Abstract
Background
Regional analgesia is more effective than conventional analgesia for controlling pain and may facilitate rehabilitation after large joint replacement in the short term. It remains unclear if regional anaesthesia improves functional outcomes after joint replacement beyond three months after surgery.
Objectives
To assess the effects of regional anaesthesia and analgesia on long‐term functional outcomes 3, 6 and 12 months after elective major joint (knee, shoulder and hip) replacement surgery.
Search methods
We performed an electronic search of several databases (CENTRAL, MEDLINE, EMBASE, CINAHL), and handsearched reference lists and conference abstracts. We updated our search in June 2015.
Selection criteria
We included randomized controlled trials (RCTs) comparing regional analgesia versus conventional analgesia in patients undergoing total shoulder, hip or knee replacement. We included studies that reported a functional outcome with a follow‐up of at least three months after surgery.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We contacted study authors for additional information.
Main results
We included six studies with 350 participants followed for at least three months. All of these studies enrolled participants undergoing total knee replacement. Studies were at least partially blinded. Three studies had a high risk of performance bias and one a high risk of attrition bias, but the risk of bias was otherwise unclear or low.
Only one study assessed joint function using a global score. Due to heterogeneity in outcome and reporting, we could only pool three out of six RCTs, with range of motion assessed at three months after surgery used as a surrogate for joint function. All studies had a high risk of detection bias. Using the random‐effects model, there was no statistically significant difference between the experimental and control groups (mean difference 3.99 degrees, 95% confidence interval (CI) − 2.23 to 10.21; P value = 0.21, 3 studies, 140 participants, very low quality evidence).
We did not perform further analyses because immediate adverse effects were not part of the explicit outcomes of any of these typically small studies, and long‐term adverse events after regional anaesthesia are rare.
None of the included studies elicited or reported long‐term adverse effects like persistent nerve damage.
Authors' conclusions
More high‐quality studies are needed to establish the effects of regional analgesia on function after major joint replacement, as well as on the risk of adverse events (falls).
Plain language summary
Pain control using local anaesthetics to improve surgical results after shoulder, hip and knee replacement surgery
Review question
Doctors can use regional analgesia (injection of local anaesthetics near the nerves or the surgical site) rather than conventional pain control after surgery. Does this choice improve long‐term function after elective major joint (knee, shoulder and hip) replacement? We conducted this systematic review to explore controversy about the use of regional analgesia amidst efforts to limit healthcare costs and demonstrate value for interventions.
Background
People undergo knee, shoulder and hip joint replacement to improve their mobility, pain and function. Local anaesthetics are injected near the nerves or the surgical site to decrease pain after surgery. Regional analgesia has been shown to lead to better surgical results (joint range of motion, strength, ability to walk, climb stairs, etc.) in the days immediately after surgery. It is unclear whether this effect lasts beyond three months.
Study characteristics
We searched electronic databases and abstracts for relevant studies. The search was last updated in June 2015. We found six studies, with 350 participants followed‐up for at least three months. All of these studies enrolled participants undergoing knee replacement.
Key results
Because the studies did not report the same outcomes, it was difficult to pool the results. We could only pool three out of six randomized controlled trials studying regional anaesthesia for better function after total knee replacement. Pooling data from 140 participants, with range of motion assessed at three months after surgery, there was no statistically significant difference between the use of regional analgesia and conventional pain control with intravenous medications.
None of the included studies examined long‐term adverse effects like persistent nerve damage.
Quality of the evidence
We deemed the quality of the evidence to be very low, as the included studies were not considered to be of high calibre and included few participants.
Conclusions
We do not have enough information to determine if regional anaesthesia improves function after major joint replacement or not. We need more and better clinical trials to decide whether there is an effect of regional analgesia on the surgical results after total shoulder, hip or knee replacement surgery. We need more research to understand if regional analgesia increases the risk of falls after joint replacement.
Summary of findings
Background
Description of the condition
Total joint replacement is the definitive treatment for joint‐destroying conditions such as osteoarthritis and rheumatoid arthritis. Replacement arthroplasty or joint replacement surgery is an orthopaedic surgical procedure that replaces an arthritic or dysfunctional joint surface with an artificial prosthesis. Joint replacement is offered when conservative therapy does not alleviate severe pain or dysfunction of the joint. The number of total replacements of large joints (shoulder, hip or knee) has increased dramatically over the past two decades and will continue to increase as the population ages. However, patients can experience severe postoperative pain that can impede rehabilitation.
Description of the intervention
Provision of regional analgesia, either by a continuous or single‐injection technique, controls pain by blocking the conduction of pain impulses from the periphery to the central nervous system. It has been hypothesized that regional anaesthesia blocks afferent pain impulses that cause muscle spasms during postsurgical ambulation or when using the extremity after surgery. This may be why regional analgesia allows for improved short‐ and long‐term knee flexibility and mobility (Singelyn 1998). The control intervention is a combination of non‐steroidal anti‐inflammatory drugs (NSAIDs) and opioids. NSAIDs reduce swelling and inflammation in the operative site and reduce pain perception in the central nervous system, but used alone, they are sometimes insufficient for controlling pain. NSAIDs also have significant side effects (gastrointestinal bleeding, renal injury) and are often contraindicated, leaving physicians to rely on opioids alone. Opioids act on opioid receptors in the central nervous system and are potent analgesics but have significant side effects including respiratory depression, constipation, nausea and vomiting, which limit their use and patient compliance.
How the intervention might work
Decreasing pain after surgery might facilitate rehabilitation and lead to earlier return of motion with better range of motion and motor power. Improved pain control allows for better patient participation during rehabilitation, for example in range of motion exercises. Early mobilization is probably one of the factors determining long‐term joint function, the outcome of interest. Some studies have shown that regional analgesia can increase passive knee flexion for a few months postoperatively (Capdevila 1999). This should allow for a more productive rehabilitation programme. Increased postoperative analgesia allows for earlier rehabilitation, which in turn should decrease the negative effects of immobilization on muscles and joints. This may help prevent long‐term stiffness of the joint and functional disability (Akeson 1987).
Why it is important to do this review
We are conducting this systematic review to explore controversy about the use of regional analgesia amidst efforts to limit healthcare costs and demonstrate value for each intervention. It is unclear if regional techniques improve long‐term function enough to justify the extra cost and effort involved.
There have been a number of controlled clinical trials evaluating regional analgesia for long‐term functionality after major joint surgery, with often conflicting results. Individual randomized controlled trials (RCTs) point to an improvement of both short‐ and long‐term function with regional anaesthesia. Some studies appear to contradict these findings, making it unclear whether the benefits extend beyond the immediate postoperative period in terms of improved surgical outcome. Decreasing pain after major joint surgery has been shown to improve range of motion in the short term (Capdevila 1999; Ilfeld 2006; Ilfeld 2011; Macfarlane 2009), but it is unclear whether these positive effects persist beyond a few months.
Regional anaesthesia and analgesia are, however, associated with additional expenses—in equipment, time and personnel. They might also impede rehabilitation because of the sensory and motor block they cause and, in the case of lower extremities, increase the risk of falls (Feibel 2009; Ilfeld 2010).
The above points suggest that there is enough evidence to warrant a Cochrane systematic review on the effect of regional anaesthesia for total joint replacement surgery, with important implications in terms of justifying the extra cost involved in providing extended postoperative regional analgesia after total joint replacement. So far, this evidence has not been synthesized.
Individual studies, especially if they contradict each other, fail to inform practitioners and patients and are unhelpful for healthcare decision makers. If we can show that regional anaesthesia improves long‐term functional outcomes based on the pooled data of several RCTs, this evidence might alter clinical anaesthesia practice with a resultant improvement in long‐term functional outcomes after major joint surgery in the ageing population.
While our preliminary search found some studies on long‐term functional outcomes after total knee replacement, we could not identify studies after shoulder or hip replacement with long‐term follow up. Our review may expose a lack of research on regional anaesthesia for long‐term function after shoulder and hip surgery for total joint replacement.
Objectives
To assess the effects of regional anaesthesia and analgesia on long‐term functional outcomes 3, 6 and 12 months after elective major joint (knee, shoulder and hip) replacement surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with a randomized controlled study design that were single‐blinded (outcome assessor) trials. Because regional anaesthesia causes numbness of the affected body part, neither participant nor anaesthesia provider could be reliably blinded to the intervention.
We excluded studies that were not randomized. We initially aimed to exclude studies in which the outcome assessor (that is, the nurse or surgeon evaluating the joint function 3, 6 or 12 months after the surgery) was not blinded, but given the paucity of studies, we ended up including studies in which there was no clear statement as to whether the outcome assessor was blinded to group allocation. This is clearly stated in the 'Risk of bias' tables for each study.
Types of participants
We included studies on participants undergoing elective total joint replacement surgery of the knee (TKR), hip (THR) or shoulder (TSR).
We excluded studies including children or participants undergoing revision arthoplasty.
Types of interventions
We included studies that compared continuous or single‐injection postoperative regional analgesia with placebo. We did not include studies comparing one regional technique versus another.
Regional analgesia included epidural anaesthesia, plexus or nerve blocks, or wound or joint infiltration. This was either the primary comparison in the study or a comparison between subgroups. Studies were included without regard to the type of anaesthesia used (general or regional) for the surgery itself. Pharmacological multimodal analgesia (non‐steroidal anti‐inflammatory drug (NSAID), paracetamol, gabapentin or pregabalin, ketamine, etc.) may or may not have been used, but they were comparable in the experimental and control groups.
Types of outcome measures
We only included studies that reported some assessment of the functional outcome of the operated joint for at least three months following surgery.
Primary outcomes
Our primary outcome was the improvement in joint function 3, 6 or 12 months after joint replacement.
There are outcome scores specific for each joint. We planned to use the appropriate score for each joint: The most commonly used scores are the Knee Society Score following total knee arthroplasty (Knee Society), the Harris Hip Score following total hip arthroplasty (Harris 1969), and the Constant Score following total shoulder arthroplasty (Constant 1987), but only one study used these indicators, and we could not obtain the information needed to score the included participants from the published data or the authors. Each joint has its specificities (surgical and anaesthetic techniques, outcomes), so we did not consider pooling data from knee, hip and shoulder operations. The outcome should have been the difference between the preoperative and the postoperative score, that is, the improvement in function.
Only one study reported a global function score. Three studies, however, reported the knee range of motion, or maximal flexion, and that was also used as a surrogate outcome for knee function.
Secondary outcomes
Quality of life: appropriate score such as the Short Form 36 Health Survey (SF‐36) or the Western Ontario and McMaster Universities Arthritis Index (WOMAC; Woolacott 2012), based on the data available in the articles and from the author
-
Immediate complications, as well as complications at 3, 6 and 12 months. Complications were to be split into:
medico‐surgical (myocardial infarction (MI), congestive heart failure (CHF), infection, pneumonia, etc.); and
block‐related (falls, local anaesthetic (LA) toxicity, localized infection, persistent neuropathy, etc.).
However, none of the studies reported long‐term complications in a systematic fashion. In fact, several studies did not even report why participants were lost to follow‐up.
Persistent pain upon movement may also be an important functional outcome after joint replacement surgery. Another Cochrane review (Andreae 2012) was recently published on regional anaesthesia for chronic pain after surgery.
Search methods for identification of studies
We performed an electronic search of commonly used databases and handsearched reference lists of relevant studies as well as conference abstracts.
Electronic searches
In order to be relevant to the current practice, we limited our search to 15 years prior to our first round of searching (in November 2012). We searched the most recent issue of the Cochrane Central Registrar of Controlled Trials (CENTRAL; 2015 Issue 6; see Appendix 1 for the detailed search strategy); MEDLINE on OvidSP (January 1997 to June 2015, see Appendix 2); EMBASE on Ovid SP (January 1997 to June 2015, see Appendix 3); and CINAHL on EBSCOhost (January 1997 to June 2015, see Appendix 4). We combined a free text search with a controlled vocabulary search and imposed no language restrictions.
Searching other resources
We searched the reference lists of identified relevant studies for additional citations. We searched conference abstracts (2011 to 2014) of the American Society of Anesthesiologists, the American Society of Regional Anesthesia and Pain Medicine, the European Society of Anaesthesiology and the European Society of Regional Anaesthesia & Pain Therapy.
We also searched two trial registries (http://www.controlledtrials.com and http://www.clinicaltrials.gov) for relevant studies. The last search was conducted in June 2015.
Data collection and analysis
We present a diagram illustrating the process of the searches and selection (Figure 1), and we followed the recommendations of the QUORUM and PRISMA statements (Moher 1999; Moher 2010).
1.
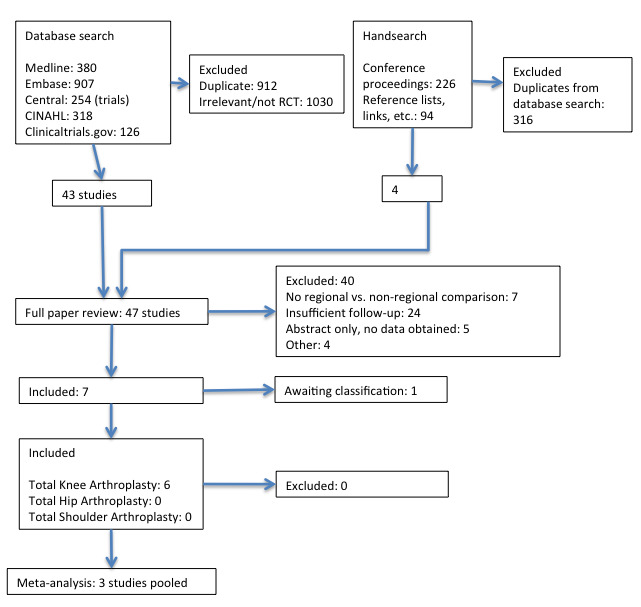
Study flow diagram
Selection of studies
Two review authors (AA and GS) screened the abstracts of all publications obtained from the results of the above searches. We independently screened all titles and abstracts for eligibility. For trials that appeared to be eligible RCTs, we obtained and inspected the full text to assess their relevance based on preplanned criteria for inclusion. For the full‐text studies reviewed, we noted the reasons for study exclusion (See Excluded studies).
In the case of disagreement between AA and GS, a third author (MHA) acted as a referee. We reached decisions by consensus, with MHA casting the decisive vote if needed. If necessary information was not available in the published article, we contacted the corresponding author to request it.
Data extraction and management
We developed a standard data collection form (Appendix 5). We recorded details of trial design, preoperative assessment, interventions and outcome measures. We performed a pilot run and revised the data sheet accordingly. For each study, two authors (AA and GS) independently extracted information and entered the data in Review Manager (RevMan 5.3).
We extracted the composite function score used as the primary outcome data for the joint being studied, as well as the range of motion when available.
The following secondary outcomes were extracted, when provided.
Quality of life (appropriate score such as the SF‐36 or the WOMAC, based on the data available in the articles and from the authors).
Complications (medico‐surgical and block‐related) at 3, 6 and 12 months.
Two authors (AA and GS) independently extracted, collected and recorded data in the data extraction form. We resolved any discrepancies between the data extracted through discussion. If we were unable to reach a consensus, we consulted a third author (MHA). If information on data or trial methodology was not reported in the published articles, GS or AA contacted the corresponding author of the relevant trial to solicit the missing information.
Assessment of risk of bias in included studies
Two review authors (AA, GS) independently evaluated each report that met the inclusion criteria. We contacted authors for missing information regarding their methods. We graded study quality on the basis of a checklist of design components: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. We accepted single‐blinding as the obvious effects of regional anaesthesia made blinding of participants and caregivers all but impossible. We initially insisted on outcome observer blinding, but given the paucity of studies, we ended up including studies in which there was no clear statement as to whether the outcome assessor was blinded to group allocation.
We achieved consensus by informal discussion. A third author (MHA) served as final arbiter when needed.
We considered a trial as having a low risk of bias if we concluded that all domains were adequate. We considered a trial as having a high risk of bias if one or more domain was inadequate or unclear. We listed all omitted studies and detailed the reason for each exclusion (Moher 1999).
We reported the 'Risk of bias' table as part of the Characteristics of included studies table.
Measures of treatment effect
Joint function was measured with continuous outcome scores. We endeavoured to use the appropriate outcome score specific for each joint (for example, the Knee Society Score following total knee arthroplasty), clearly stating the minimal clinically significant difference for the score used for each joint in the review.
We evaluated primary and secondary outcomes separately for each joint (hip, knee, shoulder), feeling that the anatomical and physiological differences between shoulder, hip and knee surgery argue against pooling or comparing studies on different joints (Higgins 2011).
We expected that studies would not all report outcomes using the same scale, even if they focused on the same joint. In this case, we planned to employ the standardized mean difference as our summary statistic. As discussed in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions, these outcome scales, while not actual continuous outcomes, can be reviewed using the same statistical methods (Higgins 2011). The use of different scales does not reflect real differences in variability among study populations but rather subjective preferences by the study authors. For studies that did not report a continuous score, we attempted to obtain the information needed to score the included participants, either by extracting it from the published data or soliciting it from the authors. However, available data were insufficient to do so.
For the secondary outcomes, lack of data also precluded any meaningful analysis.
Quality of life was also to be expressed as a score; as discussed above, the standardized mean difference would have been used if studies used different scales.
Complications and adverse events (medico‐surgical and block‐related) are dichotomous events, and we would have used the risk ratio as a measure to compare experimental group versus control group risk.
We would have used the mean difference or the standardized mean difference, as appropriate.
Unit of analysis issues
We intended to use the participant as our unit of analysis rather than each operated joint. If studies included participants operated on bilaterally, for example the left and the right knee on the same day, we would have used (and would have expected the authors to report) an average of the joint scores of both knees. However, no such studies were included.
We excluded (or given the nature of the condition and intervention studied we did not expect to find) cluster‐randomized trials, cross‐over trials, or individuals undergoing more than one intervention. In the case of studies with multiple treatment arms, we pooled the groups that used different techniques of regional analgesia and the groups that did not use regional analgesia. In the case that there were multiple observations at different time points for the same outcome, we chose the one closest to the interval being studied.
Dealing with missing data
We contacted the corresponding authors of included studies to obtain any missing information and clarify data inconsistencies. If we encountered significant skew in the outcome data, we solicited individual patient data from the primary authors. Unfortunately, we could obtain additional data only from one author (Nader 2012).
We conducted an intention‐to‐treat (ITT) analysis.
Assessment of heterogeneity
We addressed heterogeneity between studies by stratifying studies based on the joint replaced, as described above (see Measures of treatment effect). In addition, we grouped studies based on differences in rehabilitation and the type of regional analgesia interventions, for example, continuous versus single injection peripheral nerve blocks, or epidural analgesia versus peripheral nerve blocks, as discussed below (see Subgroup analysis and investigation of heterogeneity).
We investigated study heterogeneity between studies on each joint using a Chi2 test and calculation of the I2 statistic (Higgins 2002). However, we used the random‐effects model regardless of the I2 statistic for the reasons discussed (see Data synthesis).
Assessment of reporting biases
We contacted authors to request missing data. We countered time lag bias by repeating our search just prior to the submission of our work. To prevent language bias, we did not impose a language restriction.
We could not examine publication bias using graphical and statistical tests (funnel plot, Egger’s test), as planned, as there were fewer than 10 studies in each subgroup.
Data synthesis
We intended to calculate the standardized mean difference (SMD) of the various continuous scores between the groups stratified for each joint, as described in chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we found skewed data, we planned to use individual patient data for our evidence synthesis, but we were unable to procure the data from the authors. Hence, we did not perform a meta‐analysis of our primary long‐term functional outcome.
We planned to pool the standardized mean differences using the random‐effects model for meta‐analysis, which would better incorporate the clinical heterogeneity typical among small studies investigating the long‐term effects of regional anaesthesia (Andreae 2012).
We chose the random‐effects model because we wanted to address the question, what is the average intervention effect? (Higgins 2011). We assumed that the different studies were estimating randomly different yet related intervention effects (Higgins 2011). By choosing the more conservative random‐effects model, confidence intervals for the average intervention effect would be wider. We anticipated between‐study heterogeneity (including but not limited to subtle variance in regional anaesthesia delivery) and preferred the random‐effects model because of its more cautious estimate of any treatment effect (DerSimonian 1986).
We extracted dichotomous data on adverse effects, yet these were typically reported inconsistently and anecdotally. We pooled the adverse effects (falls) in the studies that reported that outcome.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analysis on several potential sources of heterogeneity: choice of anaesthesia for the joint replacement proper, adjuvant medications and multimodal pain therapy, differences in rehabilitation, exclusion criteria and comorbidities.
We accepted different choices of local anaesthetics as comparable treatments because we considered the pharmacodynamic differences between the typical local anaesthetics used to be negligible. Likewise, the pharmacokinetic differences are obliterated by the use of continuous blocks. However, the duration of action of a single‐shot block differs substantially from a continuous infusion, just as an epidural technique differs from a plexus or nerve block technique; hence we considered a subgroup analysis to investigate the differences between single‐injection versus continuous analgesia interventions, and between epidural versus plexus or nerve block techniques.
We investigated the sensitivity of our effect measure to the exclusion of studies that did not have similar pain control schemes in both groups. An example would be studies in which an NSAID was administered locally in the experimental group, along with a local anaesthetic, while the control group did not receive NSAIDs by any route.
We scrutinized the forest plot for between‐study heterogeneity and considered the differences in rehabilitation that we were able to extract as potential sources of heterogeneity. When we found both pragmatic and explanatory trials, we excluded the explanatory trials to test if the pragmatic trials alone still show an effect in spite of the inclusion of a wider range of participants and hence possibly larger standard deviations.
Sensitivity analysis
We planned to carry out a sensitivity analysis on the quality characteristics of the included studies, primarily reflected by our judgments described in the risk of bias tables, to explore the influence of the high risk of bias studies on the overall pooled result. However, we did not perform this analysis because there were not enough studies, and the risk of bias was low or unclear.
We investigated whether choosing a fixed‐effect model versus a random‐effects model would have changed the result of our evidence synthesis.
Summary of findings table
We reported the data stratified according to the joint replaced and in subgroups of regional techniques as described above. We presented the data in forest plots for outcomes at 3, 6 and 12 months. Because of the small number of studies and the high heterogeneity in the techniques used and the outcomes reported, we were unable to use the principles of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (joint function, quality of life and complications) in our review or to construct a 'Summary of findings' table using the GRADE software; the 'Summary of findings' table was constructed manually. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
The Table 1 and Table 2 reflects the fact that the evidence is of very low quality and is insufficient to draw conclusions regarding the efficacy of regional analgesia to improve functional outcome following TSR, TKR or THR.
Summary of findings for the main comparison. Regional anaesthesia compared to conventional pain control for total knee replacement patients: joint functional outcome (Knee Society Score compared to preoperative value), 3 and 6 months after surgery.
| Regional anaesthesia compared to conventional pain control for total knee replacement patients | |||||
|
Patient or population: total knee replacement patients Settings: university hospital Intervention: regional analgesia Comparison: conventional pain control | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Conventional pain control | Regional analgesia | ||||
| Knee Society Score compared to preoperative value Follow‐up: 3 months |
The mean Knee Society Score compared to preoperative value in the control group was + 42.87 points | The mean Knee Society Score compared to preoperative value in the intervention group was 1.76 points higher | Impossible to estimate as no standard deviation was provided | 30 participants (1 study) | ⊕⊝⊝⊝ very lowa,b,c,d,e |
| Knee Society Score compared to preoperative value Follow‐up: 6 months |
The mean Knee Society Score compared to preoperative value in the control group was + 44.70 points | The mean Knee Society Score compared to preoperative value in the intervention group was 4.03 points higher | Impossible to estimate as no standard deviation was provided | 30 participants (1 study) | ⊕⊝⊝⊝ very lowa,b,c,d,e |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aUnclear risk of detection bias (unclear outcome observer blinding), especially as pain is a subjective assessment. bProvider and participants were unblinded, especially as pain is a subjective assessment. cHigh risk of attrition bias. Nineteen participants were enrolled, then excluded, but were not included in the analysis. dOnly one study could be included. eThe quality of the evidence was downgraded from high to very low.
Summary of findings 2. Regional anaesthesia compared to conventional pain control for total knee replacement patients: knee flexion 3 months after surgery (as a surrogate for joint functional outcome).
| Regional anaesthesia compared to conventional pain control for total knee replacement patients | ||||
| Patient or population: total knee replacement patients Settings: university Hospital Intervention: regional analgesia Comparison: conventional pain control | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | |||
| Conventional pain control | Regional Analgesia | |||
| Knee flexion Range of motion assessment Follow‐up: mean 3 months | The mean knee flexion ranged across control groups from 110 to 140 degrees of flexion | The mean knee flexion in the intervention groups was 4 higher (2.23 lower to 10.21 higher) | 140 (3 studies) | ⊕⊝⊝⊝ very lowa,b,c,d,e |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aHigh or unclear risk of detection bias (unclear outcome observer blinding), especially as pain is a subjective assessment. bProvider and participants were partially unblinded, especially as pain is a subjective assessment. cStudies showed heterogeneous results. dThe confidence interval of the effect estimate is wide. The pooled effect is not statistically significant. eThe quality of the evidence was downgraded from high to very low.
Results
Description of studies
Results of the search
We conducted the original searches in November 2012 and reran them between February and August 2013, in January 2014, and again in June 2015. The search and selection process is illustrated in a flow diagram (Figure 1).
Electronic search
The electronic search, documented in Appendix 1; Appendix 2; Appendix 3; Appendix 4, yielded a total of 1985 references matching the predefined search parameters: 254 in CENTRAL, 380 in MEDLINE, 907 in EMBASE, 318 in CINAHL, and 126 in ClinicalTrials.gov; among them were 912 duplicates. The review authors (AA and GS) screened the remaining references and excluded 1030 references as irrelevant or not RCTs.
Handsearch
In our handsearch of conference proceedings, we searched conference abstracts (January 2011 to December 2014) of the American Society of Anesthesiologists, the American Society of Regional Anesthesia and Pain Medicine, the European Society of Anaesthesiology, and the European Society of Regional Anaesthesia & Pain Therapy. We also searched two trial registries (http://www.controlledtrials.com and http://www.clinicaltrials.gov) for relevant studies, and we selected 226 references for further review. We found 94 references in the reference lists of included studies or review articles, or by following links in PubMed and Google to other relevant studies. This resulted in a total of 319 references; all but 4 were duplicates of studies identified in the database search (Bergeron 2009; Ilfeld 2009; Ilfeld 2011; Looseley 2013).
Selection process
Two review authors (AA and GS) obtained the full text of 47 articles for further assessment (see Figure 1) and selected six studies for inclusion in this review (see Characteristics of included studies table). We found one study awaiting classification (Bergeron 2009).
Data extraction
The review authors were able to resolve all disagreements with regard to data extraction, study inclusion and quality assessment by informal discussion.
Incomplete and raw data
We sought but were unable to obtain individual patient or aggregate data for Capdevila 1999, which separated patients undergoing either total knee replacement from patients undergoing knee ligament reconstruction, and hence we had to exclude this study from our analysis.
We also requested additional data for three studies (Kadic 2009; Nader 2012; Wu 2014).
Kadic 2009 gave the knee flexion results only as mean (range) and reported only the components of the WOMAC score but not the global score. The authors replied but were unable to supply the raw data with group assignments.
Nader 2012 reported knee flexion only in a graph as the difference in knee flexion from baseline; the WOMAC components were listed individually as means (range) but without a global score. Upon request, the authors provided a table listing knee range of motion (ROM) and the global WOMAC score as a mean and standard deviation.
We did not receive a reply from Dr Wu (Wu 2014).
Included studies
We identified six RCTs studying the effect of regional analgesia on functional outcome for total knee replacement (Kadic 2009; Nader 2012; Singelyn 1998; Tammachote 2013; Wu 2014; Zhang 2011) at least three months after surgery (Characteristics of included studies table), with a total of 350 participants. We did not find any study on the effect of regional anaesthesia for long‐term function after shoulder replacement. While we reviewed articles written in languages other than English, the six included studies were all published in English. We provide an overview of all included studies in Table 4 and compare the diverse timelines of their analgesic approaches in Table 5.
1. Overview of included studies.
| Study Blindinga | Population: Enrolled/analysed/followed up to at least 3 months (no. per group) | Intervention(s) | Control | Postoperative outcomes |
|
Kadic 2009 Single |
58/53/48 (27/26) | CFNB | No block | Knee function after 3 months (knee flexion, Knee Society score and WOMAC pain, stiffness and function subscale) |
|
Nader 2012 Unblinded |
62/62/60 (31/31) | CFNB (Epidural infusion until morning of POD 1) |
No block (epidural infusion until morning of POD 1) |
Knee flexion at 1, 6 and 12 months Functional outcome on POD 1, POD 2, POD 3, 1 month, 6 months, and 12 months |
|
Singelyn 1998 Unblinded |
45/45/45 (15/15/15) | CFNB Epidural analgesia |
No block | Knee flexion at 6 weeks and 3 months |
|
Tammachote 2013 Single |
59/57/57 (28/29) | Periarticular infiltration | Intrathecal morphine | ROM at 2, 6 and 12 weeks Modified Thai version of the WOMAC score (at 6 and 12 weeks) |
|
Wu 2014 Unblinded |
79/60/60 (30/30) | CFNB | No block | Knee Society Score (difference with preoperative value) at discharge, at 6 weeks, 3 months and 6 months |
|
Zhang 2011 Double |
96/80/80 (26/27/27) | Periarticular infiltration Periarticular infiltration then ropivacaine/ketorolac infusion |
Saline intra‐articular infusion | Maximum knee flexion after 7 days (early recovery) and 90 days (late recovery) |
CFNB: continuous femoral nerve block; POD: postoperative day; RCT: randomized controlled trial; ROM: range of motion; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
aUnblinded: neither participant, nor personnel, nor assessor of long‐term function were blinded; single: only assessor of long‐term function was blinded; double: participant and personnel were blinded, but assessor of long‐term function was not.
2. Timeline chart for included studies.
| Study | Surgical anaesthesia | Group | Analgesia timeline | Analgesic regimen (common to all within‐study groups) | ||||
| POD 0 | POD 1 | POD 2 | POD 3 | POD 4 | ||||
| Kadic 2009 | Spinal | Exp | CFNB | None | IV morphine PCA Diclofenac + paracetamol |
|||
| Con | None | |||||||
| Nader 2012 | Spinal‐epidural | Exp | CFNB + epidural | CFNB | None | Hydrocodone / paracetamol PRN If needed, extended‐release oxycodone + hydromorphone PRN |
||
| Con | Epidural | None | ||||||
| Singelyn 1998 | GA | Exp | Epidural | None | IV morphine PCA IV propacetamol PRN IM piritramide PRN |
|||
| Exp | CFNB | None | ||||||
| Con | IV morphine PCA | None | ||||||
| Tammachote 2013 | Spinal | Exp | Periarticular infiltration | None | IV ketorolac PCA until POD 3, then PO naproxen and tramadol PRN PO paracetamol and amitriptyline |
|||
| Con | Intrathecal morphine | None | ||||||
| Wu 2014 | Spinal | Exp | CFNB | None | Paracetamol + sustained release diclofenac Oral opioids (codeine or morphine) Metoclopramide, senna and famotidine to prevent side effects |
|||
| Con | IV morphine PCA | None | ||||||
| Zhang 2011 | GA | Exp | Periarticular infiltration | Placebo | None | Celecoxib IV morphine PCA IM morphine PRN |
||
| Exp | Ropivacaine + ketorolac infusion | None | ||||||
| Con | Placebo | None | ||||||
CFNB: continuous femoral nerve block; Con: Control; Exp: Experimental; GA: general anaesthesia;IM: intramuscular;IV: intravenous; PCA: patient‐controlled analgesia; POD: postoperative day;PRN: as needed
Design
Three of the six studies were only single‐blinded (Kadic 2009; Tammachote 2013; Zhang 2011), while three were unblinded (Nader 2012; Singelyn 1998; Wu 2014). Due to the nature of the interventions and their obvious numbing effect (participants received either a continuous femoral nerve block, an epidural catheter, or no regional technique), blinding of participants or personnel would have been difficult.
In Zhang 2011, participants in all three groups were blinded, as they received (or not) periarticular infiltration, then an infusion of either ropivacaine or ketorolac, or saline, through an intra‐articular catheter. However, the personnel caring for the person in the operating theatre were not blinded regarding group allocation.
Only three studies mentioned an attempt to blind the assessor for the long‐term outcome to group assignment (Ilfeld 2009; Ilfeld 2011; Kadic 2009).
Descriptive characteristics of participants
Reporting of participants' demographics was too heterogenous to pool data across included studies, but the participants had comparable characteristics and were mostly in their sixth decade. Most studies included more female than male participants, but there were exceptions, such as Zhang 2011, which included similar numbers of males and females.
We found no studies on children.
Intervention
We give an overview of the regional analgesic technique and the timing in Table 5.
Four studies used a continuous femoral nerve block in the experimental group (Kadic 2009; Nader 2012; Singelyn 1998; Wu 2014). Singelyn 1998 used a three group parallel design with a second experimental group (postoperative epidural analgesia).
Two studies used periarticular infiltration (Tammachote 2013; Zhang 2011). Zhang 2011 had two experimental groups: an intra‐articular catheter was inserted in all participants during surgery, and participants were randomly assigned to receive either saline or ropivacaine and ketorolac.
Control
In five studies, the control group received no block (Kadic 2009; Nader 2012; Singelyn 1998; Tammachote 2013; Wu 2014), although in Nader 2012 all participants received an epidural infusion until the morning of postoperative day one (POD 1), and in Tammachote 2013, the spinal included administration of intrathecal morphine.
In Zhang 2011, control participants did not receive periarticular infiltration intraoperatively, but they did receive a saline infusion through an intra‐articular catheter.
Analgesic regimens were identical for experimental and control groups in all studies, with wide variations between studies. Indeed, they featured combinations of intravenous (IV) morphine patient‐controlled analgesia (PCA), oral opioids, oral NSAIDs or Cox‐2 inhibitors, IV ketorolac PCA, oral paracetamol or IV propacetamol, and even oral amitriptyline in Tammachote 2013.
Primary outcomes
The included studies reported a variety of outcomes at 3, 6 and 12 months after joint replacement (Table 6).
3. Summary of reported results.
| Study | Outcomes | Results | ||
| Control | Experimental | P valuea | ||
| Primary total knee replacement | ||||
| Kadic 2009 | Knee flexion at 3 months | 110.0 (range 100.0 to120.0) | 110.0 (range 110.0 to 112.5) | NS |
| Knee Society Score (knee component) at 3 months | 83.2 (± 13.2) | 83.8 (± 12.8) | 0.87 | |
| Knee Society Score (function component) at 3 months | 58.5 (± 21.2) | 61.2 (± 29.3) | 0.70 | |
| WOMAC (pain) at 3 months | 80.0 (± 18.7) | 83.8 (± 12.9) | 0.39 | |
| WOMAC (stiffness) at 3 months | 71.3 (± 22.4) | 75.6 (± 17.4) | 0.44 | |
| WOMAC (function) at 3 months | 71.8 (± 19.5) | 80.4 (± 10.5) | 0.05 | |
| Nader 2012b | Knee flexion at 6 months | 124.47 (± 8.32) | 123.31 (± 9.53) | 0.61 |
| Knee flexion at 12 months | 124.56 (± 7.67) | 124.50 (± 10.37) | 0.98 | |
| WOMAC at 6 months | 11.04 (± 12.50) | 14.18 (± 15.70) | 0.39 | |
| WOMAC at 12 months | 6.76 (± 7.26) | 17.39 (± 17.84) | 0.003 | |
| Singelyn 1998 | Knee flexion at 3 months | 116 (± 11) | 121 (± 12) | 0.24 |
| Tammachote 2013 | Knee flexion at 3 months | 140 (± 4) | 139 (± 4) | 0.35 |
| WOMAC at 3 months | 9 (± 4) | 9 (± 4) | 1.00 | |
| Wu 2014 | Knee Society Score (difference with preoperative value) at 3 months | + 42.87 | + 44.63 | 0.78 |
| Knee Society Score (difference with preoperative value) at 6 months | + 44.7 | + 48.73 | 0.51 | |
| Zhang 2011 | Knee flexion at 3 months | 105.7 (3.4) | 112.2 (3.8) | P value < 0.001 |
NS: non‐significant; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index. aP value calculated using a two‐tailed unpaired t‐test. bThe Nader study showed a very significant difference between WOMAC scores at 12 months, while there was no difference at 6 months. The authors concluded that this finding was probably due to confounders such as subsequent surgical procedures: "[P]atients in the CFA [continuous femoral analgesia] group had undergone additional orthopaedic procedures more frequently, which may have impacted the functional recovery and health‐related quality of life following the procedure from this study. A possible explanation for the greater incidence of addition procedures in the CFA group may be due to the greater patient satisfaction scores with their initial surgery."
Wu 2014 reported Knee Society Scores at three and six months. Kadic 2009 used the Knee Society Score at three months, but reported the results in two components rather than a total score, making pooling impossible. We were not able to obtain raw data from the authors.
Only four studies (Kadic 2009; Singelyn 1998; Tammachote 2013; Zhang 2011) reported the knee range of motion at three months, but Kadic 2009 only gave the data as mean (range). Nader 2012 only reported the difference in knee flexion from baseline at 1, 6 and 12 months on a graph, but we were able to obtain data on knee range of motion (ROM) at 6 and 12 months from the authors.
Secondary outcomes
Nader 2012 reported each individual component of the WOMAC score as mean (range); the authors supplied a table with the total WOMAC score at 6 and 12 months.
Adverse effects were not part of the explicit outcomes in any of the studies, and no included study elicited long‐term adverse effects. Adverse effect reporting was thus subject to a large selective reporting bias. Reported short‐term adverse events are summarized in Table 7. Because of this large selective reporting bias, and because this review aimed to compare long‐term outcomes rather than immediate postoperative complications, further analysis was not performed.
4. Adverse events.
| Study | Adverse events attributable to the regional anaesthesia technique | Common short‐term adverse events not directly related to regional anaesthesia | ||||
| Control | Experimental | Event | Experimental | Control | P valuea | |
| Kadic 2009 | None | None | PONV | 2 | 13 | 0.0007 |
| Drowsiness | 0 | 3 | 0.11 | |||
| Constipation | 0 | 4 | 0.05 | |||
| Urine retention | 0 | 1 | 0.49 | |||
| Nader 2012 | 1 fall (Exp); NS | 4 DVTs (Con); P = 0.04 3 positive joint aspirates (Exp); P = 0.08 |
Nausea | 0 | 0 | 1.00 |
| Vomiting | 0 | 0 | 1.00 | |||
| Pruritus | 2 | 1 | 1.00 | |||
| Singelyn 1998 | 6 catheter technical issues (Epidural); P < 0.001 vs. CFNB and IV
PCA Falls, LA toxicity, etc.: not reported |
Not discussed | PONV | 9 (5/4) | 6 | 0.52 |
| Hypotension | 1 (0/1) | 0 | 1.00 | |||
| Urine retention | 6 (0/6) | 2 | 0.70 | |||
| Tammachote 2013 | Not discussed | No wound problem Other complications not discussed |
PONV | 10 | 20 | 0.02 |
| Pruritus | 3 | 11 | 0.03 | |||
| Urine retention | 0 | 0 | 1.00 | |||
| Respiratory depression | 0 | 0 | 1.00 | |||
| Wu 2014 | None | 5 DVTS (2 Exp, 3 Con) 5 cases of bleeding requiring transfusion (2 Exp, 3 Con) 5 shocks in ward (3 Exp, 2 Con) 2 wound infections (1 Exp, 1 Con) |
PONV | 8 | 19 | 0.009 |
| Urine retention | 3 | 8 | 0.18 | |||
| Dizziness | 5 | 12 | 0.08 | |||
| Desaturation | 0 | 1 | 1.00 | |||
| Pruritus | not reported | |||||
| Zhang 2011 | None | 6 DVTs (2 Exp1, 2 Exp2, 2 Con): NS | PONV | 8 (4/4) | 7 | 0.36 |
| Pruritus | 2 (1/1) | 1 | 1.00 | |||
| Urine retention | 2 (1/1) | 2 | 0.60 | |||
| Respiratory depression | 1 (0/1) | 1 | 1.00 | |||
| Pooled 199 in Exp groups 158 in Control groups |
not applicable | not applicable | PONV | 37/199 | 67/158 | < 0.0001 |
| Pruritus | 7/169 | 13/128 | 0.06 | |||
| Urine retention | 11/199 | 13/158 | 0.40 | |||
Con: control group; DVT: deep vein thrombosis; Exp: experimental group; PONV: postoperative nausea and vomiting.
aP values were calculated using a two‐tailed Fisher’s exact test.
Excluded studies
A summary of the excluded studies and reasons for their exclusion is presented in the Characteristics of excluded studies table. We excluded 40 studies: 7 studies compared two regional techniques, 24 had insufficient follow‐up, 5 were only reported in abstract form (and we were unable to obtain the data needed for inclusion), and 4 studies were excluded for other reasons (retrospective study, non‐randomized, no regional technique used, letter to the editor commenting on another article).
Risk of bias in included studies
The risk of bias is detailed in the 'Risk of bias' tables (Characteristics of included studies), the 'Risk of bias' graph (Figure 2) and the methodological quality summary (Figure 3).
2.
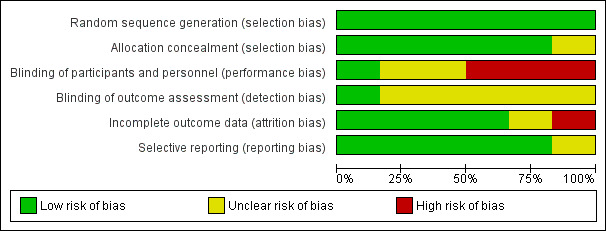
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
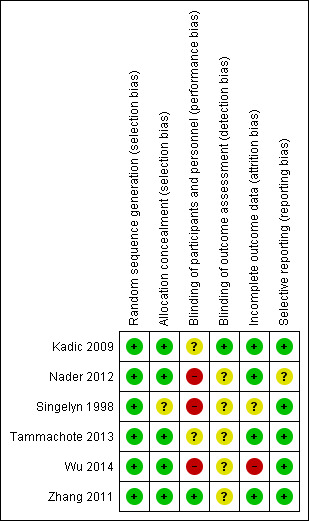
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We deemed the risk of selection bias risk to be low for all studies except Wu 2014, in which 19 of the 79 participants were excluded after entering the study because of technical issues or unrelated medical events. The 60 analysed participants were thus a (possibly healthier) subset of the included participants. All studies except Kadic 2009 used computer‐generated randomization sequences and all but Singelyn 1998 used sealed opaque envelopes to conceal group assignments. Singelyn 1998 did not clearly state how the group allocation was concealed until the participant received the chosen intervention.
Blinding
In Zhang 2011, participants and providers were blinded to group allocation. However, in four studies participants and personnel were not blinded, and participants in the intervention group received a femoral catheter or an epidural (Kadic 2009; Nader 2012; Singelyn 1998; Wu 2014). In Tammachote 2013, participants allocated to the intervention and control group could not be easily distinguished, as there was no catheter left in place, but there is no mention of efforts to maintain blinding of the participants or personnel.
We found a high risk for detection bias in the three studies we pooled (Figure 2). Kadic 2009 reported that outcome assessors were blinded to group allocation. The other studies did not mention blinding of the outcome assessor, and therefore it is likely that information about group allocation was available from the chart.
Incomplete outcome data
In all studies but one (Singelyn 1998), a number of participants were lost to long‐term follow‐up, but there was no explanation as to why participants did not return for assessment. Singelyn 1998 did not clearly state how many participants were followed up to three months, but the table showing knee flexion suggests that all participants (15 in each group) were assessed at three months. Again, Wu 2014 excluded 19 out of 79 participants after enrolment and completion of all or most of the study procedures, but data are not available for these participants.
Selective reporting
We only included three of the five studies investigating functional outcomes after knee replacement in our data synthesis, pointing to a high risk of reporting bias (Singelyn 1998; Tammachote 2013; Zhang 2011). The small numbers of studies found for each joint replacement subgroup precluded a formal analysis of publication bias using a funnel plot or the test proposed by Egger 1997.
All other studies reported the outcomes stated in the methods section of the article, but none of them elicited long‐term adverse effects, and their reporting was likely subject to selective reporting bias.
Other potential sources of bias
None of the common other causes of bias, such as design‐specific, baseline imbalance, blocked randomization or increased diagnostic activity, were relevant to the included studies.
Effects of interventions
The heterogeneity of interventions and outcome reporting hindered evidence synthesis.
Primary outcome (Table 3)
Improvement in joint function after total knee replacement
Wu 2014 reported global Knee Society Scores at three and six months (Table 1). Kadic 2009 used the Knee Society Score, but reported the results in two components, with no significant difference between groups (Table 6). As we could not obtain raw data from the authors, we were unable to pool the results of these two studies.
Knee range of motion as a surrogate measure of joint function
We pooled three RCTs studying regional analgesia for improvement after TKR in 140 participants, with range of motion assessed at three months after surgery (Singelyn 1998; Tammachote 2013; Zhang 2011) (Table 2).We estimated a mean difference in range of motion of 3.99 degrees (95% CI − 2.23 to 10.21; P value = 0.21). We analysed results using the random‐effects model because heterogeneity was high. For the same reason, and because we pooled only three studies that included small numbers of participants and did not express outcomes using a global function score, we downgraded the outcome to very low quality. There was no statistically significant difference between the experimental and control groups (Analysis 1.1).
1.1. Analysis.
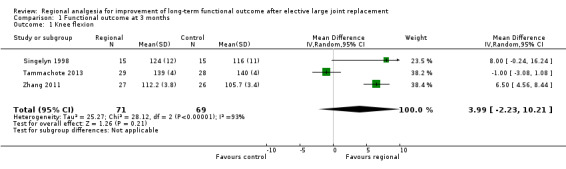
Comparison 1 Functional outcome at 3 months, Outcome 1 Knee flexion.
We pooled the three studies even though one used a continuous femoral nerve block, while the other two used periarticular infiltration, because they all achieved the outcome of interest (improved long‐term function) by the same mechanism (improved pain control, which allowed more active rehabilitation) (Shoji 1990). In our sensitivity analysis, we removed Singelyn 1998 and pooled only studies that used periarticular infiltration (Tammachote 2013; Zhang 2011) (Analysis 1.2), but the result did not change; there was a mean difference in range of motion of 2.76 degrees (95% CI − 4.59 to 10.11; P value = 0.46).
1.2. Analysis.
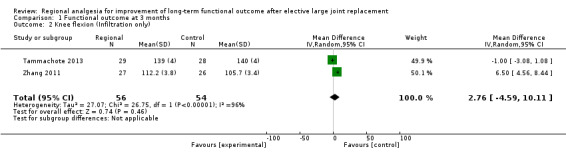
Comparison 1 Functional outcome at 3 months, Outcome 2 Knee flexion (Infiltration only).
Only these three studies reported the knee range of motion at three months. Kadic 2009 presented the data as mean (range), and Nader 2012 reported the difference in knee flexion from baseline. We found no studies reporting on range of motion beyond three months after surgery.
Secondary outcomes
Quality of life scores after total knee replacement
Nader 2012 reported the individual components of the WOMAC score as mean (range), and we obtained a total score from the authors, with a significant difference in WOMAC score at 12 months but not at 6 months. The authors concluded that this finding was probably due to confounders such as subsequent surgical procedures: "[P]atients in the CFA [continuous femoral analgesia] group had undergone additional orthopaedic procedures more frequently, which may have impacted the functional recovery and health‐related quality of life following the procedure from this study. A possible explanation for the greater incidence of addition procedures in the CFA group may be due to the greater patient satisfaction scores with their initial surgery."
Adverse events
Reported adverse events are summarized in Table 7. We did not perform further analyses because immediate adverse effects were not part of the explicit outcomes of any of these typically small studies, and long‐term adverse events after regional anaesthesia are rare.
Assessment of heterogeneity
We found evidence for heterogeneity between the three RCTs with range of motion outcomes at three months after total knee surgery using different regional anaesthesia techniques (Singelyn 1998; Tammachote 2013; Zhang 2011). The I2 statistic obtained a value of 93%, which suggests considerable heterogeneity (Higgins 2002). There were too few studies to do a subgroup analysis by regional anaesthesia intervention, as we had planned.
Sensitivity analysis of model assumptions
The effect estimates of our evidence synthesis is highly sensitive to the choice of fixed‐effect model versus random‐effects model for pooling (data not presented). The diversity of interventions (Overall completeness and applicability of evidence), the suggestion of considerable heterogeneity (I2 statistic = 93%) and our a priori choice for a random‐effects model made a sensitivity analysis moot (Data synthesis). Choosing a fixed‐effect model would be inappropriate for this data synthesis, even if this approach suggested a statistically significant effect.
Discussion
Summary of main results
The clinical heterogeneity between trials, the incomplete data, and the disparate outcome reporting hindered evidence synthesis for our primary outcome: long‐term function at 3, 6 and 12 months after joint surgery. We found six RCTs following a total of 350 participants for at least three months. Only one study (Wu 2014) reported Knee Society Scores at three and six months. We could only pool data from three RCTs enrolling a total of 140 participants on functional outcomes after total knee replacement, using knee flexion (range of motion) as a surrogate for functional outcome (Singelyn 1998; Tammachote 2013; Zhang 2011). The standardized mean difference was not statistically significant between the experimental and the control groups. The available evidence is insufficient to judge whether regional analgesia improves long‐term function after major joint surgery.
Overall completeness and applicability of evidence
Paucity of the evidence
There was no study reporting on functional outcomes after shoulder replacement or hip replacement. Our evidence synthesis is only based on a few studies with relatively few participants.
Heterogeneity between interventions
The three studies we pooled used different regional anaesthesia techniques, various local anaesthetic agents and doses. In particular, Singelyn 1998 used a continuous femoral nerve block while the other two used periarticular infiltration. However, we feel that their pooling is still justified, because we hypothesize that their effect is mediated through better pain control, which in turn facilitates better patient participation in rehabilitation activities. It does not matter which regional technique is used to achieve this goal. We found evidence for considerable heterogeneity (I2 statistic = 93%), but the paucity of studies precluded a subgroup analysis by regional anaesthesia intervention as we had envisioned in the protocol (Atchabahian 2012). In any event, removing Singelyn 1998 and pooling only Tammachote 2013 and Zhang 2011 (Analysis 1.2) does not change the result.
It is obviously questionable whether knee flexion is as good an outcome as a global function score. However, many patients have degenerative joint disease of other lower extremities, and over the 12 months following their knee replacement surgery, they may undergo other joint replacement procedures (Nader 2012). This could significantly alter their functional status (walking, climbing stairs), so knee flexion is a better reflection of the functional outcome of the specific joint replacement surgery being evaluated.
The dearth of available studies, the reporting bias and the heterogeneity of reporting and outcomes among the included studies hindered evidence synthesis. Hence, we feel it would be premature to dismiss the hypothesis that regional anaesthesia improves long‐term function. Even for TKR, for which we found six studies, there was considerable heterogeneity in the regional anaesthesia interventions administered and the outcomes reported (Description of studies).
In a subsequent update of this review, we will seek to obtain individual data from the study authors to perform an individual patient data meta‐analysis. We may also investigate the effect of regional anaesthesia on quality of life scores (WOMAC) and long‐term pain outcomes, as these are intrinsically linked with function in orthopaedic surgery.
We excluded two studies because all participants received regional analgesia for the first 24 hours and were then administered placebo or local anaesthetic depending on their group assignment (Ilfeld 2009; Ilfeld 2011). Thus, there was no real comparison of regional analgesia versus non‐regional analgesia, but rather a study of the beneficial effect of extended nociceptive perioperative blockade. In an update of our review, if we find more studies with a similar design, we will consider performing a separate analysis of studies evaluating the effect of short versus prolonged regional analgesia. Given our hypothesis that regional analgesia could enhance outcome by facilitating rehabilitation, prolonged analgesia should have a more pronounced effect than analgesia for the first 24 hours.
No study detailed the rehabilitation protocol used, so it was impossible to compare them between studies. However, if they were to differ significantly this could represent a major cause of heterogeneity. Another cause of heterogeneity was the other analgesic medications used, which varied widely between studies (see Table 5).
Adverse events
Because long‐term adverse events after regional anaesthesia are rare (Barrington 2011), and the studies are typically small, our review was not suited to detect possible differences in adverse event rates between the experimental and control groups. Also, as long‐term adverse events were not part of the explicit outcomes of any study and were therefore not reported systematically, we are concerned about publication and reporting bias. These considerations weaken any conclusions based on detected associations between adverse event rates and the regional interventions in systematic reviews.
A recent database analysis on over 190,000 patients who underwent elective TKR failed to show an increased risk of fall in patients who had received a peripheral nerve block, and it actually found a reduced rate of falls in patients who received neuraxial anaesthesia rather than general anaesthesia (Memtsoudis 2014).
A systematic review examining the risk for falls after major orthopaedic surgery with peripheral nerve blockade suggested that, based on the review of five studies (1595 participants), only continuous lumbar plexus blockade was associated with a statistically significant increase in the risk for falls (Peto odds ratio (OR) 3.85; 95% CI (1.52 to 9.72); P value = 0.005; I2 = 0%; Johnson 2013).
Quality of the evidence
The risk of bias graph (Figure 2) gives an overview of the methodological weaknesses of the included studies, and these are also detailed in the methodological quality summary (Figure 3). We noted several important limitations in the quality of the evidence. The three pooled studies that reported knee flexion at three months all had a high risk of detection bias. The nature of the interventions typically made participant blinding effectively impossible. Only studies comparing the timing and duration of regional analgesia such as Zhang 2011 were able to achieve some measure of blinding. Hence, performance bias may weaken the conclusions of our review. The placebo effect may be particularly strong for pain outcomes, and its power remains unknown for long‐term outcomes. We caution that our evidence synthesis is based on only a few small studies. Because of the high heterogeneity and the fact that we pooled only three studies including small numbers of participants, none of which expressed outcomes using a global function score, the outcome was downgraded to very low quality.
Potential biases in the review process
Reporting and selection bias
Not all outcome data were available for inclusion (Results of the search; Assessment of reporting biases). This potentially introduced bias in our review and may reflect publication bias. The small numbers of studies found in each subgroup precluded a formal analysis of publication bias by using a funnel plot or the test proposed by Egger 1997.
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic reviews or meta‐analyses study the effect of regional analgesia on long‐term function. Two previous systematic reviews discussed whether regional analgesia improves functional outcome after TKR or THR (Macfarlane 2009; MacFarlane 2009b) but did not focus on long‐term functional outcome nor include most of the studies we cover here, as they were published after 2009.
A recent review suggested that neuraxial anaesthesia could decrease the 0‐ to 30‐day mortality for patients undergoing surgery with intermediate to high cardiac risk compared to general anaesthesia (Guay 2014).
Authors' conclusions
Implications for practice.
The fragmentary and heterogeneous data of very low quality from the included studies are insufficient to recommend regional analgesia to improve long‐term surgical outcome after TKR, THR or TSR, beyond the immediate postoperative period.
Implications for research.
Future clinical trials
Participants
We need more RCTs on the effects of regional analgesia on the outcomes of TSR, THR and TKR, with results reported as well‐recognized functional scores and individual patient data available for subsequent meta‐analysis.
Interventions
We need to study the effects of newer regional analgesia interventions, such as the adductor canal block for TKR.
Control groups
Studies should compare an experimental regional anaesthesia intervention with a conventional pain control comparator, not only to another regional intervention. Efforts should be made to blind participants and personnel to the intervention, for example by sham procedures (inserting catheters and randomizing the participants to local anaesthetic or saline infusion).
Outcomes in clinical studies
Outcomes should include well‐established scores such as the Knee Society Score, and investigators should report results as mean (SD) to make it possible to pool data with other studies. Where full details and data cannot be provided due to print journal space limitations, they should be published in an appendix or online repository. Knee range of motion should be reported as both an absolute value and as the improvement from the preoperative value. Knee flexion could also be used as a dichotomous outcome, depending on whether patients achieve 110 degrees of flexion, as this seems to be the value necessary for many activities of daily life, such as sitting and going up and down stairs (Rowe 2000).
Researchers could perform long‐term functional follow‐up of the participants included in trials already performed and for which only short‐term data was collected and published.
More studies should use quality‐of‐life assessments.
Other outcomes of interest include patient‐related outcome measures such as pain (visual analogue scale, or VAS), patient experience during the postoperative mobilization programme and willingness to undergo a similar procedure, as well as cost assessment of the intervention.
Research on adverse effects
Studies should include adverse effects, separated by group, as primary outcomes, including an analysis of patient‐specific risk factors (age, mental status) for certain adverse effects like falls.
Study design
Future studies should employ methods to address patient attrition, for example intention‐to‐treat analysis.
Feedback
Wylde 2011, 17 September 2015
Summary
I was just adding this review to UK DUETs, and was inspecting the ongoing studies table and you list Wylde 2011 as ongoing. Shouldn't this be in awaiting assessment?
Reply
When the review was in the editorial process the study by Wylde was not published (Wylde 2011). It has just been published (Wylde 2015). The authors will now be excluding the Wylde 2015 paper on the basis of the following:
Wylde 2015 conducted and reported 2 RCTs: (1) knees and (2) hips.
The knee RCT did not meet the review inclusion criteria because they compared two regional anaesthesia techniques, therefore this RCT was excluded. (In this RCT they measured, but did not report range of motion).
The hip RCT met the review inclusion criteria because they compared regional versus non‐regional anaesthesia techniques, but they did not measure range of motion, therefore this RCT was excluded.
Contributors
Summary
Mark Fenton, Editor UK DUETS, NICE, UK
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Arthur Atchabahian, Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU School of Medicine, New York, NY, USA
What's new
| Date | Event | Description |
|---|---|---|
| 3 December 2015 | Amended | Wylde 2015 was published based on the studies described in Wylde 2011. This paper includes two studies performed on patients undergoing total hip and total knee replacements. The comparison was between "standard care” and local infiltration plus standard care. For hip patients, standard care did not include any regional analgesia, and thus the comparison was between a regional technique (infiltration) and a non‐regional technique. However, no functional data (our primary outcome) was collected, and thus that study was excluded. For knee patients, standard care included a femoral nerve block, and thus the comparison was between two regional techniques, which is an exclusion criterion. Although functional data (range of motion) was collected, the study should be excluded because it does not compare one regional versus one non‐regional technique. Therefore, the whole paper should be excluded. The study was added to the Excluded studies. |
Notes
Review amended October 13 2015 (see Feedback 1)
We would like to thank Mark Neuman (content editor), Marialena Trivella (statistical editor), and Edward R Mariano, Santosh Rath and Ulrik Grevstad (peer reviewers) for their help and editorial advice during the preparation of the protocol (Atchabahian 2012) for the systematic review.
Acknowledgements
We would like to thank Mark Neuman (content editor), Nathan Pace (statistical editor), Joanne Guay, Christian S Meyhoff, Santosh Rath (peer reviewers) and Janet Wale (consumer editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. CENTRAL (The Cochrane Library) search strategy
#1 MeSH descriptor: [Analgesia] this term only #2 MeSH descriptor: [Analgesia, Epidural] explode all trees #3 (regional near analg*) .:ti,ab or (analg* and rehabilitat*) #4 #1 or #2 or #3 #5 MeSH descriptor: [Arthroplasty, Replacement] explode all trees #6 ((joint* or arthroplasty or arthritic or shoulder or hip or knee) near replac*) or (joint* and replac* and (surg* or procedur* or operat*)) or (joint* and (long or short) and (flexibility or mobility or functionality)) or (articular near (range or motion)) #7 #5 or #6 #8 #4 and #7
Appendix 2. MEDLINE search strategy via OvidSP
1. Analgesia/ or exp Analgesia, Epidural/ or (regional adj3 analg*).ti,ab. or (analg* and rehabilitat*).mp. 2. exp Arthroplasty, Replacement, Hip/ or exp Arthroplasty, Replacement, Knee/ or exp Arthroplasty, Replacement/ or exp Arthroplasty, Replacement, Shoulder/ or "Range of Motion, Articular"/ or ((joint* or arthroplasty or arthritic or shoulder or hip or knee) adj3 replac*).af. or (joint* and replac* and (surg* or procedur* or operat*)).mp. or (joint* and (long or short) and (flexibility or mobility or functionality)).mp. or (articular adj3 (range or motion)).mp. 3. 1 and 2 4. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. analgesia/ or exp epidural anaesthesia/ or (regional adj3 analg*).ti,ab. or (analg* and rehabilitat*).mp. 2. exp hip arthroplasty/ or exp knee arthroplasty/ or exp arthroplasty/ or "joint characteristics and functions"/ or ((joint* or arthroplasty or arthritic or shoulder or hip or knee) adj3 replac*).af. or (joint* and replac* and (surg* or procedur* or operat*)).mp. or (joint* and (long or short) and (flexibility or mobility or functionality)).mp. or (articular adj3 (range or motion)).mp. 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. CINAHL (EBSCOhost) search strategy
S1 ( (MH "Analgesia") OR (MM "Analgesia, Epidural") ) OR ( (regional N3 analg*) or (analg* and rehabilitat*) ) S2 ( (MH "Arthroplasty, Replacement, Hip") OR (MH "Arthroplasty, Replacement") OR (MH "Arthroplasty, Replacement, Knee") OR (MH "Arthroplasty, Replacement, Shoulder") OR (MH "Range of Motion") ) OR ( ((joint* or arthroplasty or arthritic or shoulder or hip or knee) N3 replac*) or (joint* and replac* and (surg* or procedur* or operat*)) or (joint* and (long or short) and (flexibility or mobility or functionality)) or (articular N3 (range or motion)) ) S3 S1 and S2
Appendix 5. Data collection form
CAM Review Group
Study Selection, Quality Assessment & Data Extraction Form
Person Extracting Data: AA GS MHA HS ‐‐‐‐‐‐
| ID # | First author | Journal/Conference Proceedings, etc. | Year | PMID/Identifier unpublished |
Study eligibility
| Intervention | Non‐Regional Comparator | Functionality |
| Regional/No/Unclear | Yes/No/Unclear | Movement/No/Unclear |
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information/reason to be inserted into ‘Table of excluded studies’. Done: |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | ID# | Author(s) | Journal/Conference Proceedings etc | Year |
PMID/ Identifier |
| A | The paper listed above | ||||
| B | Further papers | ||||
| C |
Participants and trial characteristics
| Trial characteristics | ||
| Further details | ||
| Single centre / multi centre | ||
| Country / Countries | ||
| Trial design | Randomized Yes No Controlled Yes No Placebo Alternative Treatment Cross‐Over Parallel Prospective Retrospective Groups: 1 2 3 More ‐‐‐‐ |
|
| Participant characteristics | |
| Further details | |
| Total Hip replacement Total shoulder replacement Total Knee Replacement Other |
|
| @ Screening/enrolment: @ Randomization: @ Endpoint: |
|
| Age (mean, SD) | Each group all patients |
| Paediatric Population % | 0 |
| Sex of participants (men/women) | Exp Control |
| Inclusion Criteria | |
| Exclusion Criteria | |
| Comorbidities |
| Intervention | |
| Regional Nerve Block | Epidural Femoral Block Interscalene other |
| Active Ingredient | Ropivacaine Bupivacaine Other |
| Describe the Regional Technique administered | |
| Comparator | |
| Comparator | Placebo General Anesthesia Other Describe: |
| Comments | |
| Outcomes | |
| Follow up period(s) | Pre First@___ second@___ third@____ fourth@____ |
| Outcome(s) | Dichotomous Continuous Ordinal Responder data Primary: Secondary: |
Methodological quality
Free Text Comments:
| Selection bias/Allocation of intervention | ||
| State here method used to generate allocation and reasons for grading | Grade (circle) | Bias likely |
| Allocation is clearly described. An accepted randomized method is used.
Randomization is done at appropriate time point. Details: |
Adequate (Random) | No |
| Inadequate/ non‐randomized |
Yes | |
| Unclear | Unclear | |
|
Performance bias/Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | ||
| State here method used to conceal allocation and reasons for grading | Grade (circle) | Bias likely? |
| Concealment of allocation is explained. Provider and participants are
unaware of allocation throughout treatment/observation period,
respectively. Detail: ___________________________________________________________ |
Adequate | No |
| Inadequate Not pertinent |
Yes | |
| Unclear | Unclear | |
| Detection bias/Blinding | Bias likely | ||
| Person responsible for participants care | Yes/No/Unclear | ||
| Participant | Yes/No/Unclear | ||
| Outcome assessor | Yes/No/Unclear | ||
| Other (please specify) _____________________ |
Yes/No/Unclear | ||
| Comments: | |||
|
Attrition bias/Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| Loss to follow up? | Reported reported as none Uncertain/not reported not applicable |
| ITT Analysis? | Reported not reported no Uncertain not applicable |
| PP Analysis? | Reported not reported Uncertain not applicable |
| Were withdrawals described? | Yes No Not clear |
| How were lost participants/withdraw accounted for: | Last observation carried forward Information collected at end of study Uncertain/not reported Excluded |
| Comments | |
| Bias likely | Yes/No/Unclear |
|
Selective Reporting Did the study authors report the primary outcomes or is the reported significant outcome a secondary outcome, while the primary outcome of the study was negative. | |
| Primary outcome stated | Prior to study begin, e.g. in protocol After completion of study, e.g. in publication |
| Outcome was the primary outcome of the reported study | Yes No Unclear |
|
Conflict of Interest and Sponsors Is there a statement on potential conflict of interests or sponsors and did these have an undue influence on the analysis of the data | |
| Conflict of interest | Statement on COI: Yes No |
| No indication of relevant COI Unclear Possible conflict of interest apparent |
|
| Sponsors | No report on sponsors Unclear information on sponsor Sponsor reported and detailed information |
| Possible undue influence apparent Unclear Undue sponsor influence unlikely |
|
Data extraction
Primary Outcome:
Responder Data
| Table 1: Responder data @ _ weeks/months |
Treatment Group | Comparison Group | Third Group | Total |
| Number randomized | ||||
| Number analysed ITT | ||||
| Number analysed PP |
Dichotomous Data not reported
| Table 1: Comparison @ _ weeks/months |
Treatment Group | Comparison Group | Third Group | Total |
| Number randomized | ||||
| Number analysed ITT | ||||
| Number analysed PP |
| Table 2: Comparison @ _ weeks/months |
Treatment Group | Comparison Group | Third Group | Total |
| Number randomized | ||||
| Number analysed ITT | ||||
| Number analysed PP |
Other outcomes: not reported
| Outcome/Instrument | Treatment Group Value (+/‐SD) |
Comparison Group Value (+/‐SD) |
Between Group Comparison Absolute difference, confidence interval [] and P value |
Withdrawals and adverse events (report number of participants)
not reported
| Treatment Group | Comparison Group | |
| Any adverse event not reported |
||
| Withdrawals due to adverse events | ||
| Withdrawals due to any reason | ||
| Comments: | ||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
Authors contacted once/twice by email & letter Response Yes/No
| Free text space for writing actions such as contact with study authors and changes |
References to other trials/data
Are there any references to published or unpublished data in this article?
| Code each paper | Author(s) | Journal / Conference Proceedings, etc. | Year | PMID/Identifier | Published |
| A | Yes/No | ||||
| B | Yes/No | ||||
| C | Yes/No |
Data and analyses
Comparison 1. Functional outcome at 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knee flexion | 3 | 140 | Mean Difference (IV, Random, 95% CI) | 3.99 [‐2.23, 10.21] |
| 2 Knee flexion (Infiltration only) | 2 | 110 | Mean Difference (IV, Random, 95% CI) | 2.76 [‐4.59, 10.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kadic 2009.
| Methods | Partially blinded, randomized, controlled trial Random allocation to a group by drawing a sealed envelope from a pile Follow‐up: 3 months |
|
| Participants | Subjects: 58 participants enrolled (53 analysed, 48 followed up to 3
months) from one university hospital: Radboud University Nijmegen Medical
Centre in the Netherlands Operation: primary total knee replacement 2 groups (of 27 and 26 people) Mean age (SD): group 1, 67.4 (± 12); group 2, 66.8 (± 11) Sex: group 1, 7 males and 20 females; group 2, 7 males and 19 females |
|
| Interventions | Standardized anaesthetic: spinal using 15 mg of 5 mg/mL isobaric
bupivacaine
Standard analgesic regimen using paracetamol, diclofenac and a morphine IV PCA (bolus 1 mg, lockout 6 minutes) |
|
| Outcomes |
Catheter position was tested immediately after insertion using lidocaine; however, there is no information as to whether any of the blocks needed additional troubleshooting. |
|
| Funding source | Not provided | |
| Declarations of interest | None reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "For the randomization procedure, 58 sealed envelopes enclosed a note of either the study or the control group. A blinded operating room nurse drew an envelope, which allocated the patient to one of the groups." |
| Allocation concealment (selection bias) | Low risk | As above: random draw procedure with sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding for participants or personnel assessing participants during their hospital stay |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The knee function was assessed by a blinded, independent physician, 3 months after surgery at the orthopaedic outpatient centre." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "[F]ive patients were excluded from the analysis due to protocol violations
(one study, two control), a severe adverse reaction to morphine (one
control) and for not meeting the inclusion criteria (one study)." At the 3‐month assessment, data from only 48 patients are given (21 in the study group, and 17 in the control group), but no explanation is given as to why 5 more patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Knee Score and WOMAC components reported as mean and SD |
Nader 2012.
| Methods | Unblinded, randomized, controlled trial Computer‐generated random allocation sequence Follow‐up: 12 months |
|
| Participants | Subjects: 62 participants enrolled (60 followed up to 12 months) from one
university hospital: Northwestern University, Chicago, IL Operation: primary total knee replacement 2 groups (of 31 people each) Age: group 1, 65 (IQR 60 to 76); group 2, 64 (IQR 60 to 71) Sex: group 1, 13 males and 18 females; group 2, 7 males and 24 females |
|
| Interventions | All participants received spinal‐epidural anaesthesia (10 mg of 5 mg/mL
isobaric bupivacaine intrathecally, epidural test dose 3 mL of 15 mg/mL
lidocaine with 1:200,000 epinephrine) followed by epidural analgesia
(continuous infusion of 3 mL/h of 1mg/mL bupivacaine with hydromorphone 10
mcg/mL, patient boluses of 3 mL with a lockout time of 15 minutes and an
hourly maximum of 15 mL) until the morning of POD 1
No adjuvants Oral postoperative analgesic regimen in both groups: hydrocodone 10 mg plus paracetamol 325 mg every 4 to 6 hours for pain as needed; if participants could not achieve a VRSP < 4, the analgesic regimen was changed to sustained release oxycodone 10 mg every 12 hours plus oral hydromorphone 2 mg every 4 hours for breakthrough pain; not different between groups |
|
| Outcomes | Primary outcome: knee flexion at 1, 6 and 12 months Secondary outcomes: patient satisfaction during hospital stay, amount of opioid analgesic consumed, verbal rating of pain during hospital stay and follow‐up visits, health‐related quality of life, and functional outcome on POD 1, POD 2, POD 3, and at 1 month, 6 months, and 12 months after surgery Effectiveness of the regional analgesia is not reported |
|
| Funding source | Stryker Instruments, Inc. and departmental funds | |
| Declarations of interest | None reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was determined using a computer‐generated random allocation sequence" |
| Allocation concealment (selection bias) | Low risk | "[G]roup membership was concealed by placing the assignment slip in an opaque envelope that was not opened until after informed consent was obtained." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Participants in the CFA group received a femoral perineural catheter while participants in the OOA group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. This is not explicitly stated, but it is possible that the investigators and the surgeon assessing the participants had access to the records that would show whether each participant had received a continuous femoral block. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant in each group lost to follow‐up after 1 month |
| Selective reporting (reporting bias) | Unclear risk | The difference in knee flexion on POD 3 and at 1 month is significant but
only reported graphically, with no values given. While the components of the WOMAC score are reported as median (range), the scores themselves are not reported. |
Singelyn 1998.
| Methods | Unblinded, randomized, controlled trial Computer‐generated list of random permutations Follow‐up: 3 months |
|
| Participants | Subjects: 45 participants enrolled (not stated how many followed‐up to 3
months) from one university hospital: St. Luc Hospital in Brussels,
Belgium Operation: primary total knee replacement 3 groups (of 15 people each) Age and sex distribution not provided: "Population data (age, weight, height, gender ratio) were comparable in all groups." |
|
| Interventions | Standardized general anaesthesia for surgical procedure Analgesia for the first 48 hours:
In all groups, if VAS ≥ 1, 1 g of propacetamol was given. In groups 2 and 3, if VAS was unchanged after 30 min, 10 to 20 mg of IM piritramide and an opioid mu‐agonist were given |
|
| Outcomes |
|
|
| Funding source | Not provided | |
| Declarations of interest | None reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random permutations; no detail as to whether the list was exposed or concealed |
| Allocation concealment (selection bias) | Unclear risk | Concealment technique not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding mentioned; observers could easily see whether the participant had an epidural catheter, a femoral catheter, or none |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding mentioned; observers could refer to the chart to know what type of analgesia the participant had received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Tables suggest that no participant was lost to follow‐up, but that is not clearly stated. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Tammachote 2013.
| Methods | Partially blinded, randomized, controlled trial Computerized block randomization; sealed opaque envelopes Follow‐up: 12 weeks |
|
| Participants | Subjects: 59 participants enrolled (57 analysed and followed up to 12
weeks) from one university hospital: Thammasat University, Pathumthani,
Thailand Operation: primary total knee replacement 2 groups (of 28 and 29 people) Mean age (SD): control group, 69 (± 8); experimental group, 70 (± 7) Sex: control group, 8 males and 20 females; experimental group, 3 males and 26 females |
|
| Interventions | Spinal anaesthesia using 2.5 mL of 5 mg/mL isobaric bupivacaine
100 mg bupivacaine (5 mg/mL, 20 mL), 5 mg morphine sulphate (5 mL), 0.6 mg epinephrine (0.6 mL), 30 mg ketorolac tromethamine (1 mL), and 73.4 mL saline (total volume: 100 mL). The first 25 mL were injected into the posterior knee capsule and soft tissue around the medial and lateral collateral ligaments before implantation. Quadriceps muscle, retinacular tissues, pes anserinus, and suprapatellar and infrapatellar fat pad, then infiltrated with the rest of the mixture. Postoperative analgesia:
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding source | Not provided | |
| Declarations of interest | None reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized block randomization was done by an independent research assistant (TY) who otherwise was not engaged in the study. The anaesthesiologist assistant nurse, who opened sealed opaque envelopes containing the randomization result, allocated patients into each group." |
| Allocation concealment (selection bias) | Low risk | "The anaesthesiologist assistant nurse, who opened sealed opaque envelopes containing the randomization result, allocated patients into each group." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel was not blinded. It is unclear whether participants were blinded or aware of their group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A clinical investigator (SK) who was not aware of the randomization
collected demographic data, preoperative clinical conditions using
predesigned data sheets, and perioperative data, and entered them in a
database." However, there is no mention of blinding of the assessor of the long‐term outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two knees were excluded from analysis because one had drain dislodgement before 48 hours and the other had a periprosthetic fracture (Lewis and Rorabeck Type 2) from an accident 6 weeks after surgery." |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
Wu 2014.
| Methods | Unblinded, randomized, controlled trial Computer‐generated random sequence, sealed opaque envelopes Follow‐up: 6 months |
|
| Participants | Subjects: 79 participants enrolled (60 followed up to 6 months) from one
hospital: Yan Chai Hospital, Hong Kong, China Operation: primary total knee replacement 2 groups (of 30 people each); 19 others excluded for various reasons before or after entering the study Mean age (SD): group 1, 68.9 (± 7.5); group 2, 68.8 (± 6.4) Sex: group 1, 8 males and 22 females; group 2, 8 males and 22 females |
|
| Interventions | Spinal anaesthesia (incompletely standardized: 58 participants received
hyperbaric bupivacaine, one received levobupivacaine, and one isobaric
bupivacaine; the volume also varied: 2.5 ± 0.3 mL in one group and 2.6 ± 0.3
mL in the other) Identical standardized multimodal analgesic regimen (if not contraindicated) including paracetamol, sustained release diclofenac, opioids (codeine or morphine), and drugs to prevent the side effects of the NSAIDs and opioids. Unclear how many surgeons performed the procedures.
Participants were not mobilized until POD 1 |
|
| Outcomes |
|
|
| Funding source | Not provided | |
| Declarations of interest | None reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “These 60 patients were randomized to the CFNB and PCA groups (30 patients in each), using computer‐generated random numbers. Subjects were divided into two groups (odd against even numbers generated by the computer)." |
| Allocation concealment (selection bias) | Low risk | "The case allocation was concealed in sealed envelopes and the mode of analgesia revealed to case anaesthetist and patient after the patient was included in the study.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether the outcome assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "In all, 79 patients were recruited but 19 were excluded for various reasons . . . [R]eplacements were recruited using the permuted block technique." Some patients were excluded after entering the study for reasons such as failed spinal, infusion stopped, catheter dislodged, manpower limits, etc. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported, except for the Timed Up and Go test because of missing data |
Zhang 2011.
| Methods | Unblinded, randomized, controlled trial Computer‐generated random sequence, sealed opaque envelopes Follow‐up: 3 months |
|
| Participants | Subjects: 96 participants enrolled (80 followed‐up to 3 months) from one
hospital: Affiliated Hospital of Ningxia Medical University, Yinchuan,
China Operation: primary total knee replacement 3 groups (initial size 32 each; for analysis, groups of 26, 27 and 27) Mean age (SD): group 1, 68 (± 9); group 2, 66 (± 8); group 3, 68 (± 9) Sex: group 1, 12 males and 14 females; group 2, 13 males and 14 females; group 3, 13 males and 14 females |
|
| Interventions | General anaesthesia by the same anaesthetist (unclear whether
standardized) Celecoxib 200 mg orally the night before surgery, and twice daily on PODs 1 through 7 All surgeries performed by the same surgeon Before joint closure, an intra‐articular epidural 18G catheter was inserted, with the catheter tip running along the medial femoral condyle, usually on raw bone, medial to the metal femoral component
*Infiltration: "[A] mixture of ropivacaine 150 ml (2 mg/mL) and ketorolac 1 mL (30 mg/mL) was prepared. Of this, 50 mL was loaded into a 50‐mL syringe and used to infiltrate the skin and subcutaneous tissues in equal proportions along the whole length of the wound. The remaining 101 mL had adrenaline 0.5 mL (1 mg/mL) added, giving a total volume of 102 mL and was loaded into two further 50‐mL syringes, which were used to infiltrate below the deep tissues around the ligaments, the capsule incision and the synovium." A compression bandage and ice packs were applied around the knee joint during the first postoperative day Morphine IV PCA with 1 mg bolus and 6 min lockout but maximal 1‐hour dose of 5 mg, for 48 hours If pain remained 'intolerable', an extra dose of morphine between 5 and 10 mg was injected intramuscularly (and repeated if necessary) until a pain score of ≤ 3 was obtained |
|
| Outcomes |
|
|
| Funding source | Not provided | |
| Declarations of interest | No conflict of interest reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were divided randomly into three groups (CLIA, SLIA and controls), by a biostatistician blinded to the nature of the study, using a computer‐generated random sequence concealed in consecutively numbered opaque sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used to conceal group allocation. "Assessment of patients for inclusion and preparation of the drug treatments were performed by an investigator not otherwise involved in data collection." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Assessment of patients for inclusion and preparation of the drug
treatments were performed by an investigator not otherwise involved in data
collection. Injection of the drug treatments and the recording of
postoperative data were performed by another investigator who was blinded to
the study groups." All participants had a catheter in place. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Postoperative knee pain was evaluated by specially trained pain nurses,
who were not involved in the patients’ treatment." It is unclear, however,
whether those nurses were blinded to the participant's group allocation. No description of blinding for functional recovery evaluation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
|
| Selective reporting (reporting bias) | Low risk | All stated outcomes are reported in a detailed fashion |
CFA: continuous femoral analgesia; CFNB: continuous femoral nerve block; CLIA: continuous local infiltration analgesia; DVT: deep vein thrombosis; IQR: inter‐quartile range; IM: intramuscular;IV: intravenous POD: post‐operative day; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; OOA: Oral opioid analgesia; PCA: intravenous patient‐controlled analgesia; PO: orally; SD: standard deviation; SLIA: single‐injection local infiltration analgesia; VAS: visual analogue scale (for pain); VRSP: verbal rating score for pain.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Affas 2011 | Comparison of two regional techniques with no control group; insufficient follow‐up |
| Allen 1998a | Insufficient follow‐up (2 days) |
| Allen 1998b | Insufficient follow‐up (24 hours) |
| Andersen 2008 | Insufficient follow‐up (48 hours) |
| Andersen 2013 | Comparison of two regional techniques |
| Baranović 2011 | Insufficient follow‐up (24 hours) |
| Biboulet 2004 | Insufficient follow‐up (24 hours) |
| Borghi 2002 | Insufficient follow‐up (24 hours) |
| Bozkurt 2009 | Insufficient follow‐up (72 hours) |
| Capdevila 1999 | The study included participants who underwent both total knee replacement and knee ligament reconstruction. Despite several requests, we could not obtain the original data to use only the results of the participants having undergone a TKR |
| Chan 2013 | Abstract only. Results could not be obtained from the authors |
| Chelly 2001 | Insufficient follow‐up (5 days) |
| Chloropoulou 2013 | Insufficient follow‐up (24 hours) |
| Cucereanu Badica 2010 | Abstract only. Insufficient follow‐up (48 hours) |
| Divella 2012 | Insufficient follow‐up (3 days) |
| Hoffmann‐Klefer 2008 | Insufficient follow‐up (48 hours) |
| Ilfeld 2009 | All participants received regional analgesia for the first 24 hours, and were then administered placebo or local anaesthetic depending on their group assignment. Thus, there was no real comparison of regional analgesia vs. non‐regional analgesia. |
| Ilfeld 2011 | All participants received regional analgesia for the first 24 hours, and were then administered placebo or local anaesthetic depending on their group assignment. Thus, there was no real comparison of regional analgesia vs. non‐regional analgesia. |
| Kampe 2001 | Insufficient follow‐up (32 hours) |
| Lang 1999 | Letter to the editor commenting on Allen 1998b |
| Lee 2012 | Insufficient follow‐up (48 hours) |
| Looseley 2013 | Insufficient follow‐up (6 weeks). Abstract only |
| Marino 2009 | Insufficient follow‐up (48 hours) |
| McCarthy 2011 | Abstract only, not published as article. Follow‐up of 48 hours, therefore data was not sought out. |
| Mejia‐Terrazas 2007 | Insufficient follow‐up (48 hours) |
| Mistraletti 2006 | Comparison of two regional techniques; insufficient follow‐up |
| Nigam 2011 | Not a regional analgesia technique |
| Ning 2009 | Unable to obtain article; however, from abstract, comparison of 2 regional techniques and insufficient follow‐up (24 hours) |
| Perrier 2010 | Insufficient follow‐up (48 hours); participants undergoing surgery for hip fracture rather than elective joint replacement |
| Reinhardt 2014 | Comparison of intra‐articular infusion of local anaesthetic vs. epidural plus femoral nerve block. Insufficient follow‐up (24 hours) |
| Singelyn 1999 | Non‐randomized; 48‐hour follow‐up |
| Singelyn 2005 | Insufficient follow‐up (48 hours) |
| Sites 2004 | Insufficient follow‐up (24 hours) |
| Souron 2003 | Insufficient follow‐up (48 hours) |
| Taicher 2010 | Abstract only; retrospective review |
| Tang 2010 | Insufficient follow‐up (48 hours) |
| Tang 2010b | Insufficient follow‐up (48 hours) |
| Wang 2002 | Insufficient follow‐up (until discharge) |
| Williams 2013 | Intra‐articular infusion of local anaesthetic at a low rate (2 mL/h), insufficient to affect postoperative pain scores or morphine use |
| Wylde 2015 | For patients in the total knee replacement group, the comparison was between two regional techniques. Range‐of‐motion data was collected but not published. For patients in the total hip replacement group, only pain and quality‐of‐life data were collected. |
Characteristics of studies awaiting assessment [ordered by study ID]
Bergeron 2009.
| Methods | Partially blinded, randomized, controlled trial "Eligible patients were randomly assigned to treatment groups in blocks of different sizes (4, 6, and 8), according to a list pre‐prepared by the study epidemiologist (AV)." Follow‐up: 1 year (follow‐up of a subset of participants from a previously published study) |
| Participants | Subjects: 60 participants enrolled (27 analysed and followed‐up to one
year) from one university hospital: Jewish General Hospital, McGill
University, Montreal, Canada Operation: primary total knee replacement 3 groups (of 19, 20 and 20 people, with one participant excluded after randomization because of postoperative confusion) Age: femoral group, 65.1 (SEM 2.0); obturator group, 72 (SEM 1.8); control group, 67 (SEM 1.3) Sex: femoral group, 4 males and 15 females; obturator group, 4 males and 16 females; control group, 5 males and 15 females |
| Interventions |
|
| Outcomes |
|
| Notes | Unclear which participants were followed‐up until 1 year. More than 50% lost to follow‐up. Assessor and participant blinded, but anaesthesiologist unblinded. |
HSS: hospital for special surgery;IM: intramuscular; IV: intravenous; L2 ‐L5: lumbar; PACU: post anaesthesia care unit; PCA: patient‐controlled analgesia; POD: postoperative day; SEM: standard error of the mean
Differences between protocol and review
The following differences exist between the protocol (Atchabahian 2012) and review.
Types of studies: We initially wanted to exclude studies in which the outcome assessor was not blinded, but given the paucity of studies, we ended up including studies in which there was no clear statement as to whether the outcome assessor was blinded to group allocation.
Types of outcome measures: In the protocol, we planned to use as our primary outcome the functional result of the considered joint as evaluated by a global function score. As only one study reported such a score, we also conducted a comparison using the range of motion of the knee at three months as a surrogate.
Electronic searches: rather than adapting our MEDLINE search strategy for other databases, we developed separate search terms for each database (see Appendix 1, Appendix 3, Appendix 4).
Secondary outcomes: the WOMAC score was added to the quality of life scores, as no study used the SF‐36.
Contributions of authors
Arthur Atchabahian (AA), Gary Schwartz (GS), Charles B Hall (CBH), Claudette M Lajam (CMJ), Michael H Andreae (MHA)
Conceiving the review: AA
Co‐ordinating the review: AA and MHA
Undertaking handsearches: AA and GS
Screening search results: AA, GS and CMJ
Organizing retrieval of papers: AA and GS
Screening retrieved papers against inclusion criteria: AA, CMJ and GS
Appraising quality of papers: AA, CMJ and GS
Abstracting data from papers: AA, CMJ and GS
Writing to authors of papers for additional information: AA, GS
Providing additional data about papers: AA, MHA and GS
Obtaining and screening data on unpublished studies: AA, CMJ and GS
Data management for the review: AA, MHA and GS
Entering data into Review Manager (RevMan 5.3): AA and GS
RevMan statistical data: AA, MHA and CBH
Other statistical analysis not using RevMan: MHA and CBH
Double entry of data: AA and GS
Interpretation of data: AA, MHA, CMJ and GS
Statistical inferences: CBH, AA and MHA
Writing the review: AA, GA, CMJ and MHA
Securing funding for the review: AA
Performing previous work that was the foundation of the present study: AA and MHA
Guarantor for the review (one author): AA
Person responsible for reading and checking review before submission: AA and GS
Sources of support
Internal sources
Departmental, USA.
External sources
-
National Institutes of Health (NIH), USA, Other.
- This publication was supported in part by the CTSA Grant 1 UL1 TR001073‐01, 1 TL1 TR001072‐01, 1 KL2 TR001071‐01 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Declarations of interest
Arthur Atchabahian: none known
Gary Schwartz: none known
Charles B Hall: none known
Claudette M Lajam: none known
Michael H Andreae: see Sources of support
Edited (no change to conclusions)
References
References to studies included in this review
Kadic 2009 {published data only}
- Kadic L, Boonstra MC, Waal Malefijt MC, Lako SJ, Egmond J, Driessen JJ. Continuous femoral nerve block after total knee arthroplasty?. Acta Anaesthesiologica Scandinavica 2009;53(7):914‐20. [PUBMED: 19388886] [DOI] [PubMed] [Google Scholar]
Nader 2012 {published and unpublished data}
- Nader A, Kendall MC, Wixson RL, Chung B, Polakow LM, McCarthy RJ. A randomized trial of epidural analgesia followed by continuous femoral analgesia compared with oral opioid analgesia on short‐ and long‐term functional recovery after total knee replacement. Pain Medicine 2012;13(7):937‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Singelyn 1998 {published data only}
- Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient‐controlled analgesia with morphine, continuous epidural analgesia, and continuous three‐in‐one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesthesia and Analgesia 1998;87(1):88‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tammachote 2013 {published data only}
- Tammachote N, Kanitnate S, Manuwong S, Yakumpor T, Panichkul P. Is pain after TKA better with periarticular injection or intrathecal morphine?. Clinical Orthopaedics and Related Research 2013;471(6):1992‐9. [PUBMED: 23397315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu JW, Wong YC. Elective unilateral total knee replacement using continuous femoral nerve blockade versus conventional patient‐controlled analgesia: perioperative patient management based on a multidisciplinary pathway. Hong Kong Medical Journal 2014;20(1):45‐51. [PUBMED: 24021935] [DOI] [PubMed] [Google Scholar]
Zhang 2011 {published data only}
- Zhang S, Wang F, Lu ZD, Zhang L, Jin QH. Effect of single‐injection versus continuous local infiltration analgesia after total knee arthroplasty: a randomized, double‐blind, placebo‐controlled study. Journal of International Medical Research 2011;39(4):1369‐80. [PUBMED: 21986137] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Affas 2011 {published data only}
- Affas F, Nygårds EB, Stiller CO, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthopaedica 2011;82(4):441‐7. [PUBMED: 21561303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1998a {published data only}
- Allen HW, Liu SS, Ware PD, Nairn CS, Owens BD. Peripheral nerve blocks improve analgesia after total knee replacement surgery. Anesthesia and Analgesia 1998;87(1):93‐7. [PUBMED: 9661553] [DOI] [PubMed] [Google Scholar]
Allen 1998b {published data only}
- Allen JG, Denny NM, Oakman N. Postoperative analgesia following total knee arthroplasty: a study comparing spinal anesthesia and combined sciatic femoral 3‐in‐1 block. Regional Anesthesia and Pain Medicine 1998;23(2):142‐6. [PUBMED: 9570601] [PubMed] [Google Scholar]
Andersen 2008 {published data only}
- Andersen LØ, Husted H, Otte KS, Kristensen BB, Kehlet H. High‐volume infiltration analgesia in total knee arthroplasty: a randomized, double‐blind, placebo‐controlled trial. Acta Anaesthesiologica Scandinavica 2008;52(10):1331‐5. [PUBMED: 19025523] [DOI] [PubMed] [Google Scholar]
Andersen 2013 {published data only}
- Andersen HL, Gyrn J, Møller L, Christensen B, Zaric D. Continuous saphenous nerve block as supplement to single‐dose local infiltration analgesia for postoperative pain management after total knee arthroplasty. Regional Anesthesia and Pain Medicine 2013;38(2):106‐11. [PUBMED: 23222363] [DOI] [PubMed] [Google Scholar]
Baranović 2011 {published data only}
- Baranović S, Maldini B, Milosević M, Golubić R, Nikolić T. Peripheral regional analgesia with femoral catheter versus intravenous patient controlled analgesia after total knee arthroplasty: a prospective randomized study. Collegium Antropologicum 2011;35(4):1209‐14. [PUBMED: 22397261] [PubMed] [Google Scholar]
Biboulet 2004 {published data only}
- Biboulet P, Morau D, Aubas P, Bringuier‐Branchereau S, Capdevila X. Postoperative analgesia after total‐hip arthroplasty: comparison of intravenous patient‐controlled analgesia with morphine and single injection of femoral nerve or psoas compartment block. a prospective, randomized, double‐blind study. Regional Anesthesia and Pain Medicine 2004;29(2):102‐9. [PUBMED: 15029544] [DOI] [PubMed] [Google Scholar]
Borghi 2002 {published data only}
- Borghi B, Laici C, Iuorio S, Casati A, Fanelli G, Celleno D, et al. Epidural vs general anaesthesia [Anestesia epidurale vs generale]. Minerva Anestesiologica 2002;68(4):171‐7. [PUBMED: 12024077] [PubMed] [Google Scholar]
Bozkurt 2009 {published data only}
- Bozkurt M, Yilmazlar A, Bilgen OF. Comparing the effects of analgesia techniques with controlled intravenous and epidural on postoperative pain and knee rehabilitation after total knee arthroplasty [Article in Turkish]. Eklem Hastalıkları ve Cerrahisi Dergisi 2009;20(2):64‐70. [PUBMED: 19619108] [PubMed] [Google Scholar]
Capdevila 1999 {published data only (unpublished sought but not used)}
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, D'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91(1):8‐15. [PUBMED: 10422923] [DOI] [PubMed] [Google Scholar]
Chan 2013 {published data only}
- Chan EY, Teo YH, Ng CTL, Ng EL, Yin TWS, Assam PN, et al. Effect of femoral nerve block on ambulation following total knee arthroplasty. Annals of the Academy of Medicine of Singapore 2013;42(9 Suppl):S287. [Google Scholar]
Chelly 2001 {published data only}
- Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, et al. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. Journal of Arthroplasty 2001;16(4):436‐45. [PUBMED: 11402405] [DOI] [PubMed] [Google Scholar]
Chloropoulou 2013 {published data only}
- Chloropoulou P, Iatrou C, Vogiatzaki T, Kotsianidis I, Trypsianis G, Tsigalou C, et al. Epidural anesthesia followed by epidural analgesia produces less inflammatory response than spinal anesthesia followed by intravenous morphine analgesia in patients with total knee arthroplasty. Medical Science Monitor 2013;19:73‐80. [PUBMED: 23353589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucereanu Badica 2010 {published data only}
- Cucereanu Badica IG, Pavelescu D, Badica L, Barbilian R, Grintescu I. The efficacy of fascia iliaca compartment block (FICB) for postoperative analgesia after hip arthroplasty (HA). Regional Anesthesia and Pain Medicine 2010;35(5):E80. [Google Scholar]
Divella 2012 {published data only}
- Divella M, Cecconi M, Fasano N, Langiano N, Buttazzoni M, Gimigliano I, et al. Pain relief after total hip replacement: oral CR oxycodone plus IV paracetamol versus epidural levobupivacaine and sufentanil. A randomized controlled trial. Minerva Anestesiologica 2012;78(5):534‐41. [PUBMED: 22327039] [PubMed] [Google Scholar]
Hoffmann‐Klefer 2008 {published data only}
- Hofmann‐Kiefer K, Eiser T, Chappell D, Leuschner S, Conzen P, Schwender D. Does patient‐controlled continuous interscalene block improve early functional rehabilitation after open shoulder surgery?. Anesthesia and Analgesia 2008;106(3):991‐6. [PUBMED: 18292451] [DOI] [PubMed] [Google Scholar]
Ilfeld 2009 {published data only}
- Ilfeld BM, Ball ST, Gearen PF, Mariano ER, Le LT, Vandenborne K, et al. Health‐related quality of life after hip arthroplasty with and without an extended‐duration continuous posterior lumbar plexus nerve block: a prospective, 1‐year follow‐up of a randomized, triple‐masked, placebo‐controlled study. Anesthesia and Analgesia 2009;102(2):586‐91. [PUBMED: 19608835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilfeld 2011 {published data only}
- Ilfeld BM, Shuster JJ, Theriaque DW, Mariano ER, Girard PJ, Loland VJ, et al. Long‐term pain, stiffness, and functional disability after total knee arthroplasty with and without an extended ambulatory continuous femoral nerve block: a prospective, 1‐year follow‐up of a multicenter, randomized, triple‐masked, placebo‐controlled trial. Regional Anesthesia and Pain Medicine 2011;36(2):116‐20. [PUBMED: 21425510] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampe 2001 {published data only}
- Kampe S, Randebrock G, Kiencke P, Hünseler U, Cranfield K, König DP, et al. Comparison of continuous epidural infusion of ropivacaine and sufentanil with intravenous patient‐controlled analgesia after total hip replacement. Anaesthesia 2001;56(12):1189‐93. [PUBMED: 11736778] [DOI] [PubMed] [Google Scholar]
Lang 1999 {published data only}
- Lang SA. Postoperative analgesia following total knee arthroplasty: a study comparing spinal anesthesia and combined sciatic femoral 3‐in‐1 block. Regional Anesthesia and Pain Medicine 1999;24(1):97. [PUBMED: 9952105] [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee JJ, Choi SS, Lee MK, Lim BG, Hur W. Effect of continuous psoas compartment block and intravenous patient controlled analgesia on postoperative pain control after total knee arthroplasty. Korean Journal of Anesthesiology 2012;62(1):47‐51. [PUBMED: 22323954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Looseley 2013 {published data only}
- Looseley A, Pappin D, Knight T, Warman P, McEwen A, Key W, et al. A randomized, observer blinded, trial of intrathecal diamorphine vs femoral nerve block for postoperative analgesia following primary total knee arthroplasty. British Journal of Anaesthesia 2013;111(2):309P‐320P. [Google Scholar]
Marino 2009 {published data only}
- Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total hip arthroplasty. A randomized controlled trial. Journal of Bone and Joint Surgery, American Volume 2009;91(1):29‐37. [PUBMED: 19122076] [DOI] [PubMed] [Google Scholar]
McCarthy 2011 {published data only}
- McCarthy DM, Galbraith J, Loughnane F, Shorten G, Iohom G. A comparison of the efficacy of local anaesthetic wound infiltration versus intrathecal morphine for postoperative analgesia following total knee arthroplasty. European Journal of Anaesthesiology 2011;28(48S):124. [Google Scholar]
Mejia‐Terrazas 2007 {published data only}
- Mejia‐Terrazas GE, Zaragoza‐Lemus G, Gaspar‐Carrillo SP. Postoperative analgesia for total knee arthroplasty; a comparative study [Analgesia postoperatoria para cirugía de rodilla, estudio comparativo]. Revista Mexicana de Anestesiología 2007;30(4):197‐200. [Google Scholar]
Mistraletti 2006 {published data only}
- Mistraletti G, Cuadra‐Fontaine JC, Asenjo FJ, Donatelli F, Wykes L, Schricker T, et al. Comparison of analgesic methods for total knee arthroplasty: metabolic effect of exogenous glucose. Regional Anesthesia and Pain Medicine 2006;31(3):260‐9. [PUBMED: 16701193] [DOI] [PubMed] [Google Scholar]
Nigam 2011 {published data only}
- Nigam AK, Taylor DM, Valeyeva Z. Non‐invasive interactive neurostimulation (InterX™) reduces acute pain in patients following total knee replacement surgery: a randomised, controlled trial. Journal of Orthopaedic Surgery and Research 2011;24(6):45. [PUBMED: 21864362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ning 2009 {published data only}
- Ning HJ, Xu HT, Yuan HB, Li YK, Shi XY. Low‐dose naloxone combined with sufentanil and ropivacaine for postoperative patient‐controlled epidural analgesia in elderly patients undergoing total hip replacement. Academic Journal of Second Military Medical University 2009;30(1):65‐8. [Google Scholar]
Perrier 2010 {published data only}
- Perrier V, Julliac B, Lelias A, Morel N, Dabadie P, Sztark F. Influence of the fascia iliaca compartment block on postoperative cognitive status in the elderly [Bloc iliofascial et troubles cognitifs postopératoires chez la personne âgée]. Annales Française d'Anesthésie et de Réanimation 2010;29(4):283‐8. [PUBMED: 20122812] [DOI] [PubMed] [Google Scholar]
Reinhardt 2014 {published data only}
- Reinhardt KR, Duggal S, Umunna BP, Reinhardt GA, Nam D, Alexiades M, et al. Intraarticular analgesia versus epidural plus femoral nerve block after TKA: a randomized, double‐blind trial. Clinical Orthopaedics and Related Research 2014;472(5):1400‐8. [PUBMED: 24163093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singelyn 1999 {published data only}
- Singelyn FJ, Gouverneur JM. Postoperative analgesia after total hip arthroplasty: i.v. PCA with morphine, patient‐controlled epidural analgesia, or continuous "3‐in‐1" block? a prospective evaluation by our acute pain service in more than 1,300 patients. Journal of Clinical Anesthesia 1999;11(7):550‐4. [PUBMED: 10624638] [DOI] [PubMed] [Google Scholar]
Singelyn 2005 {published data only}
- Singelyn FJ, Ferrant T, Malisse MF, Joris D. Effects of intravenous patient‐controlled analgesia with morphine, continuous epidural analgesia, and continuous femoral nerve sheath block on rehabilitation after unilateral total‐hip arthroplasty. Regional Anesthesia and Pain Medicine 2005;30(5):452‐7. [PUBMED: 16135349] [DOI] [PubMed] [Google Scholar]
Sites 2004 {published data only}
- Sites BD, Beach M, Gallagher JD, Jarrett RA, Sparks MB, Lundberg CJ. A single injection ultrasound‐assisted femoral nerve block provides side effect‐sparing analgesia when compared with intrathecal morphine in patients undergoing total knee arthroplasty. Anesthesia and Analgesia 2004;99(5):1539‐43. [PUBMED: 15502061] [DOI] [PubMed] [Google Scholar]
Souron 2003 {published data only}
- Souron V, Delaunay L, Schifrine P. Intrathecal morphine provides better postoperative analgesia than psoas compartment block after primary hip arthroplasty. Canadian Journal of Anaesthesia 2003;50(6):574‐9. [PUBMED: 12826549] [DOI] [PubMed] [Google Scholar]
Taicher 2010 {published data only}
- Taicher BM, Scarfo K, Gandhi K, Viscusi ER. Economic perspective of three common analgesic modalities for total knee arthroplasty. Regional Anesthesia and Pain Medicine 2010;35(5):468. [Google Scholar]
Tang 2010 {published data only}
- Tang S, Xu Z, Huang Y, He K, Qian W, Weng X, et al. Effect of continuous femoral nerve block on pain score, rehabilitation and stress response after total knee arthroplasty. Regional Anesthesia and Pain Medicine 2010;35(5):E181‐2. [Google Scholar]
Tang 2010b {published data only}
- Tang S, Xu ZH, Huang YG, He K, Ren LY, Qian WW, et al. Comparison of the influences of continuous femoral nerve block and patient controlled intravenous analgesia on total knee arthroplasty. Acta Academiae Medicinae Sinicae 2010;32(5):574‐8. [DOI] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang H, Boctor B, Verner J. The effect of single‐injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Regional Anesthesia and Pain Medicine 2002;27(2):139‐44. [PUBMED: 11915059] [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only}
- Williams D, Petruccelli D, Paul J, Piccirillo L, Winemaker M, Beer J. Continuous infusion of bupivacaine following total knee arthroplasty: a randomized control trial pilot study. The Journal of Arthroplasty 2013;28(3):479‐84. [PUBMED: 23123039] [DOI] [PubMed] [Google Scholar]
Wylde 2015 {published data only (unpublished sought but not used)}
- Wylde V, Lenguerrand E, Gooberman‐Hill R, Beswick AD, Marques E, Noble S, et al. Effect of local anaesthetic infiltration on chronic postsurgical pain after total hip and knee replacement: the APEX randomised controlled trials. Pain 2015;156(6):1161‐70. [PUBMED: 25659070] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bergeron 2009 {published data only}
- Bergeron SG, Kardash KJ, Huk OL, Zukor DJ, Antoniou J. Functional outcome of femoral versus obturator nerve block after total knee arthroplasty. Clinical Orthopaedics and Related Research 2009;467(6):1458‐62. [PUBMED: 19224305] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Akeson 1987
- Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clinical Orthopaedics and Related Research 1987;219:28‐37. [MEDLINE: ] [PubMed] [Google Scholar]
Andreae 2012
- Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007105.pub2; PUBMED: 23076930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrington 2011
- Barrington MJ, Snyder GL. Neurologic complications of regional anesthesia. Current Opinion in Anaesthesiology 2011;24(5):554‐60. [PUBMED: 21869680] [DOI] [PubMed] [Google Scholar]
Constant 1987
- Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clinical Orthopaedics and Related Research 1987;214:160‐4. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feibel 2009
- Feibel RJ, Dervin GF, Kim PR, Beaulé PE. Major complications associated with femoral nerve catheters for knee arthroplasty: a word of caution. The Journal of Arthroplasty 2009;24(6 Suppl):132‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guay 2014
- Guay J, Choi P, Suresh S, Albert N, Kopp S, Pace NL. Neuraxial blockade for the prevention of postoperative mortality and major morbidity: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD010108.pub2; PUBMED: 24464831] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. British Medical Journal 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 1969
- Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end‐result study using a new method of result evaluation. The Journal of Bone & Joint Surgery. American Volume 1969;4:737‐55. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;15;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ilfeld 2006
- Ilfeld BM, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple‐masked, placebo‐controlled study. Anesthesiology 2006;105:999‐1007. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ilfeld 2010
- Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesthesia and Analgesia 2010;111(6):1552‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2013
- Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB. Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta‐analysis. British Journal of Anaesthesia 2013;110(4):518‐28. [PUBMED: 23440367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knee Society
- Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clinical Orthopaedics and Related Research 1989;248:9‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Macfarlane 2009
- Macfarlane AJ, Prasad GA, Chan VWS, Brull R. Does regional anesthesia improve outcome after total knee arthoplasty?. Clinical Orthopaedics and Related Research 2009;467:2379‐402. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacFarlane 2009b
- Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anaesthesia improve outcome after total hip arthroplasty? A systematic review. British Journal of Anaesthesia 2009;103(3):335‐45. [PUBMED: 19628483] [DOI] [PubMed] [Google Scholar]
Memtsoudis 2014
- Memtsoudis SG, Danninger T, Rasul R, Poera J, Gerner P, Stundner O, et al. Inpatient falls after total knee arthroplasty: The role of anesthesia type and peripheral nerve blocks. Anesthesiology 2014;120(3):551‐63. [PUBMED: 24534855] [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwod S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta analyses of randomised controlled trials: The QUOROM statement. The Lancet 1999;354:1896‐900. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. International Journal of Surgery 2010;8(5):336‐41. [PUBMED: 20171303] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowe 2000
- Rowe PJ, Myles CM, Walker C, Nutton R. Knee joint kinematics in gait and other functional activities measured using flexible electrogoniometry: how much knee motion is sufficient for normal daily life?. Gait and Posture 2000;12(2):143‐55. [PUBMED: 10998612] [DOI] [PubMed] [Google Scholar]
Shoji 1990
- Shoji H, Solomonow M, Yoshino S, D'Ambrosia R, Dabezies E. Factors affecting postoperative flexion in total knee arthroplasty. Orthopedics 1990;13(6):643‐9. [PUBMED: 2367246] [DOI] [PubMed] [Google Scholar]
Woolacott 2012
- Woolacott NF, Corbett MS, Rice SJC. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology 2012;51(8):1440‐6. [PUBMED: 22467082] [DOI] [PubMed] [Google Scholar]
Wylde 2011
- Wylde V, Gooberman‐Hill R, Horwood J, Beswick A, Noble S, Brookes S, et al. The effect of local anaesthetic wound infiltration on chronic pain after lower limb joint replacement: a protocol for a double‐blind randomised controlled trial. BMC Musculoskeletal Disorders 2011;12:53. [PUBMED: 21352559] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Atchabahian 2012
- Atchabahian A, Schwartz G, Hall CB, Lajam CM, Andreae MH. Regional analgesia for improvement of long‐term functional outcome after elective large joint replacement. Cochrane Database of Systematic Reviews 12, Issue 12. [DOI: 10.1002/14651858.CD010278] [DOI] [PMC free article] [PubMed] [Google Scholar]


