Highlight
AtNUDX9, a GDP-d-Man pyrophosphohydrolase in Arabidopsis, is involved in the regulation of GDP-d-Man levels affecting ammonium sensitivity via modulation of protein N-glycosylation in the roots.
Key words: Ammonium response, Arabidopsis, GDP-d-mannose pyrophosphohydrolase, l-ascorbic acid, protein N-glycosylation, Nudix hydrolase.
Abstract
GDP-d-mannose (GDP-d-Man) is an important intermediate in ascorbic acid (AsA) synthesis, cell wall synthesis, protein N-glycosylation, and glycosylphosphatidylinositol-anchoring in plants. Thus, the modulation of intracellular levels of GDP-d-Man could be important for maintaining various cellular processes. Here an Arabidopsis GDP-d-Man pyrophosphohydrolase, AtNUDX9 (AtNUDT9; At3g46200), which hydrolysed GDP-d-Man to GMP and mannose 1-phosphate, was identified. The K m and V max values for GDP-d-Man of AtNUDX9 were 376±24 μM and 1.61±0.15 μmol min–1 mg–1 protein, respectively. Among various tissues, the expression levels of AtNUDX9 and the total activity of GDP-d-Man pyrophosphohydrolase were the highest in the roots. The GDP-d-Man pyrophosphohydrolase activity was increased in the root of plants grown in the presence of ammonium. No difference was observed in the levels of AsA in the leaf and root tissues of the wild-type and knockout-nudx9 (KO-nudx9) plants, whereas a marked increase in N-glycoprotein levels and enhanced growth were detected in the roots of KO-nudx9 plants in the presence of ammonium. These results suggest that AtNUDX9 is involved in the regulation of GDP-d-Man levels affecting ammonium sensitivity via modulation of protein N-glycosylation in the roots.
Introduction
GDP-d-mannose (GDP-d-Man) is an activated sugar nucleotide that is required for many aspects of plant metabolism. This compound is necessary for the biosynthesis of ascorbic acid (AsA), an essential antioxidant in plants, and some different structural carbohydrates for the synthesis of cell wall polysaccharides, as a precursor (Bonin et al., 1997; Conklin et al., 1999) (see Supplementary Fig. S1 at JXB online). GDP-d-Man is also essential for post-translational modifications such as protein N-glycosylation and glycosylphosphatidylinositol (GPI)-anchoring (Qian et al., 2007; Jadid et al., 2011) and for the synthesis of GDP-l-fucose, a constituent of many structural polysaccharides and glycoproteins (Reiter and Vauzin, 2001). Three enzymes located in the cytosol, phosphomannose isomerase (PMI), phosphomannose mutase (PMM), and GDP-d-mannose pyrophosphorylase (GMP/VTC1), have been linked to the biosynthesis of GDP-d-Man (see Supplementary Fig. S1 at JXB online). Of these, VTC1 catalyses the reversible pyrophosphorylation: d-Man 1-phosphate+GTP↔GDP-d-Man+pyrophosphate (Conklin et al., 1999). Arabidopsis vtc1-1 mutants, which accumulate low levels of AsA (approximately 35% that of wild-type plants), show pleiotropic phenotypes, such as altered defence in response to oxidative stress and hormone homeostasis, and inhibited growth and development (Conklin et al., 1996, 2000; Lukowitz et al., 2001; Pastori et al., 2003). On the other hand, a mutation in the gene encoding enzymes downstream of VTC1 in the AsA biosynthetic pathway, l-galactose-1-phosphate phosphatase (GPP/VTC4), whose products are only used to synthesize AsA (see Supplementary Fig. S1 at JXB online), hardly showed such pleiotropic phenotypes (Qin et al., 2008). Thus, it appears that some of these pleiotropic phenotypes of vtc1 cannot be directly attributed to an AsA deficiency, suggesting the importance of maintaining appropriate levels of GDP-d-Man in cells.
A relationship has recently been reported between the metabolism of GDP-d-Man and sensitivity to ammonium ( ), a major nitrogen source for plants. A previous study demonstrated that root growth by vtc1-1 mutants was stunted in the presence of , whereas they developed roots similar to those of wild-type plants in the absence of (Barth et al., 2010). The vtc1-1 mutants were also found to have N-glycosylation defects, enhanced programmed cell death, and some cell-cycle defects in the presence of NH4 + (Qin et al., 2008; Kempinski et al., 2011). Root elongation was previously shown to be sensitive to in the mutants of DPMS1 (dpms1-1), which acts downstream of VTC1 and mediates the biosynthesis of dolichol phosphate mannose (Dol-phosphate-Man), required for the synthesis of N-glycoproteins and GPI-anchored proteins (Jadid et al., 2011). These previous findings suggested not only that root growth in the presence of was regulated by protein N-glycosylation, but also that subcellular levels of GDP-d-Man affected various cellular processes possibly via the modulation of cell-wall synthesis, protein N-glycosylation, and GPI-anchoring. However, it currently remains unclear how GDP-d-Man levels are regulated.
Nudix hydrolases are distributed in all living organisms and constitute a large family of proteins that share a highly conserved amino acid sequence, GX 5EX 7REUXEEXGU, in which U is an aliphatic, hydrophobic residue, although several examples exist with altered consensus sequences (Bessman et al., 1996; McLennan, 2006; Xu et al., 2006). Nudix hydrolases play protective, regulatory, and signalling roles in various metabolic pathways by hydrolysing a wide variety of substrates that contain a nucleoside diphosphate linked to some other moiety, X, such as oxidized nucleotides, dinucleoside polyphosphates, nucleotide sugars, coenzymes, and capped RNAs (Bessman et al., 1996; McLennan, 2006; Kraszewska, 2008, Yoshimura and Shigeoka, 2015). Arabidopsis has 28 genes (AtNUDX1–27 and AtDCP2) that encode the Nudix hydrolase homologues distributed to the cytosol and organelles such as chloroplasts and mitochondria (Ogawa et al., 2005, 2008). Although Nudix hydrolases in Arabidopsis were initially named as ‘AtNUDT’, we renamed them ‘AtNUDX’ because the Arabidopsis gene was not numbered with reference to the closest orthologue of human gene, although both genes were named as NUDT (Yoshimura and Shigeoka, 2015). Various AtNUDXs have been shown to have pyrophosphohydrolase activities with a wide range of substrate specificities; 8-oxo-7,8-dihydro-2′-(deoxy) guanosine 5′-triphosphate (AtNUDX1), ADP-d-ribose (AtNUDX2 and 7), ADP-d-glucose (AtNUDX14), NAD(P)H (AtNUDX6, 7, and 19), coenzyme A (AtNUDX11 and 15), thiamine diphosphate (AtNUDX20), FAD (AtNUDX23), guanosine-3′,5′-tetraphosphate (AtNUDX26), long-chain diadenosine polyphosphates (ApnA)(AtNUDX13, 25, 26, and 27), and mRNA caps (AtDCP2) (Ogawa et al., 2005, 2008, 2009; Munoz et al., 2006; Yoshimura et al., 2007; Gunawardana et al., 2008; Ishikawa et al., 2009, 2010a, b ; Ito et al., 2012a, b; Maruta et al., 2012; Goyer et al., 2013). However, numerous AtNUDXs (AtNUDX3–5, 8, 9, 12, 16–18, 21, 22, and 24) do not exhibit this activity toward any substrates analysed previously (Ogawa et al., 2005, 2009). GDP-d-Man was identified as a substrate for the Nudix hydrolases, Orf1.9 and Orf191, in Escherichia coli (Frick et al., 1995; Xu et al., 2006), suggesting that these enzymes had the potential to be involved in the metabolism of GDP-d-Man.
In the present study, the cytosolic GDP-d-Man pyrophosphohydrolase (AtNUDX9; At3g46200) was identified in Arabidopsis plants. The enzymatic properties and tissue-specific expression of AtNUDX9 were investigated as well as the sensitivity of AtNUDX9-disrupted plants. In addition, the levels of AsA and glycoprotein were analysed in the disrupted plants. The present results indicated that AtNUDX9 is involved in the metabolism of GDP-d-Man through the hydrolysis of GDP-d-Man, which then modulates responses.
Materials and methods
Expression and purification of recombinant AtNUDX proteins
The recombinant forms of cytosolic AtNUDXs (AtNUDX1–11 and 25) were produced using E. coli strain BL21 (DE3) pLysS cells transformed with pET16b/AtNUDX1–3 and 5–7, pCold II/AtNUDX4, 9–11, and 25, and pCold TF/AtNUDX8 following previously described methods (Ogawa et al., 2005). The respective proteins were purified from the extracts using a His Trap HP column (GE Healthcare, Little Chalfont, UK). Protein contents were determined by the Bradford method, using bovine serum albumin as a standard (Bradford, 1976). The molecular masses of the recombinant AtNUDX proteins were consistent with the predicted values calculated from the amino acid sequence of the mature protein plus the hexahistidine-tag (see Supplementary Fig. S2 at JXB online).
Enzyme assay
GDP-d-Man and GDP-l-fucose were purchased from YAMASA CORPORATION (Chiba, Japan). GDP-d-glucose was purchased from Sigma (St Louis, MO, USA). The hydrolytic activities of the recombinant forms of AtNUDXs toward GDP-d-Man, GDP-d-glucose, and GDP-l-fucose were assayed according to a previously described method (Ogawa et al., 2005). The reaction mixture (60 μl) containing 50mM TRIS–HCl (pH 8.0), 5mM MgCl2, 100 μM substrate, and 1.0 μg of the purified recombinant protein, was incubated at 37 °C for 10min. In the assay for K m, GDP-d-Man was added at 50 μM to 2mM to the reaction. In the assay for divalent cation-dependency, Mg2+ was substituted with Cu2+, Zn2+, Ca2+, or Mn2+ (1mM or 5mM each). The reaction was terminated by adding 10 μl of 100mM EDTA. The mixture was then analysed by HPLC using a COSMOSIL C18 column (4.6×250mm, Nacalai Tesque, Kyoto, Japan) at a flow rate of 0.6ml min–1 for the mobile phase buffer, which contained 73mM KH2PO4, 5mM tetrabutylammonium dihydrogenphosphate, and 12.5% methanol. The substrates (GDP-d-Man, GDP-d-glucose, and GDP-l-fucose) and reaction product (GMP) were detected by their UV absorbance at 260nm. Blanks without either enzyme or divalent cation were run in parallel.
Due to interference of GDP-d-Man detection by UV absorbance at 260nm by contaminants in the plant extract, GDP-d-Man pyrophosphohydrolase activities in crude extracts from Arabidopsis seedlings were determined by detecting d-Man 1- phosphate using high performance anion exchange chromatography coupled with the pulsed amperometric detection (HPAEC-PAD) system using the ICS-3000 ion chromatography system (Dionex, Sunnyvale, CA). The leaves (0.2g) of Arabidopsis plants were homogenized with 0.5ml of 100mM TRIS–HCl (pH 8.0) containing 20% glycerol. After centrifugation (20 000×g) for 20min at 4 °C, the supernatant was used to analyse enzymatic activity. The reaction mixture (10 μl) was automatically injected onto the column of CarboPac PA1 guard (2×50mm) and CarboPac PA1 (2×250mm), and eluted with the NaOH/sodium acetate gradient. The sodium acetate gradient was increased from 0mM to 200mM between 16min and 24min with a flow rate set at 0.25ml min–1. Detection of the reaction product (d-Man 1-phosphate) was achieved by a pulsed amperometric cell using an electrochemical detector equipped with a working gold electrode and combined pH-Ag/AgCl reference electrode.
Semi-quantitative RT-PCR analysis
Total RNA extracted from various tissues of 5-week-old Arabidopsis plants, including rosette leaves, stems, cauline leaves, inflorescences, and roots, was purified with the QuickGene RNA cultured cell Kit S (KURABO, Osaka, Japan), and then treated with DNase I to eliminate any DNA contamination (Takara, Shiga, Japan). First-strand cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan) with an oligo(dT) primer. cDNAs encoding AtNUDX9 and Actin8 were semi-quantitatively amplified by PCR using the following primer sets; AtNUDX9-F (5′-GTGTTCGAGCTTCTTCCATGGCG-3′), AtNUDX9-R (5′-CCCCCAGGGAATACATAGTGTCC-3′), Actin8-F (5′-GAGATCCACATCTGCTGG-3′), and Actin8-R (5′-GCTGAGAGATTCAGGTGCCC-3′). The Actin8 transcript was used as a constitutive control. PCR conditions were as follows: 32 cycles for AtNUDX9, and 20 cycles for Actin8. Thermal control for amplification was defined by cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s; and a final extension at 72 °C for 10min. The PCR products were analysed on 2% agarose gels. The equal loading of each amplified gene sequence was determined with the control Actin8 PCR product.
Western blot analysis
Western blot analysis was carried out as described previously by Yoshimura et al. (2004). Protein bands were detected by an anti-AtNUDX9 polyclonal rabbit antibody prepared using the recombinant protein as the primary antibody and an anti-rabbit IgG-horseradish peroxidase conjugate (Bio-Rad) as the secondary antibody.
Plant materials and growth conditions
The mutants used in this study were derived from the wild-type Arabidopsis Col-0 ecotype. The knockout (KO)-nudx9 (SALK_025038C), knockdown (KD)-nudx9 (SALK_027992), and vtc1-1 (CS8326) mutants were obtained from the ABRC. These plants were selfed to check for segregation and to obtain a purely homozygous line. T3 or M3 seeds were harvested and used for the experiments. Surface-sterilized wild-type and mutant seeds were sown on half-strength Murashige and Skoog (MS) medium containing 1% sucrose. Plates were stratified in darkness for 2 d at 4 °C and then transferred to a growth chamber kept at 23 °C during 16h of light (100 μmol photons m–2 s–1) and at 22 °C during 8h of darkness (normal growth conditions). In the assay for the NH4 + response, surface-sterilized seeds were grown on full-strength MS medium with or without ammonium nitrate (20.6mM)(but still containing 18.8mM potassium nitrate), and grown under normal growth conditions. The seedlings were collected as samples 4h after illumination. All experiments were repeated at least three times using independent batches of plant (more than 20 seedlings) as biological replicates.
Leaf area determination
Total rosette surface area (hereafter called leaf area) was measured using Image J as described previously (Schindelin et al., 2012; Maruta et al., 2014).
Measurement of AsA and DHA levels
Plant tissue was frozen in liquid N2 and used in the AsA and DHA analyses. AsA and DHA levels were determined spectrophotometrically using AsA oxidase as described previously (Maruta et al., 2008).
N-Glycoprotein analysis
Total protein samples were prepared as described above. Proteins in the supernatant were separated by SDS-PAGE in a 12% slab gel and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) using an electroblot apparatus (model 200/2.0, Bio-Rad). The membrane was incubated for 1h in PBSCT buffer (138mM NaCl, 2.7mM KCl, 10mM Na2HPO4, 1.8mM KH2PO4, 1mM MgCl2, 1mM CaCl2, 0.5% Tween 20, pH 7.2) and then for 1h in PBSCT buffer containing 0.1 μg ml–1 ConA conjugated to horseradish peroxidase (Sigma, St Louis, MO, USA). The membrane was washed five times for 5min with PBSCT. ConA-binding glycoproteins were detected with the ECL Select Western Blotting Detection System (GE Healthcare). The ConA-specific bands were quantified using Image J software.
Statistical analyses
Statistical differences were evaluated by analysis of variance (ANOVA) using Microsoft Excel 2010 software (ver. 14.0). Differences were considered significant at a probability level of P <0.05.
Accession numbers
Arabidopsis Genome Initiative locus identifiers for the genes described in this study are as follows: AtNUDX1 (At1g68760), AtNUDX2 (At5g47650), AtNUDX3 (At1g79690), AtNUDX4 (At1g18300), AtNUDX5 (At2g04430), AtNUDX6 (At2g04450), AtNUDX7 (At4g12720), AtNUDX8 (At5g47240), AtNUDX9 (At3g46200), AtNUDX10 (At4g25434), AtNUDX11 (At5g45940), AtNUDX25 (At1g30110), VTC1 (At2g39770), Actin8 (At1g49240).
Results and discussion
Identification of AtNUDXs having pyrophosphohydrolase activity toward GDP-d-Man
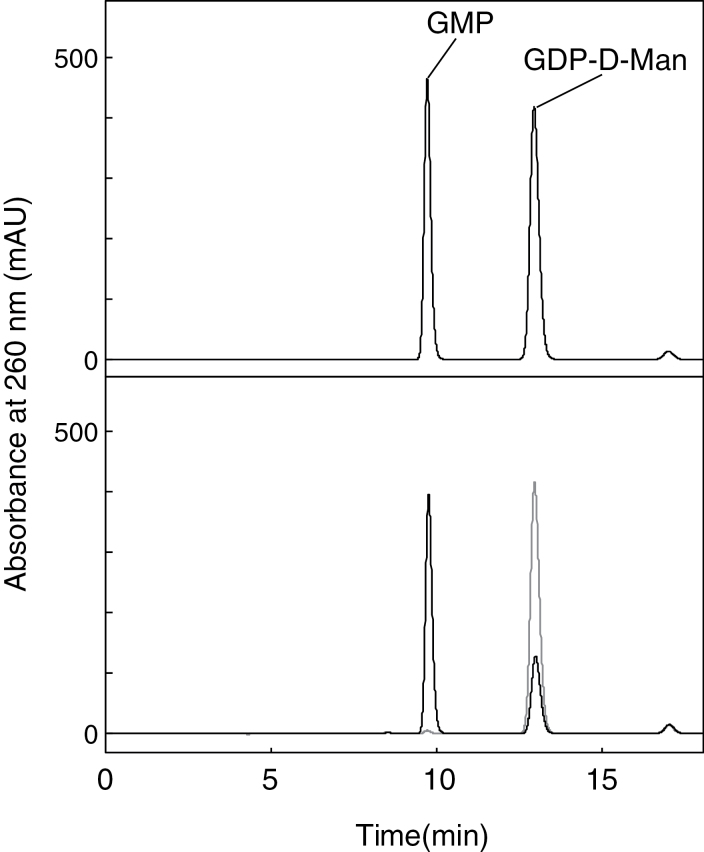
Considering the subcellular localization of the enzymes involved in the synthesis of GDP-d-Man (Wheeler et al., 1998; Conklin et al., 1999), the AtNUDX(s) having activity toward GDP-d-Man should exist in the cytosol. Thus, the purified recombinant proteins of cytosolic-type AtNUDXs (AtNUDX1–11, 25) were prepared. Their pyrophosphohydrolase activities toward GDP-d-Man in the presence of 5mM Mg2+ as a cofactor were examined by HPLC analysis as described above. No activity could be detected in recombinant AtNUDX1-8, 10, 11, and 25, but a reduction was observed in the peak of GDP-d-Man in the reaction mixture containing recombinant AtNUDX9 (Fig. 1). In addition to the peak in GDP-d-Man as a substrate, the first peak that was eluted at approximately 9.7min corresponded to that of standard GMP. The activities were linear with time and amount of the enzyme, AtNUDX9. E. coli Orf191 has been shown to hydrolyse GDP-d-Man to GDP and mannose, whereas E. coli Orf1.9 hydrolyses GDP-d-Man to GMP and d-Man 1-phosphate (Frick et al., 1995). Our results indicated that, similar to E. coli Orf1.9, AtNUDX9 exhibited pyrophosphohydrolase activity toward the diphosphate linkage in GDP-d-Man, and generated GMP and d-Man 1-phosphate as final products (see Supplementary Fig. S1at JXB online; Fig. 1). The possibility cannot be excluded that other members of the AtNUDX family have the GDP-d-Man pyrophosphohydrolases activity in the presence of other divalent cations, such as Mn2+ and Zn2+, as a cofactor. Ogawa et al. (2005) have already demonstrated that AtNUDX9 had no detectable activity toward various types of nucleotide sugars (ADP-d-ribose, ADP- and UDP-d-glucose, UDP-l-galactose), in addition to deoxynucleoside triphosphates (dNTPs), 8-oxo-7,8-dihydro- 2′-(deoxy) guanosine 5′-triphosphate, ApnA, NADH, FAD, and coenzyme A. On the other hand, E. coli Orf1.9 was reported to have activity toward not only GDP-d-Man, but also GDP-d-glucose and GDP-l-fucose with relatively low efficiency (Frick et al., 1995). However, AtNUDX9 had no detectable activity toward GDP-d-glucose and GDP-l-fucose. Therefore, it was concluded that AtNUDX9 has high specificity to GDP-d-Man as a substrate for its hydrolysis activity. This substrate and the resulting products (GDP-d-Man+H2O and d-Man 1-phosphate+GMP) corresponded partly to those of the reverse reaction of VTC1, which catalyses the reversible reaction (d-Man 1-phosphate+GTP↔GDP-d-Man+pyrophosphate) (Conklin et al., 1999) (see Supplementary Fig. S1 at JXB online). It is possible that the AtNUDX9 reaction competes with that of VTC1 and perturbs the equilibration between intracellular d-Man 1-phosphate and the GDP-d-Man levels maintained by VTC1.
Fig. 1.
Identification of GDP-d-Man degradation products by AtNUDX9. Reaction mixture containing 100 μM (2 nmol per 20 μl) GDP-d-Man was incubated with 5mM Mg2+ in the absence or presence of the purified recombinant AtNUDX9 protein at 37 °C for 10min and then subjected to HPLC with the COSMOSIL C18 column as described in the Materials and methods. The elution profile of the reaction mixture without the enzyme (grey line) or with the enzyme (black line) is shown. Positions of standards (2 nmol of GMP and 2 nmol of GDP-d-Man) are shown in the upper panel.
Enzymatic properties and kinetic parameters of AtNUDX9 as a GDP-d-Man pyrophosphohydrolase
Divalent metal ions are required for the activity of Nudix hydrolases (Mildvan et al., 2005). Therefore, the effects were examined of a series of divalent metal ions on the activity of AtNUDX9 (Table 1). No activity was detected in the absence of metal ions. The activity of AtNUDX9 was the highest in the presence of Mg2+ (5mM); it was 20–27 % in the presence of Zn2+, Mn2+, Ca2+, and Cu2+ (5mM each). The AtNUDX9 activity toward GDP-d-Man in the presence of 1mM Mg2+ was approximately 17% of that in 5mM Mg2+ (Table 1). Mg2+ is known most effectively to induce the activities of almost all of the other Nudix hydrolases from various organisms (Gasmi and McLennan, 2001; Okuda et al., 2004; Klaus et al., 2005; Ogawa et al., 2005; Xu et al., 2006; Ito et al., 2012b; Goyer et al., 2013). Considering that the typical cytosolic concentration of Mg2+ in plants is higher than that of the other divalent ions (Klaus et al., 2005), it is suggested that the divalent ion essential for AtNUDX9 activity is Mg2+.
Table 1.
The requirement of divalent cations for AtNUDX9 activity
The requirement of divalent cations for GDP-d-Man pyrophosphohydrolase activity was determined in the presence and the absence of divalent cations (5mM) at 37 °C for 10min, as described in the Materials and methods.
| Metal ions | Concentration | Relative Activity (%) GDP-d-Mannose |
|---|---|---|
| Mg2+ | 5 mM | 100 |
| 1 mM | 17.1 | |
| None | <1.0 | |
| Zn2+ | 5 mM | 20.6 |
| Mn2+ | 5 mM | 27.2 |
| Ca2+ | 5 mM | 23.9 |
| Cu2+ | 5 mM | 21.6 |
The kinetic parameters for GDP-d-Man were measured in the presence of Mg2+ (Table 2). The apparent K m and V max values for GDP-d-Man of AtNUDX9 were estimated from Lineweaver–Burk plots (see Supplementary Fig. S3 at JXB online). The K m and V max values of AtNUDX9 were 376±24 μM and 1.6±0.15 μmol min–1 mg–1 protein, respectively. The V max of AtNUDX9 was almost the same as that of E. coli orf1.9 (1.6±0.1 μmol min–1 mg–1 protein), but was markedly lower than that of E. coli Orf191 (207±9.2 μmol min–1 mg–1 protein) (Xu et al., 2006). On the other hand, the K m value of AtNUDX9 was approximately 50% lower than that of E. coli Orf191 (810±120 μM). Therefore, AtNUDX9 had moderate catalytic efficiency (k cat /K m) for GDP-d-Man, similar to E. coli orf1.9.
Table 2.
Analysis of the enzymatic properties of AtNUDX9
The standard assay was used with concentrations of 50 μM to 2mM for GDP-d-Man at 37 °C with 5mM Mg2+, as described in the Materials and methods. Data are means of three independent determinations ±SD.
| K m (mM) | V max (μmol min–1 mg–1 protein) | k cat (s–1) | k cat/K m (s–1 M–1) | |
|---|---|---|---|---|
| AtNUDX9 | 0.376±0.024 | 1.61±0.15 | 0.93±1.50 | 2.4×103 |
| E. coli orf1.9a | 0.30±0.08 | 1.6±0.1 | 0.49±0.03 | 1.6×103 |
| E. coli orf191b | 0.81±0.12 | 207±9.2 | 75±3.3 | 9.3×104 |
a Frick et al. (1995).
b Xu et al. (2006).
Previous studies reported that the activities of PMI1 and l-galactose dehydrogenase, which is involved in AsA biosynthesis, were inhibited by AsA itself, suggesting the existence of a feedback control mechanism (Mieda et al., 2004; Maruta et al., 2008), although it is debatable whether such inhibition occurs in vivo (Linster and Clarke, 2008). For example, incubation with 5mM AsA was shown to inhibit 52% of the activity of PMI1, an enzyme involved in the GDP-d-Man/AsA biosynthetic pathway (Maruta et al., 2008). The activity of AtNUDX9 remained largely unchanged even after incubation with 5mM AsA (see Supplementary Table S1 at JXB online). The activity of AtNUDX9 was only slightly inhibited by incubation with H2O2 (see Supplementary Table S1 at JXB online). These results suggest that the activity of AtNUDX9 is independent of feedback control by AsA and the cellular redox state.
Comparison of amino acid sequences between AtNUDX9 and other GDP-d-Man pyrophosphohydrolases from various organisms
The amino acid sequences of AtNUDX9 were compared with other GDP-d-Man pyrophosphohydrolases from various organisms. As shown in Fig. 2, similar to AtNUDX1 and 7, the typical Nudix hydrolases contained the highly conserved Nudix motif as the active site. However, previous studies demonstrated that E. coli orf1.9, but not orf191, had amino-acid replacements in the Nudix motif, by which the conserved two glutamic acid residues and bulky aliphatic residue (isoleucine, valine, or leucine) were substituted by other residues, suggesting that such replacements were unique to the GDP-d-Man pyrophosphohydrolase (Frick et al., 1995). Similar amino-acid replacements in the Nudix motif were also observed in the sequence of AtNUDX9, but not of the other AtNUDXs (Fig. 2), suggesting the involvement of such amino-acid replacements in the pyrophosphohydrolase activity toward the diphosphate linkage in GDP-d-Man. A conserved arginine, three glutamic acid residues, and two bulky aliphatic residues were substituted in the Nudix motif of AtNUDX9. On the other hand, AtNUDX9 showed only a slight identity (>14%) to both orf191 and orf1.9.
Fig. 2.
Alignment of partial amino acid sequences surrounding the Nudix motifs of AtNUDXs with other GDP-d-Mannose pyrophosphohydrolases from various organisms. Amino acids that were highly conserved are shown by grey boxes. Amino acids conserved in the Nudix motif GX 5EX 7REUXEEXGU (where U is a hydrophobic residue) are shown in black boxes. The sequences used here were as follows AtNUDX1, At1g68760; AtNUDX7, At4g12720; Escherichia coli Orf191, X77707; Escherichia coli Orf 1.9, L11721; AtNUDX9, At3g46200; Glycine max nudix hydrolase 9, HB828438; Medicago truncatula Nudix hydrolase, HB828440; Jatropha curcas, KDP44172; Oryza sativa hypothetical protein, AK073223; Zea mays nudix type motif 22, EU962750; Hordeum vulgare predicted protein, AK375942; Physcomitrella patens hypothetical protein, EDQ77035; Mus musculus Nudt22, AW545547; Rattus norvegicus Nudt22, BC060593; Xenopus tropicalis nudt22, CR942377; Danio rerio nudt22, BC083253; and Ostreococcus tauri hypothetical protein, CAL56042.
Nudix hydrolases containing the amino-acid replacements characteristic for the GDP-d-Man pyrophosphohydrolase from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/) were then examined. Similar to AtNUDX9, amino-acid replacements were observed in many putative Nudix hydrolases from dicots (Glycine max and Medicago truncatula), monocots (Oryza sativa, Zea mays, and Hordeum vulgare), bryophytes (Physcomitrella patens), green alga (Ostreococcus tauri), mammals (Mus musculus and Rattus norvegicus), amphibians (Xenopus tropicalis), and fish (Danio rerio) (Fig. 2). Those from dicots, monocots, bryophytes, and the green alga showed high identity (49–64%) to AtNUDX9, whereas those from mammals, amphibians, and fish were relatively low (28–31%). These results suggest that the GDP-d-Man pyrophosphohydrolase was derived from a common evolutionary origin and the enzymes from phototrophs have been developed separately from those of vertebrates.
Effect of on the AtNUDX9 expression and GDP-d-Man pyrophosphohydrolase activity
Changes in the expression levels of AtNUDX9 were analysed in various tissues of 5-week-old wild-type Arabidopsis plants. Semi-quantitative RT-PCR analyses detected the expression of AtNUDX9 in all tissues tested, including the rosette leaf, cauline leaf, stem, root, and inflorescence (Fig. 3). Among these tissues, the highest expression levels were detected in the roots. This result was inconsistent with previous findings (Ogawa et al., 2005) and has been attributed to differences in the growth conditions used. As described below, the action of AtNUDX9 may be involved in response to . In the present study, Arabidopsis plants were grown on a half-strength MS medium containing abundant , whereas they were grown on soil in the previous study. A polyclonal antibody against the recombinant AtNUDX9 was produced to analyse protein levels. Western blot analyses showed that the AtNUDX9 antibody specifically cross-reacted with the recombinant AtNUDX9 protein (deduced molecular weights of AtNUDX9+hexahistidine-tag, 36.1kDa) (see Supplementary Fig. S4 at JXB online). Consistent with the results obtained by the semi-quantitative RT-PCR analysis, the levels of the AtNUDX9 protein (deduced molecular weights, 34.7kDa) were the highest in the roots, but were hardly detected in the rosette or cauline leaves (Fig. 3). The total activity of GDP-d-Man pyrophosphohydrolase in the extracts prepared from various tissues was assayed using the HPAEC-PAD system (Dionex). The results obtained showed that the levels of activities in respective tissues were similar to the levels of both AtNUDX9 mRNA and protein (Fig. 3). These results suggest that AtNUDX9 mainly acts in root tissues.
Fig. 3.
Expression of AtNUDX9 in various tissues of Arabidopsis. The mRNA and protein levels of AtNUDX9 and the activity of GDP-d-Man pyrophosphohydrolase in the rosette leaf (RL), stem (ST), root (RO), cauline leaf (CL), and inflorescence (IN) of 5-week-old Arabidopsis plants grown on half-strength MS medium were analysed. (A) Semi-quantitative RT-PCR analysis of AtNUDX9 mRNA. The equal loading of each amplified cDNA was determined with the control Actin8 PCR product. (B) Western blot analysis of the AtNUDX9 protein. The AtNUDX9 protein was detected using a specific polyclonal antibody raised against the recombinant AtNUDX9 protein. (C) Total GDP-d-Man pyrophosphohydrolase activity. Data are means ±SD for three individual experiments (n=3) using plants grown independently. Details of the procedures used are described in the Materials and methods. Values without a common letter are significantly different according to ANOVA (P <0.05).
In order to clarify the physiological function of AtNUDX9, two T-DNA insertion lines of AtNUDX9 (SALK_025038C; KO-nudx9 and SALK_027992; KD-nudx9) were obtained. The T-DNA insertion sites of KO-nudx9 and KD-nudx9 are shown in Fig. 4A. Insertion resulted in the complete loss and a marked decrease in the expression of AtNUDX9 in KO-nudx9 plants and KD-nudx9 plants, respectively (Fig. 4B). AtNUDX9 protein levels in the respective plants correlated well with the levels of mRNA (Fig. 4B). The activities of GDP-d-Man pyrophosphohydrolase in the KO-nudx9 and KD-nudx9 plants were 71% and 50% lower, respectively, than those in the wild-type plants (Fig. 4C). These results indicated that AtNUDX9 accounted for the majority of total GDP-d-Man pyrophosphohydrolase activity in Arabidopsis cells. Both plants grown on half-strength MS medium showed phenotypes similar to the wild-type plants under normal growth conditions (data not shown).
Fig. 4.
Characteristics of AtNUDX9-disrupted or -suppressed Arabidopsis plants. (A) Integration positions of T-DNA insertions in KO-nudx9 and KD-nudx9 plants. Exons and introns are represented by boxes and lines, respectively. The open reading frame and untranslated regions are indicated by black and grey boxes, respectively. (B) Semi-quantitative RT-PCR (top) and Western blot (bottom) analyses of the mRNA and protein levels, respectively, of AtNUDX9 in the rosette leaves of wild-type, KO-nudx9, and KD-nudx9 plants. The plants were grown on half-strength MS medium for 2 weeks under normal growth conditions and then used for the analysis. Semi-quantitative RT-PCR analysis was performed using specific primers for AtNUDX9 and Actin8. The equal loading of each amplified cDNA was determined with the control Actin8 PCR product. The AtNUDX9 protein was detected by Western blotting using a specific polyclonal antibody raised against the recombinant AtNUDX9 protein. Coomassie Brilliant Blue (CBB) staining of protein gels was used to control for protein loading. (C) Total GDP-d-Man pyrophosphohydrolase activity in the leaves of wild-type, KO-nudx9, and KD-nudx9 plants. Experimental conditions are the same as in (B). Data are means ±SD for three individual experiments (n=3) using plants grown independently. Details of the procedures used are described in the Materials and Methods. Values without a common letter were significantly different according to ANOVA (P <0.05).
Barth et al. (2010) demonstrated that a mutation in VTC1 resulted in enhanced sensitivity to independent of intracellular AsA levels. Considering the catalytic reaction of VTC1, this result suggests that sensitivity is facilitated by decreased levels of GDP-d-Man in plant cells. Qin et al. (2008) demonstrated that VTC1 activity was inhibited by the addition of . Therefore, the levels of expression and activity of AtNUDX9 in wild-type Arabidopsis plants grown on full-strength MS medium in the presence (+ , 20.6mM) or absence (– ) of ammonium nitrate were analysed. In both roots and leaves of 10-d-old wild-type plants, no difference was observed in the expression of AtNUDX9 between both media (Fig. 5A, B). However, it was found that the activity of GDP-d-Man pyrophosphohydrolase in the roots, but not in the leaves, was slightly, but significantly increased on the + medium (Fig. 5C), suggesting that the degradation of GDP-d-Man in the roots was activated in response to . To clarify whether the increased activity under the + medium was dependent on AtNUDX9, Mg2+-dependent GDP-d-Man pyrophosphohydrolase activity was assayed. When Mg2+, as the cofactor, was not added to the reaction mixture, a slight and a marked decrease in the activities were observed in the leaf and roots, respectively, of wild-type plants grown on both – and + mediums compared with those in the presence of Mg2+ (Fig. 5C). Similarly, the activities in the leaves and roots of the KO-nudx9 and KD-nudx9 plants were significantly lower than those in the wild-type plants grown on the – and + media (Fig. 5C). However, the levels of activities in the KO plants were lower than those in the wild-type plant in the absence of Mg2+ (Fig. 5). The difference in the activities could be due to carried-over Mg2+ from the cells to the reaction mixture. The GDP-d-Man pyrophosphohydrolase activity of the KO-nudx9 and KD-nudx9 plants was not increased on the + medium. The activities of GDP-d-Man pyrophosphohydrolase in the leaf and root tissues of KO-nudx9 plants grown on – medium were 51% and 53% lower, respectively, than those in the wild-type plants (Fig. 5C). On the other hand, the activities on + medium were 70% and 76% lower, respectively. These results indicated that AtNUDX9 accounted for 51% and 53% of the total GDP-d-Man pyrophosphohydrolase activity in the leaves and roots, respectively, under the absence of – and its contribution to the total activity was increased by 70% and 76% in the leaves and roots, respectively, in response to + . In addition, the AtNUDX9-dependent activities in the leaves and roots on + medium were increased by 1.7- and 2.4-fold, respectively, compared with those on – medium. On the other hand, the detection of the residual activities in the wild-type plants in the absence of Mg2+ and in the KO-nudx9 plants suggests that, in addition to AtNUDX9, other enzyme(s) having the GDP-d-Man pyrophosphohydrolase activity occurs. The difference in the levels between mRNA and protein, and activity in the wild-type plants suggest that AtNUDX9 is activated in response to possibly through a post-translational modification. However, the possibility could not be excluded that the semi-quantitative RT-PCR and Western blot analyses were less sensitive to detect the changes in expression levels of AtNUDX9 under different growth conditions. In addition, these findings, including the previous reports, suggest that the levels of GDP-d-Man are accurately regulated by the balance between the degradation and synthetic reactions, and the activation of AtNUDX9 and the inhibition of VTC1 by a high concentration of cause a decrease in the levels of GDP-d-Man.
Fig. 5.
Expression of AtNUDX9 in response to . The mRNA and protein levels of AtNUDX9 and the activity of GDP-d-Man pyrophosphohydrolase in the rosette leaf and root of Arabidopsis plants grown on full-strength MS medium in the presence (+ ) or absence (– ) of ammonium nitrate for 10 d. (A) Semi-quantitative RT-PCR analysis of AtNUDX9 mRNA in the wild-type plants. The equal loading of each amplified cDNA was determined with the control Actin8 PCR product. (B) Western blot analysis of the AtNUDX9 protein in the wild-type plants. The AtNUDX9 protein was detected using a specific polyclonal antibody raised against the recombinant AtNUDX9 protein. Coomassie Brilliant Blue (CBB) staining of protein gels was used to control for protein loading. (C) Total GDP-d-Man pyrophosphohydrolase activity in the wild-type, KO-nudx9, and KD-nudx9 plants. The activity in the absence of Mg2+ in the reaction mixture was also shown (wild type –Mg2+). Data are means ±SD for three individual experiments (n=3) using plants grown independently. Details of the procedures used are described in the Materials and Methods. Within tissue, values without a common letter are significantly different according to ANOVA (P <0.05).
AtNUDX9 is involved in response
In order to determine whether AtNUDX9 was involved in sensitivity, the growth of wild-type, KO-nudx9, and KD-nudx9 plants were compared as well as the vtc1-1 mutants on the – and + media. Consistent with previous findings, root growth in the vtc1-1 mutants was inhibited more than that by the wild-type plants on the + medium (Qin et al., 2008; Barth et al., 2010) (Fig. 6A–C). No significant difference was noted in primary root length between the wild-type plants and vtc1-1 mutants on the – medium. An inverse correlation was observed between AtNUDX9 and VTC1 with regard to the effects of on root growth. The primary root lengths of the KO-nudx9 and KD-nudx9 plants were longer than those of the wild-type plants on the + medium, but not on the – medium (Fig. 6A, C). The relative primary root elongation of KO-nudx9 and KD-nudx9 was sustained even under the + medium (Fig. 6B). Furthermore, the dry weight of roots and numbers of lateral roots of the KO-nudx9 and KD-nudx9 plants on the + medium were increased compared with those of the wild-type plants (Fig. 6E, G). These results indicated that AtNUDX9 negatively modulated root growth in response to , possibly through the hydrolysis of GDP-d-Man. The aerial parts of all the genotypes tested here showed similar phenotypes on the – medium (Fig. 6C, D). On the other hand, the aerial parts of KO-nudx9 and KD-nudx9 plants were larger than the wild-type and vtc1-1 plants on the + medium. In fact, the leaf size and dry weight of aerial parts of KO-nudx9 and KD-nudx9 plants on the + medium were significantly increased compared with those of the wild-type plants (Fig. 6E, F). Considering the high expression levels of AtNUDX9 in root tissues, it is likely that the enhanced root growth in the KO-nudx9 and KD-nudx9 plants causes facilitation of the growth of aerial parts.
Fig. 6.
Ammonium sensitivity of AtNUDX9-disrupted or -suppressed Arabidopsis plants. Experimental conditions are the same as in Fig. 5. (A, B) Primary root length and relative root elongation, respectively, of the wild-type, KO-nudx9, KD-nudx9, and vtc1-1 plants grown on + and – mediums. In the data of relative root elongation, 100% corresponds to a primary root length of respective plants grown on – medium for 10 d. (C, D) Phenotypes. Scale bars, 1 and 0.5cm, respectively. The numbers (1 to 4) indicate the position of leaves. (E, F, G) Dry weight of leaves and roots, leaf area, and numbers of lateral roots of 10-d-old plants. The positions of leaves are described as in (D). Data represent means ±SD of 9–15 replicates. Values without a common letter were significantly different according to ANOVA (P <0.05). (This figure is available in colour at JXB online.)
AtNUDX9 mutants have increased protein N-glycosylation
The levels of AsA in the leaf and root tissues of the wild-type, KO-nudx9, KD-nudx9, and vtc1-1 plants were compared in order to determine whether AtNUDX9 was involved in the regulation of the AsA biosynthesis. Consistent with previous findings, the levels of AsA in the leaf and root tissues of the vtc1-1 mutants was significantly lower than those of wild-type plants grown on both – and + mediums (Qin et al., 2008; Barth et al., 2010) (Fig. 7). On the other hand, no significant difference was observed in the levels of AsA in the leaf and root tissues of the wild-type, KO-nudx9, and KD-nudx9 plants grown on both mediums. These results suggest that the action of AtNUDX9 might be independent of the regulation of AsA biosynthesis, under at least normal growth conditions. The -sensitive phenotype in the vtc1-1 mutants has been attributed to N-glycosylation defects, indicating the importance of accurate levels of GDP-d-Man in N-glycosylation in root tissues of plants under a high concentration of (Barth et al., 2010). To investigate whether AtNUDX9 was involved in the N-glycosylation of some proteins, N-glycoprotein levels in the respective plants were compared using a peroxidase-conjugated ConA reagent, which bound to the branches of oligomannose chains on N-glycoproteins (Keller et al., 1999). Levels of N-glycoprotein in the root tissues of the KO-nudx9 and KD-nudx9 plants were markedly and slightly higher, respectively, than those in the wild-type plants grown on the + medium, but not on the – medium, although the levels were low in the vtc1-1 mutants (Fig. 8). On the other hand, no marked difference was observed in N-glycoprotein levels in the leaf tissues of the all genotypes grown on both mediums. These results indicated that increase and decrease of the levels of GDP-d-Man induced and suppressed, respectively, the rate of protein N-glycosylation in response to and, therefore, the AtNUDX9 might prevent excessive protein N-glycosylation in response to in the roots through the hydrolysis of GDP-d-Man. The vtc1-1 mutant showed hyperstimulation of efflux in the elongation zone, which was coincident with the -mediated inhibition of root elongation (Li et al., 2010), although the levels of in the mutants were similar to those in the wild-type plants (Qin et al., 2008; Barth et al., 2010). This implied that high levels of GDP-d-Man suppressed efflux and thereby enhanced tolerance to in the KO-nudx9 and KD-nudx9 plants, possibly through the facilitation of protein N-glycosylation.
Fig. 7.

Changes in the levels of AsA in AtNUDX9-disrupted or -suppressed Arabidopsis plants in response to . Experimental conditions are the same as in Fig. 5. The levels of AsA in the wild-type, KO-nudx9, KD-nudx9, and vtc1-1 plants were analysed. Data are means±SD for three individual experiments (n=3) using plants grown independently. Details of the procedures used are described in the Materials and methods. Values without a common letter were significantly different according to ANOVA (P <0.05).
Fig. 8.
Changes in the levels of N-glycoprotein in AtNUDX9-disrupted or -suppressed Arabidopsis plants in response to . Experimental conditions are the same as in Fig. 5. (A) The N-glycosylation of proteins in the wild-type, KO-nudx9, KD-nudx9, and vtc1-1 plants was evaluated using a ConA-peroxidase reagent (top). Coomassie Brilliant Blue (CBB) staining of protein gels was used to control for protein loading (bottom). The same results were obtained in three independent experiments and the photograph showed representative result. (B) Quantification of the levels of protein N-glycosylation. The ConA-specific bands were quantified using Image J software. Values without a common letter were significantly different according to ANOVA (P <0.05).
In the presence and absence of , the vtc1 mutation was reported to cause AsA deficiency in roots and leaves, while the mutation suppressed the levels of N-glycoprotein only in the roots on the + medium (Figs 7, 8), which was consistent with previous reports (Qin et al., 2008). In addition, unlike the protein N-glycosylation, the AsA levels were not changed by the lack of AtNUDX9. These findings indicated that GDP-d-Man is preferentially utilized for protein N-glycosylation rather than the AsA biosynthesis in both leaf and root tissues regardless of ammonium conditions. This was also supported by the previous findings that the reaction catalysed by VTC2, but not VTC1, was a rate-limiting step of the AsA biosynthesis in Arabidopsis (Laing et al., 2004; Bulley et al., 2009, 2012; Yoshimura et al., 2014). Thus, AtNUDX9 and VTC1 are likely to fine-tune the GDP-d-Man levels to modulate protein N-glycosylation, but not AsA biosynthesis, in the roots under -abundant conditions. Since the Dol-phosphate-Man synthase 1 (DPMS1), catalysing the transfer of Man from GDP-d-Man to Dol-phosphate-Man, the main carrier of the Man residue at the first step of protein N-glycosylation, locates on the cytoplasmic face of the endoplasmic reticulum (Helenius and Aebi, 2008, Jadid et al., 2011), the partitioning of GDP-d-Man in the cytosol would be important for the regulation of AsA biosynthesis and protein N-glycosylation. As described above, GDP-d-Man is also important for the synthesis of cell wall polysaccharides. It will be interesting to clarify how AtNUDX9 and VTC1 are involved in the regulation of carbon partitioning between AsA, N-glycosylated proteins, and cell-wall polysaccharides.
Conclusion
In plant cells, GDP-d-Man is an important sugar donor in the biosynthesis of AsA and non-cellulosic cell-wall polysaccharides as well as post-translational modifications. The GDP-d-Man pyrophosphohydrolase was identified for the first time in plants here. Our results demonstrate that AtNUDX9 plays a role in the response through the fine-tuning of GDP-d-Man levels in combination with VTC1 in the cytosol. AtNUDX9 and VTC1 constitute a futile cycle and therefore kinetic parameters, such as the K m and V max values, of those enzymes would affect the equilibration of the cycle. Although the kinetic parameters of VTC1 have not been demonstrated yet, it is likely that the production capacity of GDP-d-Man by the action of VTC1 is much higher than its hydrolysis by AtNUDX9, at least under normal conditions. On the other hand, net production of GDP-d-Man would decrease by activation of AtNUDX9 in response to environmental concentration. It was reported that the earliest cellular response to ammonium uptake is the alkalinization of the cytosol (Britto and Kronzucker, 2005) and the activity of recombinant VTC1 is reduced by alkaline pH (Qin et al., 2008). To uncover the importance of the degradation of GDP-d-Man by AtNUDX9, there is progress towards analysing the effects of the knockout and over-expression of AtNUDX9 on GDP-d-Man metabolism and responses to various growth conditions.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Biosynthetic pathway for GDP-d-Man in higher plants.
Supplementary Fig. S2. Purification of recombinant AtNUDX1–11 and 25.
SupplementaryFig. S3. Affinity of recombinant AtNUDX9 for GDP-d-Man.
Supplementary Fig. S4. Antibody titers to the AtNUDX9 protein using the anti-AtNUDX9 polyclonal antibody.
Supplementary Table S1. Effect of AsA and H2O2 on the AtNUDX9 activity.
References
- Barth C, Gouzd ZA, Steele HP, Imperio RM. 2010. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. Journal of Experimental Botany 61, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman MJ, Frick DN, O’Handley SF. 1996. The MutT proteins or ‘Nudix” hydrolases, a family of versatile, widely distributed, ‘housecleaning’ enzymes. Journal of Biological Chemistry 271, 25059–25062. [DOI] [PubMed] [Google Scholar]
- Bonin CP, Potter I, Vanzin GF, Reiter WD. 1997. The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP-d-mannose-4,6-dehydratase, catalyzing the first step in the de novo synthesis of GDP-l-fucose. Proceedings of the National Academy of Sciences, USA 94, 2085–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. 2005. Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new views on old paradigms. Plant, Cell and Environment 28, 1396–1409. [Google Scholar]
- Bulley SG, Wright M, Rommens C, et al. 2012. Enhancing ascorbate in fruits and tubers through over-expression of thel-galactose pathway gene GDP-l-galactose phosphorylase. Plant Biotechnology Journal 10, 390–397. [DOI] [PubMed] [Google Scholar]
- Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W. 2009. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. Journal of Experimental Botany 60, 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL. 1996. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences, USA 93, 9970–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL. 1999. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences, USA 96, 4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. 2000. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick DN, Townsend BD, Bessman MJ. 1995. A novel GDP-mannose mannosyl hydrolase shares homology with the MutT family of enzymes. Journal of Biological Chemistry 270, 24086–24091. [DOI] [PubMed] [Google Scholar]
- Gasmi L, McLennan AG. 2001. The mouse Nudt7 gene encodes a peroxisomal nudix hydrolase specific for coenzyme A and its derivatives. Biochemical Journal 357, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer A, Hasnain G, Frelin O, Ralat MA, Gregory JF, 3rd, Hanson AD. 2013. A cross-kingdom Nudix enzyme that pre-empts damage in thiamin metabolism. Biochemical Journal 454, 533–542. [DOI] [PubMed] [Google Scholar]
- Gunawardana D, Cheng HC, Gayler KR. 2008. Identification of functional domains in Arabidopsis thaliana mRNA decapping enzyme (AtDcp2). Nucleic Acids Research 36, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M. 2001. Intracellular functions of N-linked glycans. Science 291, 2364–2369. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Ogawa T, Hirosue E, Nakayama Y, Harada K, Fukusaki E, Yoshimura K, Shigeoka S. 2009. Modulation of the poly(ADP-ribosyl)ation reaction via the Arabidopsis ADP-ribose/NADH pyrophosphohydrolase, AtNUDX7, is involved in the response to oxidative stress. Plant Physiology 151, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Yoshimura K, Harada K, Fukusaki E, Ogawa T, Tamoi M, Shigeoka S. 2010. a AtNUDX6, an ADP-ribose/NADH pyrophosphohydrolase in Arabidopsis, positively regulates NPR1-dependent salicylic acid signaling. Plant Physiology 152, 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Yoshimura K, Ogawa T, Shigeoka S. 2010. b Distinct regulation of Arabidopsis ADP-ribose/NADH pyrophosphohydrolases, AtNUDX6 and 7, in biotic and abiotic stress responses. Plant Signaling and Behavior 5, 839–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Kato T, Maruta T, Tamoi M, Yoshimura K, Shigeoka S. 2012. b Enzymatic and molecular characterization of Arabidopsis ppGpp pyrophosphohydrolase, AtNUDX26. Bioscience, Biotechnology, and Biochemistry 76, 2236–2241. [DOI] [PubMed] [Google Scholar]
- Ito D, Yoshimura K, Ishikawa K, Ogawa T, Maruta T, Shigeoka S. 2012. a A comparative analysis of the molecular characteristics of the Arabidopsis CoA pyrophosphohydrolases AtNUDX11, 15, and 15a. Bioscience, Biotechnology, and Biochemistry 76, 139–147. [DOI] [PubMed] [Google Scholar]
- Jadid N, Mialoundama AS, Heintz D, et al. 2011. DOLICHOL PHOSPHATE MANNOSE SYNTHASE1 mediates the biogenesis of isoprenyl-linked glycans and influences development, stress response, and ammonium hypersensitivity in Arabidopsis . The Plant Cell 23, 1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Renz FS, Kossmann J. 1999. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. The Plant Journal 19, 131–141. [DOI] [PubMed] [Google Scholar]
- Kempinski CF, Haffar R, Barth C. 2011. Toward the mechanism of sensitivity mediated by Arabidopsis GDP-mannose pyrophosphorylase. Plant, Cell and Environment 34, 847–858. [DOI] [PubMed] [Google Scholar]
- Klaus SM, Wegkamp A, Sybesma W, Hugenholtz J, Gregoy JF, 3rd, Hanson AD. 2005. A Nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of bacteria and plants. Journal of Biological Chemistry 280, 5274–5280. [DOI] [PubMed] [Google Scholar]
- Kraszewska E. 2008. The plant Nudix hydrolase family. Acta Biochimica Polonica 55, 663–671. [PubMed] [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E. 2004. A highly specificl-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proceedings of the National Academy of Sciences, USA 101, 16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li BH, Kronzucker HJ, Shi WM. 2010. Root growth inhibition by in Arabidopsis is mediated by the root tip and is linked to efflux and GMPase activity. Plant, Cell and Environment 33, 1529–1542 [DOI] [PubMed] [Google Scholar]
- Linster CL, Clarke SG. 2008. L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends in Plant Science 13, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR. 2001. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences, USA 98, 2262–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Nakamura M, Matsuda S, Tamoi M, Ishikawa T, Shigeoka S. 2014. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis . Plant Science 219–220, 61–68. [DOI] [PubMed] [Google Scholar]
- Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S. 2008. Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. Journal of Biological Chemistry 283, 28842–28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Yoshimoto T, Ito D, Ogawa T, Tamoi M, Yoshimura K, Shigeoka S. 2012. An Arabidopsis FAD pyrophosphohydrolase, AtNUDX23, is involved in flavin homeostasis. Plant and Cell Physiology 53, 1106–1116. [DOI] [PubMed] [Google Scholar]
- McLennan AG. 2006. The Nudix hydrolase superfamily. Cellular and Molecular Life Sciences 63, 123–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S. 2004. Feedback inhibition of spinachl-galactose dehydrogenase byl-ascorbate. Plant and Cell Physiology 45, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW, Amzel LM. 2005. Structures and mechanisms of nudix hydrolases. Archives of Biochemistry and Biophysics 433, 129–143. [DOI] [PubMed] [Google Scholar]
- Munoz FJ, Baroja-Fernández E, Morán-Zorzano MT, Alonso-Casajús N, Pozueta-Romero J. 2006. Cloning, expression and characterization of a Nudix hydrolase that catalyzes the hydrolytic breakdown of ADP-glucose linked to starch biosynthesis in Arabidopsis thaliana . Plant and Cell Physiology 47, 926–934. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ishikawa K, Harada K, Fukusaki E, Yoshimura K, Shigeoka S. 2009. Overexpression of an ADP-ribose pyrophosphatase, AtNUDX2, confers enhanced tolerance to oxidative stress in Arabidopsis plants. The Plant Journal 57, 289–301. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ueda Y, Yoshimura K, Shigeoka S. 2005. Comprehensive analysis of cytosolic Nudix hydrolases in Arabidopsis thaliana . Journal of Biological Chemistry 280, 25277–25283. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Yoshimura K, Miyake H, Ishikawa K, Ito D, Tanabe N, Shigeoka S. 2008. Molecular characterization of organelle-type Nudix hydrolases in Arabidopsis. Plant Physiology 148, 1412–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Nishiyama Y, Morita EH, Hayashi H. 2004. Identification and characterization of NuhA, a novel Nudix hydrolase specific for ADP-ribose in the cyanobacterium Synechococcus sp. PCC 7002. Biochimica et Biophys Acta . 1699, 245–252. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. 2003. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. The Plant Cell 15, 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Yu C, Qin H, Liu X, Zhang A, Johansen IE, Wang D. 2007. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana . The Plant Journal 49, 399–413. [DOI] [PubMed] [Google Scholar]
- Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, Wang D, Wu P. 2008. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 105, 18308–18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD, Vauzin GF. 2001. Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Molecular Biology 47, 95–113. [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an opensource platform for biological-image analysis, Nature Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. 1998. The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369. [DOI] [PubMed] [Google Scholar]
- Xu W, Dunn CA, O’Handley SF, Smith DL, Bessman MJ. 2006. Three new Nudix hydrolases from Escherichia coli . Journal of Biological Chemistry 281, 22794–22798. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, Miyasaka H, Shigeoka S. 2004. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. The Plant Journal 37, 21–33. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Nakane T, Kume S, Shiomi Y, Maruta T, Ishikawa T, Shigeoka S. 2014. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis . Bioscience, Biotechnology, and Biochemistry 78, 60–66. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Ogawa T, Ueda Y, Shigeoka S. 2007. AtNUDX1, an 8-oxo-7,8-dihydro-2’-deoxyguanosine 5’-triphosphate pyrophosphohydrolase, is responsible for eliminating oxidized nucleotides in Arabidopsis . Plant and Cell Physiology 48, 1438–1449. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Shigeoka S. 2015. Versatile physiological functions of the Nudix hydrolase family in Arabidopsis. Bioscience, Biotechnology, and Biochemistry 79, 354–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.