had accumulated in non-neuronal cells rather than in neurons. At higher concentrations of CAB (e.g. 10 lmol/L), neurons were also labeled. These results suggest that CA accumulated in non-neuronal cells at low concentrations but also in neurons at higher concentrations.
Figure 6.
Carnosic acid (CA) accumulates both in neurons and in non-neuronal cells. (a, c, and e) Cortical cultures (E17, DIV21) treated with various concentrations of CAB for 20 h were stained with anti-MAP2 monoclonal antibody to identify neurons (green) and rhodamine-conjugated streptavidin antibody to detect intracellular CAB (red). Hoechst dye 33,258 was used to label nuclei (blue). (b, d, and f) Anti-S100 monoclonal antibody was used to identify non-neuronal cells (green). Yellow and white arrows indicate accumulation of CAB in non-neuronal cells and neurons, respectively. Scale bar: 25 μm.
Carnosic acid accumulation in the brain
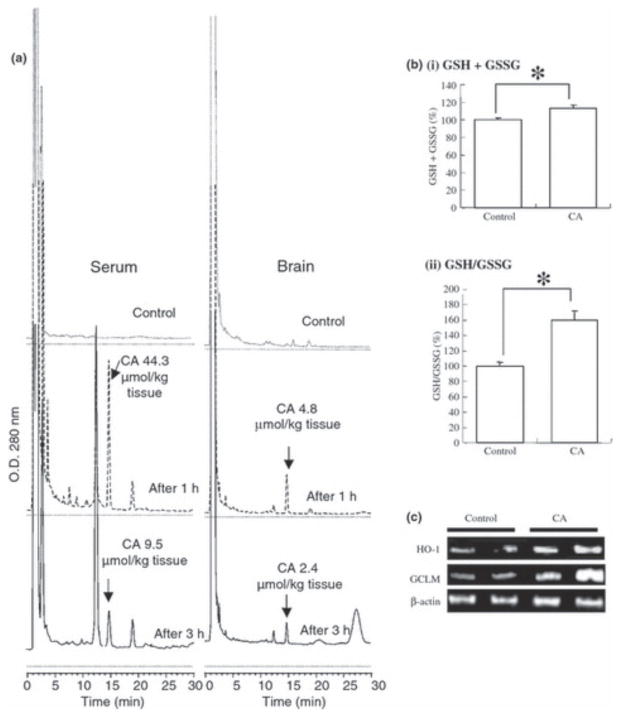
For the development of CA as a protective agent against neurodegenerative diseases, penetration into the brain is an essential requirement. To examine this aspect, we administered CA orally to mice (3 mg per 25 g mouse) and measured the level of CA (catechol-form) in serum and brain parenchyma by HPLC (Fig. 7a). Within 1 h, CA reached significant levels in the brain, suggesting that CA was able to penetrate the blood–brain barrier.
Figure 7.
Carnosic acid (CA) penetration into brain parenchyma. (a) Translocation of CA into the brain. CA was dissolved in olive oil (10 mg/mL), and 0.3 mL/mouse (representing 3 mg CA) or vehicle was then administered orally. One to 3 h later, serum and brain tissue were subjected to HPLC analysis for CA. (b) CA increased reducing equivalents of GSH in the brain. Mice were fed 0.03% CA for 1 week, and their brains were then removed, lysed, and subjected to GSH and GSSG measurement. (c) Induction of phase 2 enzymes in the brain by CA. CA was again administered orally; 8 h later, total RNA was extracted from brain tissue and subjected to RT-PCR using the indicated primers.
Next, we examined whether CA exhibited activity in the brain. Mice were allowed free access to food containing 0.03% CA for a week. Their brains were then removed, and extracts were prepared and measured for GSH and GSSG levels. Under these conditions, CA increased both total GSH + GSSG and the ratio of GSH/GSSG (Fig. 7b). During these experiments, total RNA was also extracted from the brain and subjected to RT-PCR; we found significant induction of the phase 2 enzymes HO-1 and c-GCL (Fig. 7c), consistent with the notion that CA activates the ARE, thus inducing phase 2 enzymes and increasing GSH levels. Therefore, we concluded that orally administered CA could not only reach the brain but was also effective in activating potentially neuroprotective pathways. These experiments also suggest that chemical reactions occur in vivo that convert CA, which is normally not an electrophile in its catechol or ‘pro-drug’ form, to an electrophilic (quinone) compound because CA administration resulted in induction of phase 2 enzymes in the brain. Most importantly,
Carnosic acid protects the brain from MCAO/reperfusion injury in vivo
Next, we tested whether CA could decrease the volume of cerebral infarcts after MCAO/reperfusion injury (Fig. 8). CA or vehicle (10% DMSO in PBS) was injected intraperitoneally 1 h prior to MCAO. The volume of brain infarction (corrected for possible edema) was assessed by TTC staining of coronal sections 24 h after the onset of reperfusion. MCAO induced severe brain damage in vehicle-injected mice; the infarct volume was 51.2 ± 2.4% of the ipsilateral hemisphere, similar to prior reports (Gu et al. 2002, 2005; Satoh et al. 2006). In contrast, CA significantly reduced the infarct volume (to 34.5 ± 3.6%), consistent with the notion that CA was neuroprotective in vivo.
Figure 8.
Carnosic acid (CA) protects against cerebral ischemia induced by 2-h MCAO/24-h reperfusion. (a) Representative TTC staining of brain sections after stroke in CA- versus vehicle-treated mice. CA (1 mg/kg body weight) was administrated intraperitoneally in vehicle solution (10% DMSO in PBS) 1 h prior to MCAO. After 24-h reperfusion, coronal sections, 1 mm in thickness, were prepared and stained with TTC. (b) Quantification of infarct volume by TTC staining. CA decreased infarct volume compared with vehicle-treated mice. Data represent mean ± SEM (vehicle-treated, n = 9; CA-treated, n = 9; *p < 0.05 by anova).
Discussion
In the present experiments, we found that CA is neuroprotective both in vitro and in vivo from glutamate/oxidative stress and cerebral ischemia. Concerning the chemical entity that affords this protection, the question arises if the real effector is the catechol- or quinone-type of CA? If the catechol-type CA is the effector, it must protect neurons through its antioxidant activity. In contrast, if the quinonetype is the effector, it would protect neurons by activating the Keap1/Nrf2 pathway. Although we cannot completely rule our neuroprotection via the antioxidative capacity of catechol- type CA, we believe that the main effector of protection is the quinine-type based on the experiments in the present study. This conclusion is based on the following results: (i) Quinone-type CA, but not catechol-type, activated the Keap1/Nrf2 pathway; (ii) Activation of the Keap1/Nrf2 pathway by stable expression of WT Nrf2 protected PC12h cells against oxidative stress; (iii) Inhibition of the Keap1/Nrf2 pathway by stable expression of DN Nrf2 increased oxidative stress-induced cell death and reduced protection by CA. Thus, although catechol-type CA could potentially exert antioxidant activity by donating a pair of electrons to oxygen radicals, this mechanism appears to play a limited role in the neuroprotection observed here. However, the possibility remains that other pathways may also contribute to neuroprotection.
The time course of conversion from catechol to quinone CA was rather slow in the cell-free system that we used. Then, how can the quinone form of CA be neuroprotective? One plausible explanation lies in the difference between the cell-free and cell-culture experiments employed here. As cells have many thiols, for example, on GSH and cysteine bearing proteins, quinones react rapidly with these thiols to form an adduct; removal of the quinone-type of CA by adduct formation shifts the equilibrium between free catechol and free quinone toward the quinone. Thus, conversion should be much faster in cell-based than in cell-free systems. Additionally, there are differences in thiol reactivity between GSH and various cysteine residues in proteins. For example, some proteins have potentially reactive cysteines, which can be easily converted to the thiolate anion if they are located in a motif of basic amino acids. Cysteine (151) of Keap1 is a typical example of such an active cysteine. This cysteine appears to be a sensor for electrophilic compounds. Thus, cysteine (151) of Keap1 should be more reactive with electrophilic compounds than GSH. Thus, the sustained presence of low concentrations of quinone-type CA may preferentially react with Keap1 rather than with GSH, and may thereby contribute to activation of the cell defense system. We speculate that this may be one of the reasons that CA is much less toxic than NEPP11, which itself is an electrophilic compound.
In the present series of in vitro experiments, we showed the chemical and molecular mechanisms whereby CA protected neurons against oxidative stress. CA is converted from an electrophilic precursor (or pro-electrophilic) compound to an electrophilic form, thereby activating the neuroprotective Keap1/Nrf2 pathway (Fig. 9). The chemical mechanism involves a catechol-type CA that is oxidized to a quinone type, with the carbon at position 14 [C(14)] becoming electrophilic (Fig. 2). This quinone-type CA is subject to nucleophilic attack by the cysteine thiol of GSH or various other proteins to form an adduct. Importantly, via this chemical reaction, the cysteine thiol of Keap1 protein forms a Keap1-CA adduct, resulting in release of Nrf2 protein from the Keap1/Nrf2 complex. Nrf2 can then be translocated into the nucleus, where it activates transcription of phase 2 enzymes via ARE transcriptional elements. These phase 2 enzymes improve the redox state of neurons, contributing to the antioxidant defense system.
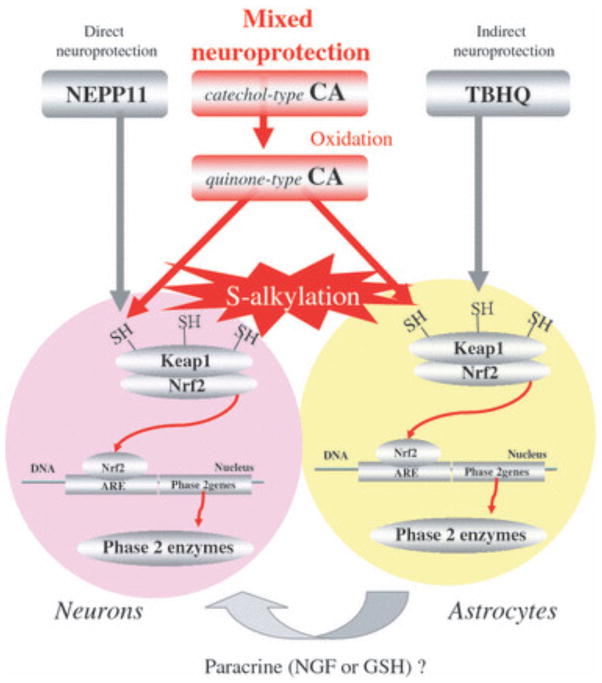
Figure 9.
Proposed mechanism of neuroprotective action of CA, NEPP11, and TBHQ. NEPP11 appears to protect neurons directly, TBHQ via effects on astrocytes, which in turn may release survival or neurotrophic factors (Ahlgren-Beckendorf et al. 1999; Kosaka and Yokoi 2003), and CA by a ‘mixed’ type of protection mediated by actions on both neurons and astrocyt
Although electrophilic compounds can possess a variety of chemical structures, the enone-type (e.g. NEPP11) and catechol-type (e.g. CA and TBHQ) represent major groups. From our prior findings and those of other groups (Kraft et al. 2004; Shih et al. 2005; Satoh et al. 2006), we have proposed that the cellular distribution in the brain of enoneand quinine-type electrophilic compounds may be different. Enone-type electrophiles, including dienones such as NEPP11, appear to act preferentially in neurons based on our earlier findings that NEPP11 accumulates in neurons as opposed to astrocytes, and consequently induces neuronal HO-1 (Satoh et al. 2006). In contrast, catechol (hydroquinone)- type electrophiles, including TBHQ, preferentially act on astrocytes, as evidenced by the fact that TBHQ activates the ARE in astrocytes and not in neurons (Kraft et al. 2004; Shih et al. 2005). In light of these findings, we proposed that the enone-type electrophile NEPP11 exerts a direct neuroprotective effect while the catechol (hydroquinone)-type TBHQ exerts a paracrine-type of neuroprotective action (Satoh and Lipton 2007). Interestingly, CA appears to afford both direct and paracrine (hence, ‘mixed’) neuroprotective effects, as discussed below.
In the immunohistochemical experiments we conducted using CAB, this labeled form of CA accumulated in nonneuronal cells at low concentrations (3 lmol/L) and in neurons at higher concentrations (10 lmol/L). However, ARE activation and consequent neuroprotection afforded by CAB were much weaker than by CA. This difference may have been because of the lower permeability of cell membranes to CAB or lower binding affinity of CAB for Keap1 protein. In light of this difference, we estimate that < 10 lmol/L CA accumulates in both non-neuronal and neuronal cells. Thus, it is reasonable to propose that CA exerts actions on both cell types, similar to the astrocyte mediated neuroprotective action previously seen for TBHQ and the neuronal-mediated neuroprotection previously observed for NEPP11.
A critical advantage of electrophilies such as NEPP11 and CA as neuroprotective compounds is their transcriptional activation of antioxidant phase 2 enzymes. This type of neuroprotection could be of potential benefit in chronic neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases. Previously, NEPP11 was shown to protect neurons via electrophilic chemical reaction (Satoh et al. 2006; Satoh and Lipton 2007). However, NEPP11 has a serious problem as a potential therapeutic agent because systemic administration can result in reaction with thiol substrates prior to reaching the intended target in the brain. It would be far better to have a pro-electrophilic compound that remains non-reactive until it is converted to an electrophile by the oxidative insult at the pathological site of its intended action. Such as drug would represent what has been termed a ‘pathologically activated therapeutic’ (Lipton 2004), and we believe that CA may constitute such an agent. We feel that CA has two clear-cut advantages over NEPP11 and similar compounds: (i) low potential toxicity because of conversion of the pro-drug to an electrophile by the oxidative insult at the pathological site and (ii) effective penetrance into brain tissue. These characteristics emanate from the chemical structure of CA. Accordingly, when the catechol is oxidized to a quinone, it becomes more hydrophobic and will tend to stay in the injured tissue.
Moreover, CA is less toxic than NEPP11 in vitro. CA manifests a large therapeutic index; i.e. 100 lmol/L CA is not toxic to neural cells, whereas as little as 1–3 lmol/L is neuroprotective. The lower toxicity of CA may also be accounted for by the fact that, unlike NEPP11, CA only becomes electrophilic at or near the site of injury. Oxidative stress plays a critical role in the progression of neurodegenerative disorders (Coyle and Puttfarcken 1993). We demonstrate here that this pathological level of oxidation can be used to activate pro-electrophilic compounds at the target site to provide neuroprotection where it is needed. Thus, this approach represents a novel strategy against neurodegenerative disorders by activating neuroprotective pro-electrophilic drugs via the very pathological activity that they are meant to combat. Moreover, to our knowledge, this study is the first demonstration of a natural product protecting neurons by thiol (S-) alkylation, resulting in activation of the Keap1/Nrf2 pathway. Thus, naturally occurring proelectrophilic compounds produced by plants are candidate neuroprotective agents for the treatment of neurodegenerative diseases.
Acknowledgments
The authors thank Professor Yasuo Nagaoka (Kansai University) and Professor Toshio Yokoi (Kobe Gakuin University) for technical suggestions and Dr Larry D. Frye for editorial help with the manuscript. This work was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan, University Education Internationalization Promotion Program (2007), those from the JSPS, Joint Project of Japan–U.S. (2004–2006) and Grand-in-aid for Scientific Research (C) (2007– 2009), and those by JST, Cooperative Program Feasibility Study for Science and Technology Incubation Programs in Advanced Regions (2006–2007) and Research for Promoting Technological seeds (2007–2008) (to TS) and TS was a Visiting Professor in Center for Neuroscience, Aging and Stem Cell Research, The Burnham Institute for Medical Research (2003–2004 and 2007). This work is also supported in part by NIH grants P01 HD29587, R01 NS43242, and R01 EY05477 (to SAL), and. SAL was a Senior Scholar in Aging Research of the Ellison Medical Foundation.
References
- 1.Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H: quinone oxidoreductase and glutathione-S-transferase in primary cultures of rat neurons and glias: role of the antioxidant/electrophile responsive element. Glia. 1999;15:131–142. [PubMed] [Google Scholar]
- 2.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a cap’n’collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 3.Ankacrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 4.Aruoma OI, Halliwell B, Aeschbach R, Loligers J. Antioxidant and pro oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 5.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-asparate or nitric oxide/superoxide in cortical cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budd SL, Tenneti L, Lishnak T, Lipton SA. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc Natl Acad Sci USA. 2000;97:6161–6166. doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi DW. Excitotoxic cell death. J Neurosci. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 9.Coyle JT, Puttfarcken P. Oxidative stress, glutamate and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 10.Dargusch R, Schubert D. Specificity of resistance to oxidative stress. J Neurochem. 2002;81:1394–1400. doi: 10.1046/j.1471-4159.2002.00950.x. [DOI] [PubMed] [Google Scholar]
- 11.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995;15:6377–6388. doi: 10.1523/JNEUROSCI.15-10-06377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 14.Gu Z, Cui J, Fridman R, Mobashery S, Strongin AY, Lipton SA. A highly specific inhibitor of metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25:6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- 16.Hong F, Freeman ML, Lieber DC. Identification of sensor cysteines in human Keap1 modified by the cancer chemopreventive agent sulforaphane. Chem Res Toxicol. 2005;18:1917– 1926. doi: 10.1021/tx0502138. [DOI] [PubMed] [Google Scholar]
- 17.Hosoya T, Maruyama A, Kang M, Kawatani Y, Shibata T, Uchida K, Itoh K, Yamamoto M. Differential responses of the Keap1-Nrf2 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;29:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 18.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosaka K, Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol Pharm Bull. 2003;26:1620–1622. doi: 10.1248/bpb.26.1620. [DOI] [PubMed] [Google Scholar]
- 21.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2- related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JM, Calkins MJ, Chan K, Kan YW, Johonson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003a;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2- related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003b;278:37948– 37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 24.Lipton SA. Concepts: turning down but not off – neuroprotection requires a paradigm shift in drug development. Nature. 2004;428:473. [Google Scholar]
- 25.Martin D, Rojo AI, Salinas M, Diaz R, Gallardo G, Alam J, De Galarreta CM, Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 26.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Cole JT. Glutamate toxicity in a neuronal cell line involves inhibition of cysteine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 27.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 28.Nakamura Y, Kumagai T, Yoshida C, Naito Y, Miyamoto M, Ohigashi H, Osawa T, Uchida K. Pivotal role of electrophilicity in glutathione S-transferase induction by tertbutylhydroquinone. Biochemistry. 2003;15:4300–4309. doi: 10.1021/bi0340090. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi S. Synaptic mechanisms of cerebellar cortical network. Trends Neurosci. 2005;28:93–100. doi: 10.1016/j.tins.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Pereira CM, Oliveira CR. Glutamate toxicity on a PC12 cell line involves glutathione (GSH) depletion and oxidative stress. Free Radic Biol Med. 1997;23:637–647. doi: 10.1016/s0891-5849(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 32.Rau O, Wurglics M, Paulke A, Zitzkowski J, Meindl N, Bock A, Dingermann T, Abdel-Tawab M, Schubert-Zsilavecz M. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006;72:881–887. doi: 10.1055/s-2006-946680. [DOI] [PubMed] [Google Scholar]
- 33.Sagara Y, Ishige K, Tsai C, Maher P. Tyrphostins protect neuronal cells from oxidative stress. J Biol Chem. 2002;277:36204– 36215. doi: 10.1074/jbc.M203895200. [DOI] [PubMed] [Google Scholar]
- 34.Satoh T, Lipton SA. Redox regulation of neuronal survival by electrophilic compounds. Trends Neurosci. 2007;30:38–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Satoh T, Furuta K, Tomokiyo K, et al. Facilitatory roles of novel compounds designed from cyclopentenone prostaglandins on neurite outgrowth-promoting activities of nerve growth factor. J Neurochem. 2000;75:1092–1102. doi: 10.1046/j.1471-4159.2000.0751092.x. [DOI] [PubMed] [Google Scholar]
- 36.Satoh T, Furuta K, Tomokiyo K, Namura S, Nakatsuka D, Sugie Y, Ishikawa Y, Hatanaka H, Suzuki M, Watanabe Y. Neurotrophic actions of novel compounds designed from cyclopentenone prostaglandins. J Neurochem. 2001;77:50–62. doi: 10.1046/j.1471-4159.2001.t01-1-00229.x. [DOI] [PubMed] [Google Scholar]
- 37.Satoh T, Baba M, Nakatsuka D, Ishikawa Y, Aburatani H, Furuta K, Ishikawa T, Hatanaka H, Suzuki M, Watanabe Y. Role of heme oxygenase-1 protein in the neuroprotective effects by cyclopentenone prostaglandin derivatives as a sustained phase of neuronal survival promoting mechanism under oxidative stress. Eur J Neurosci. 2003;17:2249–2255. doi: 10.1046/j.1460-9568.2003.02688.x. [DOI] [PubMed] [Google Scholar]
- 38.Satoh T, Okamoto S, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II Inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 41.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–136. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 42.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazawa K, Kihara T, Shen H, Shimmoyo Y, Niidome T, Sugimoto H. Distinct mechanisms underline distinct polyphenolinduced neuroprotection. FEBS Lett. 2006;580:6623–6628. doi: 10.1016/j.febslet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]