Abstract
Objectives
The HIV NNRTI rilpivirine is being evaluated as a possible agent for HIV pre-exposure prophylaxis. We have recently shown that the NNRTI efavirenz may impair endothelial function assessed as flow-mediated dilation (FMD), but whether this impairment is also found with rilpivirine is unknown. We sought to compare cardiovascular risk profiles between efavirenz and rilpivirine in healthy volunteers.
Methods
We performed a prospective, randomized, open-label trial in 40 HIV-uninfected healthy volunteers who were randomized 1: 1 to either efavirenz or rilpivirine. Vascular indices, metabolic parameters, inflammatory biomarkers and oxidative stress were measured before and after 4 weeks of treatment. This study is registered at ClinicalTrials.gov (NCT01585038).
Results
There were no significant differences in 4 week mean (SD) changes in FMD between efavirenz and rilpivirine [0.089 (3.65)% versus 0.63 (2.42)%; P = 0.77]. There were also no significant differences in 4 week changes in high-sensitivity C-reactive protein, IL-6, soluble vascular cell adhesion molecule-1, HDL-cholesterol, triglycerides or homeostasis model assessment–insulin resistance. However, efavirenz led to significant increases in total cholesterol [19.39 (23.9) versus −5.78 (16.5) mg/dL; P < 0.001], LDL-cholesterol [13.29 (19.5) versus −2.24 (13.4) mg/dL; P = 0.009] and F2-isoprostanes [92.7 (178.6) versus −101.4 (215.7) pg/mL; P = 0.019] compared with rilpivirine. Two participants from each study group discontinued prematurely for adverse events.
Conclusions
There were no significant differences in the changes in endothelial function over 1 month between the efavirenz and rilpivirine groups, although efavirenz had worse lipid changes compared with rilpivirine. Longer-term studies are required for confirmation.
Introduction
Given that the NNRTI rilpivirine is being developed as a pre-exposure prophylaxis agent,1 it is critically important to determine rilpivirine's cardiovascular safety profile. We and others have recently found that the NNRTI efavirenz may lead to dyslipidemia,2 increased oxidative stress3 and endothelial dysfunction.4 However, we did not find changes in similar parameters with the use of the NNRTI etravirine over 1 month in HIV-uninfected, healthy volunteers.5 This suggests that individual drugs within an antiretroviral class may lead to different cardiovascular risk profiles. Since rilpivirine is more structurally related to etravirine than to efavirenz, we hypothesized that rilpivirine would have a superior cardiovascular risk profile compared with efavirenz. Therefore, we performed a randomized trial in healthy individuals comparing the short-term effects on cardiovascular risk between rilpivirine and efavirenz.
Methods
Study design
We performed an open-label, randomized, controlled, single-centre trial from 2012 to 2014 at the Indiana University Medical Center in which HIV-uninfected, healthy volunteers were randomized 1: 1 to either 25 mg of rilpivirine daily or 600 mg of efavirenz daily for 4 weeks. Study procedures were performed at study entry and at week 4 of treatment. The Indiana University Institutional Review Board approved this trial. All participants provided written, informed consent. This study is registered at ClinicalTrials.gov (NCT01585038).
Study participants
Major eligibility criteria included age ≥18 years; being negative for HIV-1 at screening; being free of known cardiovascular disease; having a screening serum glucose <200 mg/dL, serum total cholesterol <260 mg/dL, negative hepatitis C antibody or estimated creatinine clearance >55 mL/min; having a screening systolic blood pressure <160 mmHg; and no history of any tobacco use within 45 days of screening.
Study procedures and outcomes
All study procedures were performed after a minimum 8 h fast. All circulating biomarkers were measured in a batch at the completion of the trial from serum samples stored at −80°C. Circulating markers of inflammation [IL-6, high sensitivity C-reactive protein (hsCRP) and soluble vascular cell adhesion molecule-1 (sVCAM-1)] and metabolism (insulin, glucose and lipid fractions) were measured using standard ELISA kits at the University of Vermont Laboratory for Clinical Biochemistry Research. The homeostasis model assessment–insulin resistance (HOMA-IR) was used to estimate insulin resistance.6 Circulating serum levels of 8-iso-prostaglandin F2α (F2-isoprostane) were measured using a standard LC-MS/MS analytical method.7
Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NTGMD) of the brachial artery to assess, respectively, endothelium-dependent and endothelium-independent conduit artery function were performed according to recommended guidelines.8 In addition, the reactive hyperemic velocity time integral (RHVTI) and FMD/RHVTI were calculated as measures of microvascular function. The images were read by a single, blinded investigator (S. K. G.) using AccessPoint 2011 (version 8.2) software (Freeland Systems, Westminster, CO, USA).
Statistical analysis
As we were only interested in determining if rilpivirine would result in improved FMD compared with efavirenz, we adopted a one-sided hypothesis for sample size estimation. We assumed that the 4 week change in FMD would be −4.0% with a variance of 25.0 in the efavirenz arm.4,9 As we expected that the changes with rilpivirine would be similar to those with etravirine, we assumed that there would be 0% change with a variance of 14.0 in the rilpivirine arm.5 Allowing for a 20% dropout rate, we enrolled 20 participants per arm. The computerized randomization list was generated by the study statistician and kept by the study pharmacist, who then provided rilpivirine or efavirenz.
Analyses were performed as ITT, but without corrections for multiple testing. Non-normal distributions were log-transformed. Continuous outcomes were analysed with Student's t-tests on raw or transformed data and categorical outcomes were analysed with Fisher's exact test due to low cell counts, which will be referred to as unadjusted analyses hereafter. Multivariable linear regressions were used for the vascular parameters to adjust for additional covariates. One-sided Student's t-test was used for unadjusted FMD analyses, while all the other tests were two-sided. Spearman and Pearson correlations were calculated, as appropriate, to evaluate the associations between continuous variables. We used one-sample t-tests to determine whether within-group changes were statistically significantly different from 0. P values <0.05 were considered statistically significant. All analyses were performed in SAS 9.3 (SAS Inc., Cary, NC, USA).
Results
The dispositions of the participants are shown in Figure S1 (available as Supplementary data at JAC Online). The baseline characteristics of the participants in each arm were well balanced (Table S1).
Changes in vascular endpoints
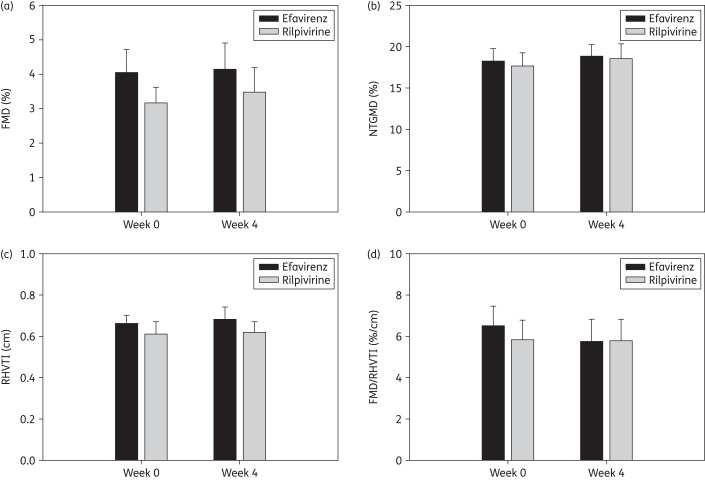
There were no statistically significant differences (all P > 0.3) in the 4 week mean (SD) changes in FMD [0.63 (2.42)% versus 0.089 (3.65)%], NTGMD [1.59 (5.69)% versus 0.45 (3.37)%], RHVTI [0.011 (0.28) m versus 0.020 (0.25) m] or FMD/RHVTI [0.54 (5.55) %/cm versus −0.67 (5.61) %/cm] between rilpivirine and efavirenz, respectively, either in unadjusted analyses or in models adjusting for baseline diameter at week 0, age, sex and race (Figure 1). There were no significant changes (all P>0.25) within groups for any of these parameters (data not shown).
Figure 1.
Vascular indices at week 0 (entry) and at week 4. There were no significant differences between groups in FMD, NTGMD, RHVTI or FMD/RHVTI in adjusted models. Vertical bars represent absolute values at each timepoint and upper whiskers represent standard errors.
Changes in F2-isoprostane and bilirubin levels
The 4 week changes in F2-isoprostane levels are shown in Table 1. Changes in F2-isoprostane levels decreased significantly in the rilpivirine group compared with the efavirenz group (P = 0.019), although there were no significant within-group changes.
Table 1.
Comparison of 4 week changes in oxidative stress (F2-isoprostanes), systemic inflammation (hsCRP, IL-6 and sVCAM-1), lipid fractions and insulin resistance between rilpivirine and efavirenz
| Biomarker | Study visit | Rilpivirine | Efavirenz | Mean difference | P value |
|---|---|---|---|---|---|
| F2-isoprostanes (pg/mL)a | entry | 164.2 (184.9) (n = 15) | 92.2 (71.8) (n = 15) | 72.0 (−35.5, 179.6) | 0.18 |
| week 4 | 83.3 (93.3) (n = 12) | 178.4 (149.2) (n = 15) | −95.0 (−196.8, 6.8) | 0.07 | |
| change from entry to week 4 | −101.4 (215.7) (n = 12) | 92.7 (178.6) (n = 14) | −194.1 (−353.7, −34.6) | 0.02 | |
| hsCRP (mg/L) | entry | 2.59 (4.88) | 2.75 (3.07) | −0.16 (−2.93, 2.60) | 0.43b |
| week 4 | 2.35 (3.37) | 3.49 (3.19) | −1.14 (−3.43, 1.16) | 0.20b | |
| change from entry to week 4 | −0.41 (6.01)c | 0.80 (2.47)c | −1.21 (−4.71, 2.30) | 0.35b | |
| IL-6 (pg/mL) | entry | 0.58 (5.37) | 2.24 (1.36) | −0.52 (−1.47, 0.43) | 0.14b |
| week 4 | 1.77 (1.88) | 4.23 (6.05) | −2.45 (−5.89, 0.98) | 0.11b | |
| change from entry to week 4 | 0.09 (2.51)c | 1.93 (5.63)c | −1.84 (−5.14, 1.46) | 0.21b | |
| sVCAM-1 (pg/mL) | entry | 580.33 (139.50) | 632.25 (133.20) | −51.92 (−139.20, 35.39) | 0.24 |
| week 4 | 541.67 (125.13) | 604.32 (182.44) | −62.65 (−168.6, 43.32) | 0.24 | |
| change from entry to week 4 | −20.92 (68.95)c | −27.62 (125.20)c | 6.70 (−62.49, 75.89) | 0.41 | |
| Total cholesterol (mg/dL) | entry | 165.20 (30.80) | 173.45 (28.81) | −8.25 (−27.34, 10.84) | 0.39 |
| week 4 | 157.00 (26.44) | 194.56 (28.72) | −37.56 (−56.25, −18.86) | <0.01 | |
| change from entry to week 4 | −5.78 (16.45) | 19.39 (23.90)c | −25.17 (−39.06, −11.27) | <0.01 | |
| LDL-C (mg/dL) | entry | 89.12 (25.38) | 97.53 (24.37) | −8.42 (−24.34, 7.51) | 0.29 |
| week 4 | 84.17 (23.57) | 110.54 (23.47) | −26.38 (−42.31, −10.44) | <0.01 | |
| change from entry to week 4 | −2.24 (13.43) | 13.29 (19.50)c | −15.54 (−26.88, −4.20) | <0.01 | |
| HDL-C (mg/dL) | entry | 60.05 (17.37) | 55.85 (17.44) | 4.20 (−6.94, 15.34) | 0.45 |
| week 4 | 59.00 (14.29) | 59.56 (18.52) | −0.56 (−11.76, 10.65) | 0.92 | |
| change from entry to week 4 | −1.28 (9.53) | 2.17 (10.35) | −3.44 (−10.18, 3.30) | 0.31 | |
| Total cholesterol/HDL-C | entry | 2.94 (0.92) | 3.35 (0.94) | −0.40 (−1.00, 0.19) | 0.18 |
| week 4 | 2.79 (0.73) | 3.57 (1.19) | −0.78 (−1.45, −0.11) | 0.02 | |
| change from entry to week 4 | −0.05 (0.49) | 0.29 (0.48)c | −0.35 (−0.67, −0.02) | 0.04 | |
| Non-HDL-C (mg/dL) | entry | 105.15 (28.14) | 117.60 (27.55) | −12.45 (−30.28, 5.38) | 0.17 |
| week 4 | 98.00 (25.58) | 135.00 (31.52) | −37.00 (−56.44, −17.56) | <0.01 | |
| change from entry to week 4 | −4.50 (17.96) | 17.22 (18.87)c | −21.72 (−34.20, −9.24) | <0.01 | |
| Triglycerides (mg/dL) | entry | 80.20 (39.13) | 101.20 (52.01) | −21.00 (−50.46, 8.46) | 0.16 |
| week 4 | 70.22 (31.32) | 122.33 (80.48) | −52.11 (−94.32, −9.90) | 0.02 | |
| change from entry to week 4 | −10.11 (31.79)c | 19.00 (62.11)c | −29.11 (−62.96, 4.74) | 0.11b | |
| HOMA-IR | entry | 1.47 (1.21) | 3.40 (5.37) | −1.93 (−4.50, 0.63) | 0.19b |
| week 4 | 2.02 (2.23) | 3.22 (4.49) | −1.19 (−3.63, 1.24) | 0.35b | |
| change from entry to week 4 | 0.60 (1.62) | −0.43 (1.50) | 1.03 (−0.03, 2.08) | 0.06b |
Data are presented as mean (SD) or as mean (95% CI). All P values are two-sided for the variables listed in this table.
aDue to incomplete assay reactions or to specimen hemolysis, the evaluable dataset with paired entry and week samples included only 14 in the efavirenz arm and 12 in the rilpivirine arm.
bP values for variables with non-normal distributions that were log-transformed prior to significance testing.
cP value for within-group change is <0.05.
In a post hoc analysis, we determined that there was a significant reduction in 4 week mean (SD) changes in total bilirubin levels in those receiving efavirenz [−0.147 (0.191) mg/dL; within-group change P = 0.006] versus those receiving rilpivirine [0.022 (0.213) mg/dL; within-group change P = 0.66] with a mean (95% CI) difference of −0.169 (−0.309, −0.030) mg/dL (P = 0.019), although there were no significant correlations (all P > 0.1) between changes in total bilirubin levels and changes in F2-isoprostane levels (data not shown).
Changes in inflammation biomarkers
Although significant within-group changes were noted for each inflammation parameter in each group, the 4 week changes in hsCRP, IL-6 and sVCAM-1 were not statistically different between the two study arms (Table 1).
Changes in metabolic markers
The levels of total cholesterol (P = 0.0008), LDL-cholesterol (LDL-C) (P = 0.0087), total cholesterol/HDL-cholesterol (HDL-C) ratio (P = 0.04) and non-HDL-C fractions (P < 0.01), but not levels of HDL-C or triglycerides, decreased significantly in the rilpivirine group compared with the efavirenz group (Table 1). There was a non-significant trend towards an increase in HOMA-IR in the rilpivirine group compared with the efavirenz group (P = 0.056). Within the efavirenz group, there were significant changes in total cholesterol, LDL-C and triglycerides; within the rilpivirine group, only triglycerides changed significantly.
Safety
Two participants were discontinued from the study in each arm due to study drug-related toxicities. In the efavirenz group, there was one discontinuation for Grade 2 rash and another for Grade 2 nausea and vomiting. In the rilpivirine group, there was one discontinuation for Grade 2 insomnia and another for Grade 2 rash. There were no Grade 3 or 4 toxicities and no serious adverse events. We did not find appreciable changes in serum creatinine in either the rilpivirine [0.008 (0.101) mg/dL; P = 0.75] or the efavirenz [0.005 (0.046) mg/dL; P = 0.68] group, with no significant difference between the two groups (P = 0.91).
Discussion
In this randomized trial, we did not find significant between-group or within-group changes in vascular function indices over 1 month in healthy individuals. We4 and others9 have previously found that efavirenz led to reductions in endothelial function in HIV-infected patients. These conflicting results could be due to an unknown interaction between HIV and efavirenz, or perhaps the combination of efavirenz with nucleoside inhibitors is required for the development of vascular impairment to emerge. Thus, both drugs appear to have inherently benign short-term endothelial effects.
However, we did find that efavirenz led to significant increases in oxidative stress (measured as circulating levels of F2-isoprostanes), total cholesterol and LDL-C compared with rilpivirine. These results corroborate previous data noting an association between efavirenz and higher F2-isoprostane3,10,11 and cholesterol levels.12 However, we did not find differences in the changes between the two study arms in three key inflammatory biomarkers, namely hsCRP, IL-6 and sVCAM-1, that have been linked to cardiovascular disease in HIV.13
This study had several strengths, including the randomized design and the diverse study population, which would increase generalizability. We acknowledge that the brief study duration might preclude finding clinically important changes in several of the studied parameters. We did not conduct a double-blinded study; the need for using rilpivirine with food and efavirenz without food would require double dummy placebos, which was considered overly complex for the purposes of this study. Although the sample size was relatively small, reductions in endothelial function with efavirenz have been found in smaller studies of similar duration.4,9
In summary, we did not detect significant short-term differences in vascular parameters, in systemic inflammation, or in tolerability between efavirenz and rilpivirine over 4 weeks in HIV-uninfected healthy volunteers. Additional study is required to determine the long-term effects of rilpivirine when given to HIV-uninfected persons as pre-exposure prophylaxis.
Funding
Drug and funding support were provided by Janssen Scientific Affairs, LLC for this investigator-initiated study (TMC278HIV4002). The sponsor had no role in the design or implementation of this work and had no input on the decision to publish its findings.
In addition, this project was supported, in part, by the Indiana Clinical and Translational Sciences Institute funded, in part, by Grant Number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Transparency declarations
S. K. G. has received unrestricted research grants from Janssen Pharmaceuticals, Gilead Sciences and Merck & Co., has received consultancy/advisory fees from ICON Clinical Research and Gilead Sciences, and has received travel support to report research findings at scientific conferences from Bristol-Myers Squibb and Gilead Sciences. All other authors: none to declare.
Author contributions
S. K. G. obtained the funding for this work, S. K. G. and Z. L. designed the trial, J. E. S. and Z. L. performed the statistical analyses, and S. K. G., J. E. S., L. M. K. and Z. L. interpreted the data and prepared the manuscript.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary data
Acknowledgements
We first thank the study participants for their generous time and effort in completing this study. We also thank Beth Zwickl and Paula Johnson for study coordination, Jule Scott for performance of the vascular imaging studies, Sheila Ellinger for regulatory assistance, Cheryl Denski for data management and Lauren Kennedy for data entry. We also are grateful for the performance of the metabolic and inflammation biomarker assays by Ms Elaine Cornell. Finally, we appreciate the efforts by Dr Allon Friedman in serving as the independent data and safety monitor for this trial.
References
- 1.Jackson AGA, Else LJ, Mesquita PMM et al. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther 2014; 96: 314–23. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Mi D, Moe SM et al. Effects of switching from efavirenz to raltegravir on endothelial function, bone mineral metabolism, inflammation, and renal function: a randomized, controlled trial. J Acquir Immun Defic Syndr 2013; 64: 279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulgan T, Morrow J, D'Aquila RT et al. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis 2003; 37: 1711–7. [DOI] [PubMed] [Google Scholar]
- 4.Gupta SK, Shen C, Moe SM et al. Worsening endothelial function with efavirenz compared to protease inhibitors: a 12-month prospective study. PLoS One 2012; 7: e45716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta SK, Mi D, Liu Z et al. Endothelial, inflammatory, coagulation, metabolic effects and safety of etravirine in HIV-uninfected volunteers. AIDS Patient Care STDS 2011; 25: 327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenski AS et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9. [DOI] [PubMed] [Google Scholar]
- 7.Kamendulis LM, Wu Q, Sandusky GE et al. Perfluorooctanoic acid exposure triggers oxidative stress in the mouse pancreas. Toxicology Reports 2014; 1: 513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corretti MC, Anderson TJ, Benjamin EJ et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39: 257–65. [DOI] [PubMed] [Google Scholar]
- 9.Kristoffersen US, Wiinberg N, Petersen CL et al. Reduction in coronary and peripheral vasomotor function in patients with HIV after initiation of antiretroviral therapy: a longitudinal study with positron emission tomography and flow-mediated dilation. Nucl Med Commun 2010; 31: 874–80. [DOI] [PubMed] [Google Scholar]
- 10.Redhage LA, Shintani A, Haas DW et al. Clinical factors associated with plasma F2-isoprostane levels in HIV-infected adults. HIV Clin Trials 2009; 10: 181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glesby MJ, Hoover DR, Raiszadeh F et al. Oxidant stress in HIV-infected women from the Women's Interagency HIV Study. Antivir Ther 2009; 14: 763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen CJ, Andrade-Villanueva J, Clotet B et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 2011; 378: 229–37. [DOI] [PubMed] [Google Scholar]
- 13.Duprez DA, Neuhaus J, Kuller LH et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7: e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.